Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Design
2.3. Growth Performance Parameters
body weight])/time (days)
body weight]/mean initial body weight)
weight + final body weight)/2]/t
2.4. Liver Sample Preparation
2.5. RNA Extraction and Reverse Transcription
2.6. Quantitative Real-Time PCR
2.7. Data Analysis
3. Results
3.1. Effects on Growth Performances
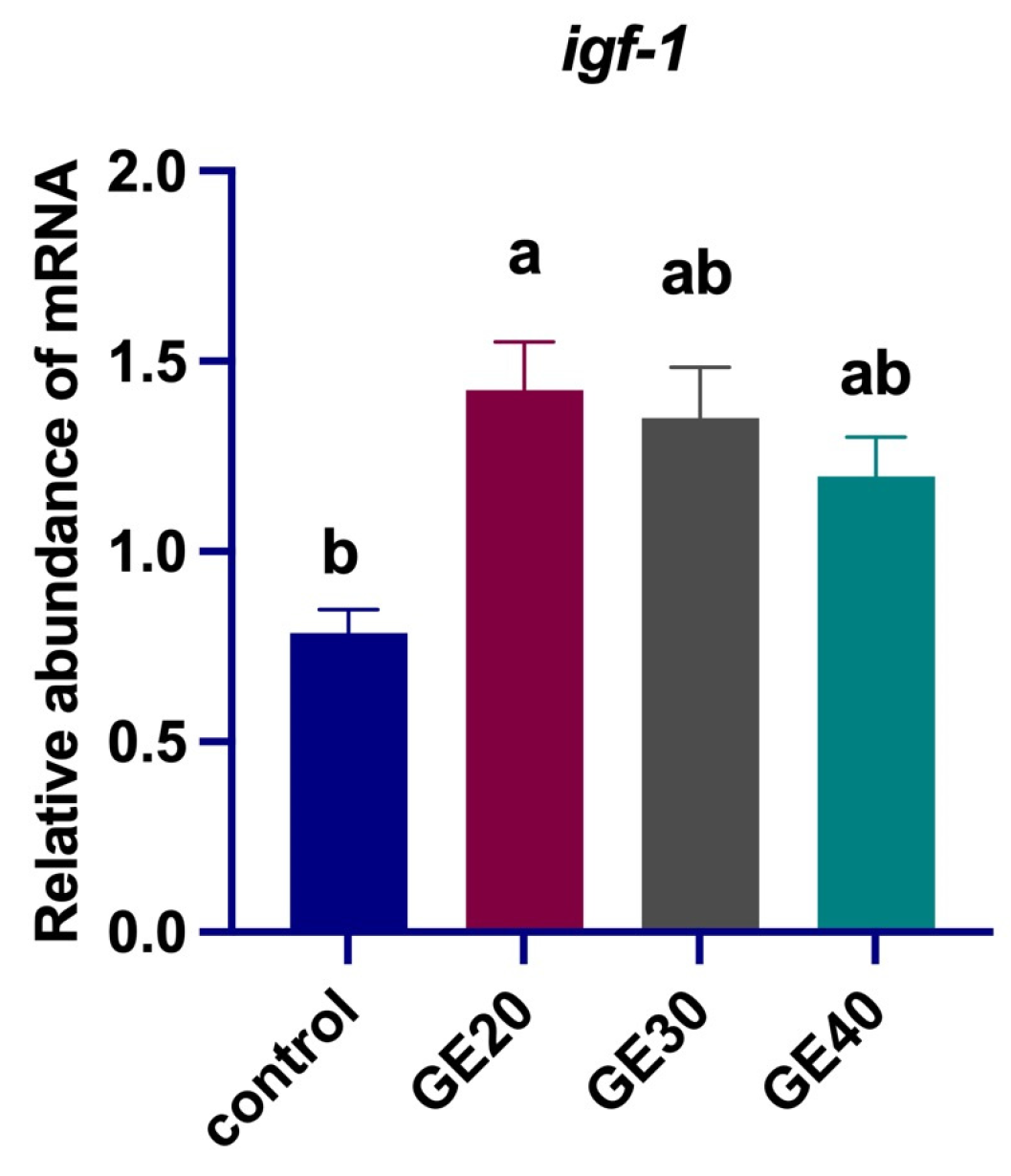
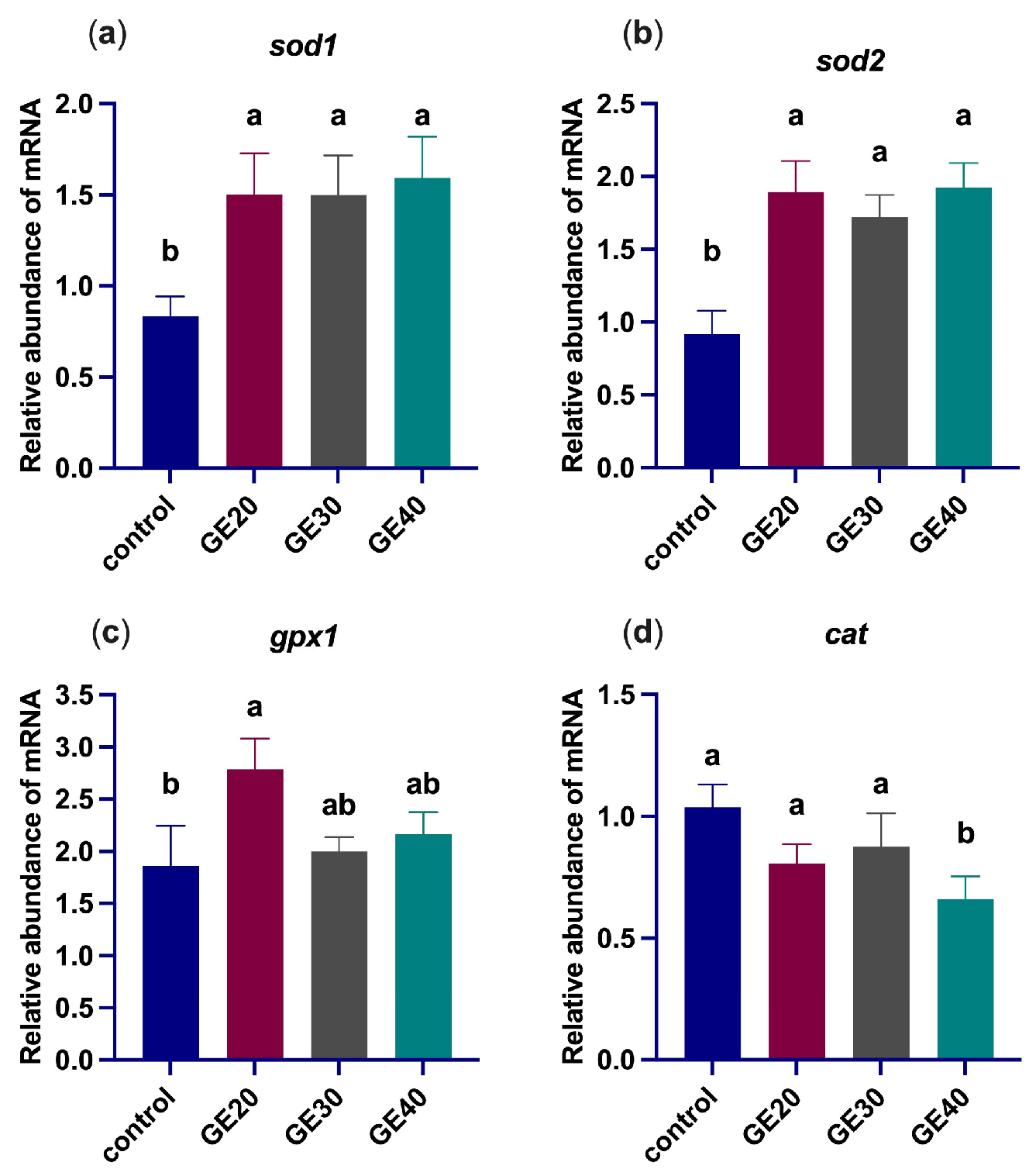
3.2. Effects on Oxidative Stress-Related Genes
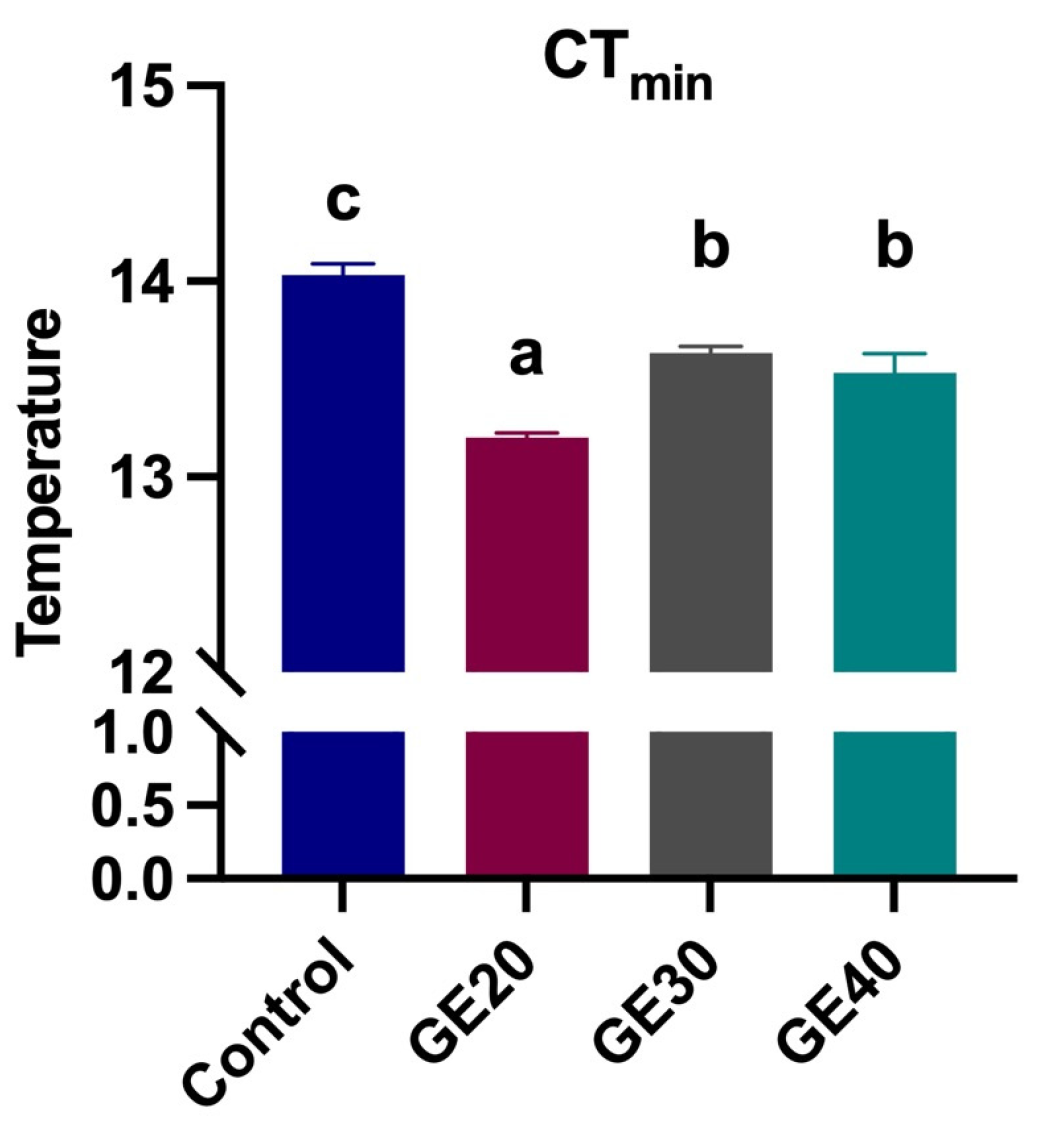
3.3. Effects on Thermal Tolerance
4. Discussion
4.1. Antioxidant Enzyme-Related Genes Expression
4.2. Growth Performances
4.3. Thermal Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoodi, B.; Aberoumand, A.; Ziaei-nejad, S.; Seyyedi, S. Effects of diets containing grape pomace on the growth, nutrition indices, and the quality traits of common carp (Cyprinus carpio). J. Food. Sci. Nutr. 2023, 11, 6660–6669. [Google Scholar] [CrossRef]
- Manam, V.K. Fish feed nutrition and its management in aquaculture. Int. J. Fish. Aquat. Stud. 2023, 11, 58–61. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; González-Aguilar, G.; Siddiqui, M.W. Plant Food by-Products: Industrial Relevance for Food Additives and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O. Antioxidant activity of plant phenols: Chemical mechanisms and biological significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Reda, F.M.; Alagawany, M.; Farag, M.R.; El-Naggar, K. The role of dietary chia seed powder in modulating cold stress-related impacts in Nile tilapia, Oreochromis niloticus. Aquaculture 2023, 567, 739246. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Kamangar, B.B.; Zarei, M.A. Effects of diets containing grape seed proanthocyanidin extract on the growth and oxidative capacity of common carp (Cyprinus carpio). Aquaculture 2021, 540, 736689. [Google Scholar] [CrossRef]
- Morante, V.H.P.; Copatti, C.E.; Souza, A.R.L.; da Costa, M.M.; Braga, L.G.T.; Souza, A.M.; de Melo, F.V.S.T.; Camargo, A.C.D.S.; Melo, J.F.B. Assessment the crude grape extract as feed additive for tambaqui (Colossoma macropomum), an omnivorous fish. Aquaculture 2021, 544, 737068. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J Pathol. Rev. Med. Microbiol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Hamidian, G.; Mardani, K.; Oushani, A.K.; Firouzamandi, M.; Esteban, M.Á.; Shohreh, P. Changes in rainbow trout (Oncorhynchus mykiss) growth and mucosal immune parameters after dietary administration of grape (Vitis vinifera) seed extract. Fish Physiol. Biochem. 2021, 47, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Sheikhzadeh, N.; Tayefi-Nasrabadi, H.; Alizadeh-Salteh, S.; Khani Oushani, A.; Firouzamandi, M.; Mardani, K. Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol. Biochem. 2020, 46, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Benedito-Palos, L.; Saera-Vila, A.; Calduch-Giner, J.-A.; Kaushik, S.; Pérez-Sánchez, J. Combined replacement of fish meal and oil in practical diets for fast growing juveniles of gilthead sea bream (Sparus aurata L.): Networking of systemic and local components of GH/IGF axis. Aquaculture 2007, 267, 199–212. [Google Scholar] [CrossRef]
- de Almeida Xavier, M.J.M. Improving Growth Performance of Fish Larvae through Early Nutrition. 2021. Available online: https://repositorio-aberto.up.pt/bitstream/10216/138509/2/520563.pdf (accessed on 20 February 2024).
- Chandhini, S.; Trumboo, B.; Jose, S.; Varghese, T.; Rajesh, M.; Kumar, V.J.R. Insulin-like growth factor signalling and its significance as a biomarker in fish and shellfish research. Fish Physiol. Biochem. 2021, 47, 1011–1031. [Google Scholar] [CrossRef]
- Tveteras, R. Global fish production data & analysis. In Proceedings of the Global Outlook for Aquaculture Leadership Conference in Guangzhou, Guangzhou, China, 19–22 September 2016; Available online: https://www.aquaculturealliance.org/wp-content/uploads/2017/06/Day1_RagnarTveteras.pdf (accessed on 21 March 2024).
- Liu, E.L.; Chen, G.R.; Chen, F.M.; Yang, S.D. Temperature causing cold damage to Asian seabass; Fisheries Research Institute, Ministry of Agriculture Taiwan: Keelung, Taiwan, 2021. [Google Scholar]
- Nazir, A.; Chen, T.Y.; Wang, P.L.; Shiao, J.C. Reconstructing habitat use, identifying origin and discrimination of the barramundi (wild and farmed) populations using otolith stable isotope analysis. Estuar. Coast. Shelf Sci. 2023, 285, 108317. [Google Scholar] [CrossRef]
- Williams, K.C.; Barlow, C.G.; Rodgers, L.; Agcopra, C. Dietary composition manipulation to enhance the performance of juvenile barramundi (Lates calcarifer Bloch) reared in cool water. Aquac. Res. 2006, 37, 914–927. [Google Scholar] [CrossRef]
- Jerry, D.R. Biology and Culture of Asian Seabass Lates Calcarifer; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Bardallo, R.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. The FEBS J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef]
- Wang, M.C.; Wang, Y.C.; Peng, H.W.; Hseu, J.R.; Wu, G.C.; Chang, C.F.; Tseng, Y.C. Resveratrol induces expression of metabolic and antioxidant machinery and protects tilapia under cold stress. Int. J. Mol. Sci. 2020, 21, 3338. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Moniruzzaman, M.; Ghosal, I.; Pegu, T.; Das, D.N.; Chakraborty, S.B. Evaluating the role of dietary plant extracts to allow adaptation to thermal stress in a cold stream ornamental fish, Botia rostrata (Günther, 1868). J. Therm. Biol. 2022, 105, 103224. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, C.; Ale, A.; Rossi, A.S.; Karakachoff, M.; Cazenave, J. Effects of cold stress on juvenile Piaractus mesopotamicus and the mitigation by β-carotene. J. Therm. Biol. 2020, 88, 102497. [Google Scholar] [CrossRef] [PubMed]
- Tejaswini, K.; Deo, A.D.; Shamna, N.; Jayant, M.; Aklakur, M.; Annadurai, R. Effect of flavanone rich lemon peel extract on feed intake and growth of Labeo rohita (Hamilton, 1822) fingerlings reared at low temperature recirculatory aquaculture system. Aquaculture 2024, 584, 740450. [Google Scholar] [CrossRef]
- Refaey, M.M.; Mehrim, A.I.; Zenhom, O.A.; Mansour, A.T. Effect of fatty acids manipulation on survival and physiological response of hybrid red tilapia under chronic cold stress. Aquaculture 2022, 561, 738663. [Google Scholar] [CrossRef]
- Ford, T.; Beitinger, T.L. Temperature tolerance in the goldfish, Carassius auratus. J. Therm. Biol. 2005, 30, 147–152. [Google Scholar] [CrossRef]
- Hu, Y.C.; Chu, K.F.; Yang, W.K.; Lee, T.H. Na+, K+-ATPase β1 subunit associates with α1 subunit modulating a “higher-NKA-in-hyposmotic media” response in gills of euryhaline milkfish, Chanos chanos. J. Comp. Physiol. B-Biochem. Syst. Environ. Physiol. 2017, 187, 995–1007. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Lin, C.H.; Lee, T.H. Cholesterol accumulation in livers of Indian medaka, Oryzias dancena, acclimated to fresh water and seawater. Front. Mar. Sci. 2022, 9, 891706. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lange, B.; Currie, K.L.; Howarth, G.S.; Stone, D.A.J. Grape seed extract and dried macroalgae, Ulva lactuca Linnaeus, improve survival of greenlip abalone, Haliotis laevigata Donovan, at high water temperature. Aquaculture 2014, 433, 348–360. [Google Scholar] [CrossRef]
- Ahmadi, A.; Bagheri, D.; Hoseinifar, S.H.; Morshedi, V.; Paolucci, M. Beneficial role of polyphenols as feed additives on growth performances, immune response and antioxidant status of Lates Calcarifer (Bloch, 1790) juveniles. Aquaculture 2022, 552, 737955. [Google Scholar] [CrossRef]
- Rashidah, A.R.; Shariff, M.; Yusoff, F.M.; Ismail, I.S. Dietary supplementation of Polygonum chinense improves the immunity of Asian seabass, Lates calcarifer (Bloch, 1790) against Vibrio harveyi infection. Fish Shellfish Immunol. Rep. 2023, 5, 100118. [Google Scholar] [CrossRef] [PubMed]
- Balu, M.; Sangeetha, P.; Haripriya, D.; Panneerselvam, C. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci. Lett. 2005, 383, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- de Mello Andrade, J.M.; Fasolo, D. Chapter 20–Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 253–265. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2–Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. [Google Scholar]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Bashir, N.; Manoharan, V.; Miltonprabu, S. Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J. Nutr. Biochem. 2016, 32, 128–141. [Google Scholar] [CrossRef]
- Liu, H.; He, J.; Chi, C.; Gu, Y. Identification and analysis of icCu/Zn-SOD, Mn-SOD and ecCu/Zn-SOD in superoxide dismutase multigene family of Pseudosciaena crocea. Fish Shellfish Immunol. 2015, 43, 491–501. [Google Scholar] [CrossRef]
- Duong, D.N.; Qin, J.G.; Harris, J.O.; Hoang, T.H.; Bansemer, M.S.; Currie, K.L.; Phan-Thien, K.Y.; Dowell, A.; Stone, D.A.J. Effects of dietary grape seed extract, green tea extract, peanut extract and vitamin C supplementation on metabolism and survival of greenlip abalone (Haliotis laevigata Donovan) cultured at high temperature. Aquaculture 2016, 464, 364–373. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Esteban, M.Á.; Abdel-Tawwab, M. Impact of grape pomace flour (GPF) on immunity and immune-antioxidant-anti-inflammatory genes expression in Labeo rohita against Flavobacterium columnaris. Fish Shellfish Immunol. 2021, 111, 69–82. [Google Scholar] [CrossRef]
- Yang, H.; Li, Y.; Wang, G.; Xie, J.; Kaneko, G.; Yu, E. Dietary grape seed proanthocyanidin extract improved the chemical composition, antioxidant capacity, myofiber growth and flesh quality of Nile tilapia muscle. Aquacult. Rep. 2023, 33, 101878. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox. Lancet 2000, 355, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Spanou, C.; Veskoukis, A.S.; Stagos, D.; Liadaki, K.; Anastasiadi, M.; Haroutounian, S.A.; Tsouka, M.; Tzanakouli, E.; Kouretas, D. Effects of grape extracts on the in vitro activity of enzymes involved in oxidative stress regulation. In Vivo 2011, 25, 657–662. [Google Scholar] [PubMed]
- Arslan, G.; Sönmez, A.Y.; Yan, K.T. Effects of grape Vitis vinifera seed oil supplementation on growth, survival, fatty acid profiles, antioxidant contents and blood parameters in rainbow trout Oncorhynchus mykiss. Aquac. Res. 2018, 49, 2256–2266. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef]
- Chien, A.; Chou, C.Y.; Cheng, Y.C.; Sheen, S.S.; Kirby, R. The optimal dietary level of dry grape extract and its effect on the growth performance and antioxidant activity of the white shrimp Litopenaeus vannamei. Aquacult. Rep. 2023, 29, 101527. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Imanpour, M.R.; Mazandarani, M.; Sanchouli, H.; Paolucci, M. Effects of dietary polyphenols on mucosal and humoral immune responses, antioxidant defense and growth gene expression in beluga sturgeon (Huso huso). Aquaculture 2020, 528, 735494. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, H.; Poolsawat, L.; Rahman, M.M.; Xu, X.; Jiang, X.; Li, X.; Tan, H.; Leng, X. Flavonoid-enriched diets improved the growth and flesh quality of grass carp (Ctenopharyngodon idellus) based on metabolomics. Aquac. Nutr. 2021, 27, 2514–2528. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. Zootecn. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Pascariu, S.; Pop, I.; Simeanu, D.; Pavel, G.; Solcan, C. Effects of wine by-products on growth performance, complete blood count and total antioxidant status in broilers. Braz. J. Poult. Sci. 2017, 19, 191–202. [Google Scholar] [CrossRef]
- Kim, E.Y.; Pai, T.K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.Y.; Han, O. Bioactive dietary polyphenols decrease heme iron absorption by decreasing basolateral iron release in human intestinal Caco-2 cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.W.; Lu, J.J.; Chen, X.H. Effects of dietary grape seed proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital. J. Anim. Sci. 2014, 13, 3357. [Google Scholar] [CrossRef]
- Rosas, V.T.; Mureb, R.A.; Monserrat, J.M.; Wasielesky, W., Jr.; Tesser, M.B. Inclusion of grape bagasse (Vitis sp.) in the diet of white shrimp (Litopenaeus vannamei) and its effects on growth and antioxidant system. Aquac. Res. 2022, 53, 4805–4813. [Google Scholar] [CrossRef]
- Donaldson, M.; Cooke, S.; Patterson, D.; Macdonald, J. Cold shock and fish. J. Fish Biol. 2008, 73, 1491–1530. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Jin, X.; Wang, Z.; Mao, C.; Guo, S.; Yan, S.; Shi, B. Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals 2022, 12, 2535. [Google Scholar] [CrossRef]
- Ibarz, A.; Martín-Pérez, M.; Blasco, J.; Bellido, D.; de Oliveira, E.; Fernández-Borràs, J. Gilthead sea bream liver proteome altered at low temperatures by oxidative stress. Proteomics 2010, 10, 963–975. [Google Scholar] [CrossRef]
- Wu, S.M.; Liu, J.H.; Shu, L.H.; Chen, C.H. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, C.; Jiang, L.; Chen, D.; Jiang, P.; Huang, B. Effects of Vitamin E on Immune Response, Antioxidant Capacity, and Liver Tissue Structure of Crucian Carp under Acute Cold Stress. Aquac. Res. 2023, 2023, 2579785. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Sun, S.X.; Zhang, H.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 235, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jeong, W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective effects and molecular mechanisms of tea polyphenols on cardiovascular diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Niu, M.; Hu, L.; Chen, L. Cold acclimation for enhancing the cold tolerance of zebrafish cells. Front. Physiol. 2022, 12, 813451. [Google Scholar] [CrossRef]
- Chung, D.J.; Schulte, P.M. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus). J. Exp. Biol. 2015, 218, 1621–1631. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′ to 3′) | Amplicon Size (bp) | Reference Number | |
|---|---|---|---|---|
| igf-1 | F | ACGAGTGCTGCTTCCAAAG | 118 | XM_018697285.1 |
| R | GGTGTTCTCGGCATGTCTG | |||
| sod1 | F | GGTCCCAATGATGCAGAGAG | 108 | XM_018691152.1 |
| R | GGTCCCAATGATGCAGAGAG | |||
| sod2 | F | TGCGGCCAGACTATGTTAAG | 200 | XM_018675982.1 |
| R | GTATCAGTGTTGGTGGTCAGT | |||
| gpx1 | F | GGCTGGGAGTGTTGAAGAG | 177 | XM_018686718.2 |
| R | TTGCTGGAGTAACGAGAGTG | |||
| cat | F | GAGTCTGCATCAGGTGTCTTT | 109 | XM_018675907.1 |
| R | CAAACTGGTTAATGCTGATGGG | |||
| elf1α | F | GTTGCCTTTGTCCCCATCTC | 130 | XM_018699049.1 |
| R | CTTCCAGCAGTGTGGTTCCA |
| Groups | Control | GE20 | GE30 | GE40 |
|---|---|---|---|---|
| Initial weight (g) | 20.1 ± 0.75 | 19.83 ± 0.75 | 20.33 ± 0.816 | 20.33 ± 1.032 |
| Final weight (g) | 80 ± 3.03 b | 93.5 ± 3.67 a | 81.3 ± 4.08 b | 81.66 ± 3.26 b |
| Weight gain (g) | 59.8 ± 3.43 b | 73.6 ± 4.17 a | 61 ± 3.74 b | 61.33 ± 3.26 b |
| SGR% (g/day) | 4.33 ± 0.038 b | 4.48 ± 0.039 a | 4.34 ± 0.04 b | 4.35 ± 0.04 b |
| FI (%/day) | 1.94 ± 0.20 | 1.92 ± 0.10 | 1.91 ± 0.19 | 1.96 ± 0.24 |
| FCR | 1.09 ± 0.04 a | 0.88 ± 0.04 c | 0.92 ± 0.06 ab | 0.96 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, S.; Ranasinghe, N.; Lee, T.-H.; Chou, C.-C. Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed. Animals 2024, 14, 2731. https://doi.org/10.3390/ani14182731
Akram S, Ranasinghe N, Lee T-H, Chou C-C. Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed. Animals. 2024; 14(18):2731. https://doi.org/10.3390/ani14182731
Chicago/Turabian StyleAkram, Salman, Naveen Ranasinghe, Tsung-Han Lee, and Chi-Chung Chou. 2024. "Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed" Animals 14, no. 18: 2731. https://doi.org/10.3390/ani14182731
APA StyleAkram, S., Ranasinghe, N., Lee, T.-H., & Chou, C.-C. (2024). Enhancement of Thermal Tolerance and Growth Performances of Asian Seabass (Lates calcarifer) Fed with Grape Extract Supplemented Feed. Animals, 14(18), 2731. https://doi.org/10.3390/ani14182731






