Remodeling of Hepatic Glucose Metabolism in Response to Early Weaning in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Feeding Management
2.2. Sample Collection
2.3. Serum Glucose and Hepatic Glycogen Content
2.4. ATP Detection
2.5. Determination of Enzyme Activity
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Targeted Liver Metabolomics
2.8. Statistical Analysis
3. Results
3.1. Body Weight, Serum Biochemical, and Glycogen Metabolism Indicators
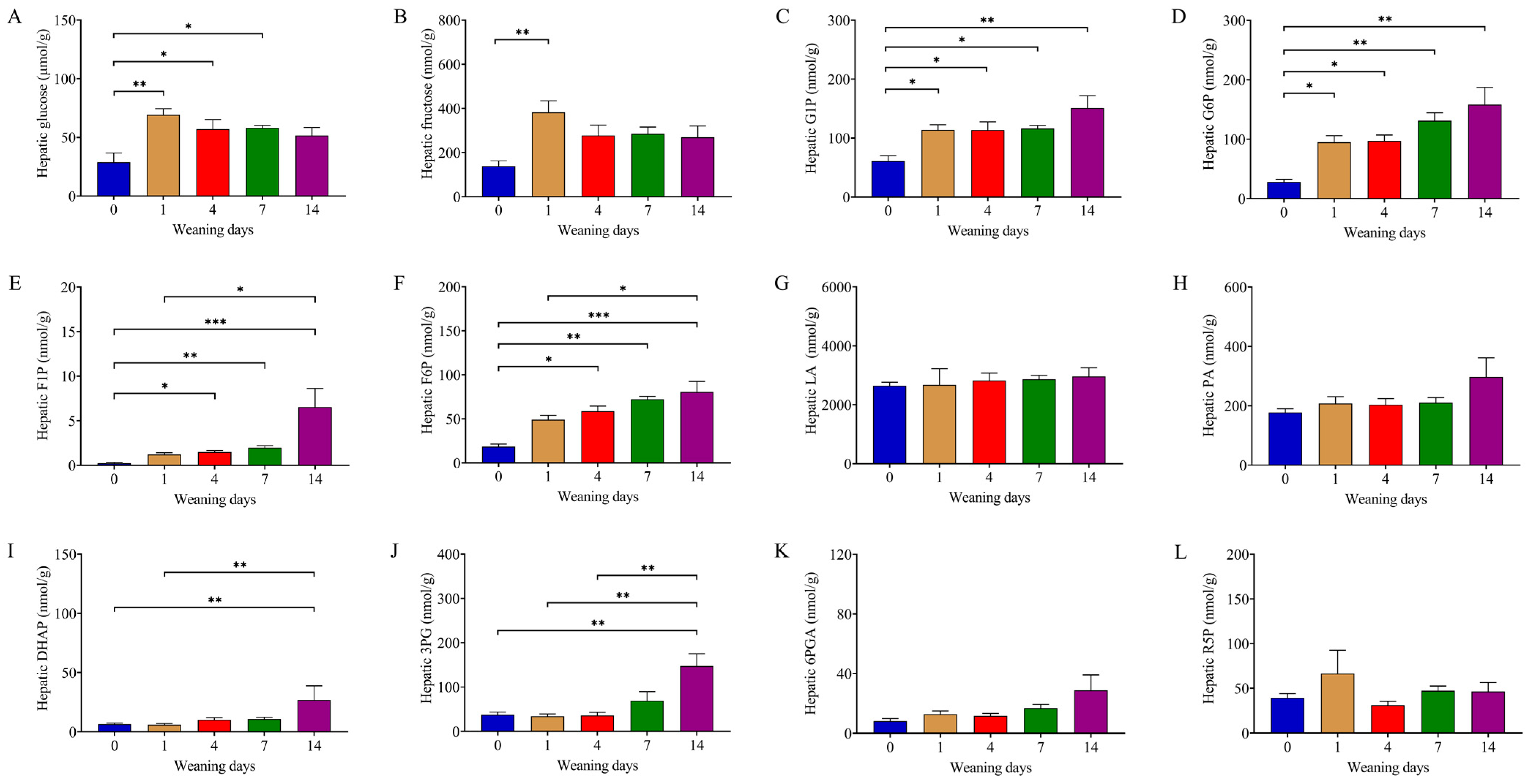
3.2. Weaning Remodeled Metabolites Related to Glycometabolism
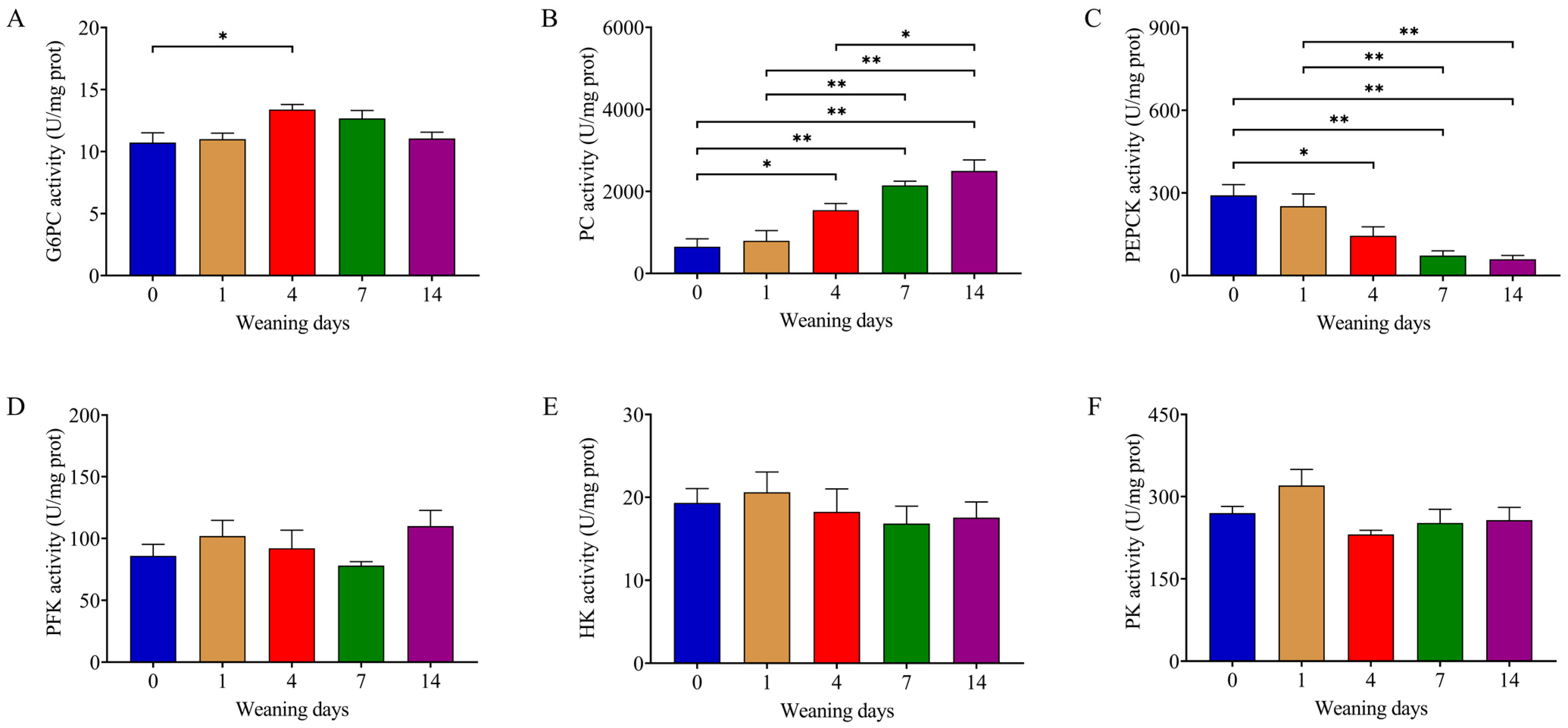
3.3. Weaning Enhanced Activity of Enzymes in Gluconeogenesis
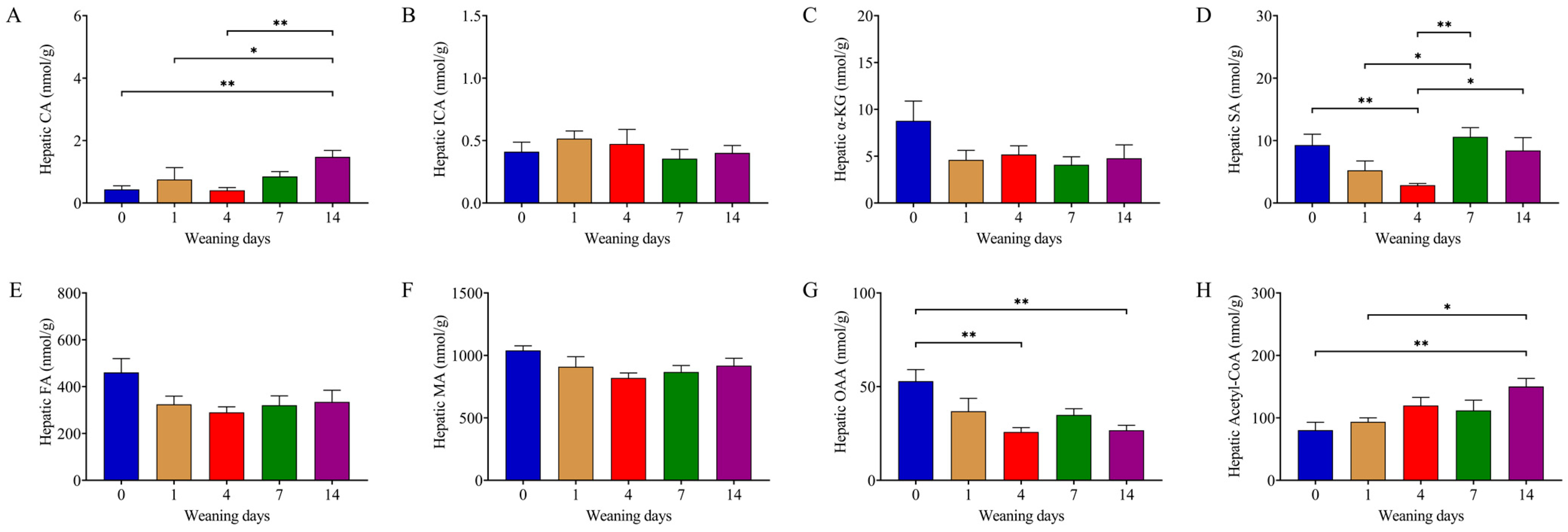
3.4. Weaning Decreased Content of Succinate in the TCA Cycle
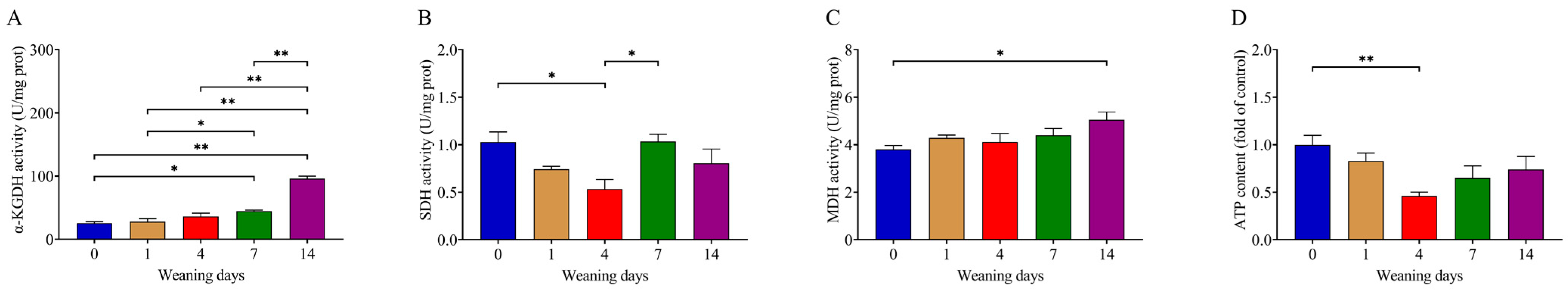
3.5. Weaning Reduced SDH Activity and ATP Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, S.; Alvarez, J.; Bicout, D.; Calistri, P.; Canali, E.; Drewe, J.; Garin-Bastuji, B.; Rojas, J.; Schmidt, G.; Herskin, M.; et al. Welfare of pigs on farm. EFSA J. 2022, 20, e07421. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.; Turpin, D.L.; Pluske, J. Weaning age and its long-term influence on health and performance. In The Suckling and Weaned Piglet; Wageningen Academic Publishers: Wageningen, The Netherlands, 2020; pp. 225–250. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A Microbiota-Derived Bacteriocin Targets the Host to Confer Diarrhea Resistance in Early-Weaned Piglets. Cell Host Microbe 2018, 24, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Faccin, J.; Laskoski, F.; Hernig, L.; Kummer, R.; Lima, G.; Orlando, U.; Gonçalves, M.; Mellagi, A.P.; Ulguim, R.; Bortolozzo, F. Impact of increasing weaning age on pig performance and belly nosing prevalence in a commercial multisite production system. J. Anim. Sci. 2020, 98, skaa031. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Crenshaw, J.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Mota Rojas, D.; Roldán Santiago, P.; Pérez Pedraza, E.; Martínez Rodríguez, R.; Henrández Trujillo, E.; Trujillo Ortega, M.E. Stress factors in weaned piglet. Vet. Mex. 2014, 10, 37–51. Available online: https://veterinariamexico.fmvz.unam.mx/index.php/vet/article/view/418 (accessed on 1 January 2024).
- Le Dividich, J.; Sève, B. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domest. Anim. Endocrinol. 2000, 19, 63–74. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef]
- Ekberg, K.; Landau, B.R.; Wajngot, A.; Chandramouli, V.; Efendic, S.; Brunengraber, H.; Wahren, J. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 1999, 48, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Rothman, D.; Magnusson, I.; Katz, L.; Shulman, R.; Shulman, G. Quantitation of Hepatic Glycogenolysis And Gluconeogenesis in Fasting Humans with 13C NMR. Science 1991, 254, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Duee, P.-H.; Pegorier, J.-P.; Darcy-Vrillon, B.; Girard, J. Glucose and Fatty Acid Metabolism in the Newborn Pig. In Advances in Swine in Biomedical Research; Plenum Press: New York, NY, USA, 1996; pp. 865–884. [Google Scholar] [CrossRef]
- Jarratt, L.; James, S.E.; Kirkwood, R.N.; Nowland, T.L. Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival. Animals 2023, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, Q.; Wang, J.; Tan, B.; Fan, Z.; Deng, Z.; Wu, X.; Yin, Y. Developmental changes in hepatic glucose metabolism in a newborn piglet model: A comparative analysis for suckling period and early weaning period. Biochem. Biophys. Res. Commun. 2016, 470, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, C.; Wang, Y.; Sun, J.; Feng, J.; Ma, Z.; Li, P.; Peng, W.; Yin, C.; Xu, G.; et al. α-Ketoglutaric acid ameliorates hyperglycemia in diabetes by inhibiting hepatic gluconeogenesis via serpina1e signaling. Sci. Adv. 2022, 8, eabn2879. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Pierce, K.; Jedrychowski, M.; Garrity, R.; Winther, S.; Vidoni, S.; Yoneshiro, T.; Spinelli, J.B.; Lu, G.; Kazak, L.; et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 2018, 560, 7716. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Li, X.; Mao, M.; Zhang, Y.; Yu, K.; Zhu, W. Succinate Modulates Intestinal Barrier Function and Inflammation Response in Pigs. Biomolecules 2019, 9, 486. [Google Scholar] [CrossRef]
- Wang, L.; Yi, D.; Hou, Y.; Ding, B.; Li, K.; Li, B.; Zhu, H.; Liu, Y.; Wu, G. Dietary Supplementation with -Ketoglutarate Activates mTOR Signaling and Enhances Energy Status in Skeletal Muscle of Lipopolysaccharide-Challenged Piglets. J. Nutr. 2016, 146, 1514–1520. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Kabasakalian, P.; Kalliney, S.; Westcott, A. Enzymatic Blood Glucose Determination by Colorimetry of N-Diethylaniline-4-Aminoantipyrine. Clin. Chem. 1974, 20, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.; Dailey, R. Determination of glycogen with the anthrone reagent. Anal. Biochem. 1966, 15, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.; Peng, Z.; Ge, Y.; Liu, J.; Wang, T.; Wang, H.; Dong, L. Early Weaning Affects Liver Antioxidant Function in Piglets. Animals 2021, 11, 2679. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, W.; Xia, J.; Wang, T.; Huang, F. Glucagon-Induced Acetylation of Energy-Sensing Factors in Control of Hepatic Metabolism. Int. J. Mol. Sci. 2019, 20, 1885. [Google Scholar] [CrossRef] [PubMed]
- Amiel, S.A. The consequences of hypoglycaemia. Diabetologia 2021, 64, 963–970. [Google Scholar] [CrossRef]
- Petersen, M.; Vatner, D.; Shulman, G. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Cappel, D.A.; Deja, S.; Duarte, J.A.G.; Kucejova, B.; Iñigo, M.; Fletcher, J.A.; Fu, X.; Berglund, E.D.; Liu, T.; Elmquist, J.K.; et al. Pyruvate-Carboxylase-Mediated Anaplerosis Promotes Antioxidant Capacity by Sustaining TCA Cycle and Redox Metabolism in Liver. Cell Metab. 2019, 29, 1291–1305. [Google Scholar] [CrossRef]
- Beale, E.G.; Harvey, B.J.; Forest, C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem. Biophys. 2007, 48, 89–95. [Google Scholar] [CrossRef]
- Eniafe, J.; Jiang, S. The functional roles of TCA cycle metabolites in cancer. Oncogene 2021, 40, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; O’Neill, L.A.J. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell 2018, 174, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Harmon, C.; Jedrychowski, M.; Xiao, H.; Garrity, R.; Tran, N.; Bradshaw, G.; Fu, A.; Szpyt, J.; Reddy, A.; et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab. 2021, 3, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.; Bisbach, C.; Robbings, B.; Sadilek, M.; Sweet, I.; Hurley, J. Succinate metabolism in the retinal pigment epithelium uncouples respiration from ATP synthesis. Cell Rep. 2022, 39, 110917. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Kelly, B.; Logan, A.; Costa, A.; Varma, M.M.; Bryant, C.; Tourlomousis, P.; Däbritz, J.H.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Attali, V.; Parnes, M.; Ariav, Y.; Cerasi, E.; Kaiser, N.; Leibowitz, G. Regulation of Insulin Secretion and Proinsulin Biosynthesis by Succinate. Endocrinology 2006, 147, 5110–5118. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Liu, K.; Lin, L.; Li, Q.; Xue, Y.; Zheng, F.; Wang, G.; Zheng, C.; Du, L.; Hu, M.; Huang, Y.; et al. Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl. Acad. Sci. USA 2020, 117, 2462–2472. [Google Scholar] [CrossRef]
- Chen, J.S.; Wu, F.; Yang, H.S.; Li, F.N.; Jiang, Q.; Liu, S.J.; Kang, B.J.; Li, S.; Adebowale, T.O.; Huang, N.; et al. Growth performance, nitrogen balance, and metabolism of calcium and phosphorus in growing pigs fed diets supplemented with alpha-ketoglutarate. Anim. Feed Sci. Technol. 2017, 226, 21–28. [Google Scholar] [CrossRef]
- Meng, M.; Zhao, X.; Huo, R.; Li, X.; Chang, G.; Shen, X. Disodium Fumarate Alleviates Endoplasmic Reticulum Stress, Mitochondrial Damage, and Oxidative Stress Induced by the High-Concentrate Diet in the Mammary Gland Tissue of Hu Sheep. Antioxidants 2023, 12, 223. [Google Scholar] [CrossRef]
- Qiu, K.; He, W.; Zhang, H.; Wang, J.; Qi, G.; Guo, N.; Zhang, X.; Wu, S. Bio-Fermented Malic Acid Facilitates the Production of High-Quality Chicken via Enhancing Muscle Antioxidant Capacity of Broilers. Antioxidants 2022, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Huang, G.; Zhang, R.; Zhang, Y.; Zhu, W.; Yu, K. Succinate communicates pro-inflammatory signals to the host and regulates bile acid enterohepatic metabolism in a pig model. Food Funct. 2022, 13, 11070–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Xiong, X.; Chen, J.; Tang, W.; He, L.; Zhang, Z.; Yin, Y.; Li, F. Intestinal accumulation of microbiota-produced succinate caused by loss of microRNAs leads to diarrhea in weanling piglets. Gut Microbes 2022, 14, 2091369. [Google Scholar] [CrossRef] [PubMed]
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 33.00 |
| Extruded corn | 22.00 |
| Fermented soybean meal | 11.20 |
| Extruded soybean | 8.00 |
| Soybean meal | 9.40 |
| Fish meal | 4.00 |
| Whey powder | 6.00 |
| Soybean oil | 1.40 |
| White granulated sugar | 1.35 |
| Choline chloride | 0.20 |
| CaHPO4 | 1.20 |
| Limestone | 0.40 |
| NaCl | 0.30 |
| L-Lys | 0.33 |
| DL-Met | 0.12 |
| L-Thr | 0.10 |
| Premix a | 1.00 |
| Total | 100.00 |
| Calculated nutrient level | |
| Metabolizable energy (MJ/kg) | 14.62 |
| Crude protein | 20.50 |
| Crude fat | 4.37 |
| Crude fiber | 2.34 |
| Crude ash | 5.80 |
| Lys | 1.17 |
| Met + Cys | 0.69 |
| Thr | 0.73 |
| Try | 0.19 |
| Calcium | 0.60 |
| Total phosphorus | 0.60 |
| Item | D0 | D1 | D4 | D7 | D14 | p-Value |
|---|---|---|---|---|---|---|
| Body weight, kg | 7.05 ± 0.16 bc | 6.58 ± 0.14 c | 6.62 ± 0.09 c | 7.34 ± 0.16 b | 9.08 ± 0.15 a | <0.001 |
| Serum glucose, mmol/L | 5.95 ± 0.46 a | 4.41 ± 0.12 b | 4.15 ± 0.33 b | 3.84 ± 0.33 b | 3.82 ± 0.26 b | <0.001 |
| Serum insulin, µIU/mL | 4.45 ± 0.16 a | 5.04 ± 0.31 a | 3.37 ± 0.17 b | 3.21 ± 0.31 b | 3.17 ± 0.10 b | <0.001 |
| Serum glucagon, pg/mL | 310.91 ± 7.08 b | 327.57 ± 4.20 b | 315.69 ± 6.09 b | 314.95 ± 3.93 b | 360.42 ± 8.18 a | <0.001 |
| Hepatic glycogen, mg/g | 40.49 ± 9.17 a | 18.38 ± 2.16 ab | 15.13 ± 2.87 b | 23.71 ± 3.17 ab | 42.25 ± 8.31 a | 0.007 |
| Hepatic GYS2, pg/mg prot | 16.84 ± 0.58 | 20.40 ± 1.50 | 19.40 ± 1.13 | 20.09 ± 1.34 | 17.63 ± 2.05 | 0.322 |
| Hepatic PYGL, pg/mg prot | 30.32 ± 1.73 b | 42.19 ± 3.37 a | 39.65 ± 1.19 ab | 41.28 ± 1.94 ab | 39.23 ± 4.58 ab | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Wang, D.; Shen, C.; Luo, Z.; Zhang, H.; Zhang, J.; Xu, W.; Xu, J. Remodeling of Hepatic Glucose Metabolism in Response to Early Weaning in Piglets. Animals 2024, 14, 190. https://doi.org/10.3390/ani14020190
Yu C, Wang D, Shen C, Luo Z, Zhang H, Zhang J, Xu W, Xu J. Remodeling of Hepatic Glucose Metabolism in Response to Early Weaning in Piglets. Animals. 2024; 14(2):190. https://doi.org/10.3390/ani14020190
Chicago/Turabian StyleYu, Chengbing, Di Wang, Cheng Shen, Zhen Luo, Hongcai Zhang, Jing Zhang, Weina Xu, and Jianxiong Xu. 2024. "Remodeling of Hepatic Glucose Metabolism in Response to Early Weaning in Piglets" Animals 14, no. 2: 190. https://doi.org/10.3390/ani14020190
APA StyleYu, C., Wang, D., Shen, C., Luo, Z., Zhang, H., Zhang, J., Xu, W., & Xu, J. (2024). Remodeling of Hepatic Glucose Metabolism in Response to Early Weaning in Piglets. Animals, 14(2), 190. https://doi.org/10.3390/ani14020190







