Effect of Amorphous Halomonas-PHB on Growth, Body Composition, Immune-Related Gene Expression and Vibrio anguillarum Resistance of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatu ♂) Juveniles
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and methods
2.1. Halomonas Fermentation
2.2. Feed Preparation and Experimental Design
2.2.1. Experiment I
2.2.2. Experiment II
2.3. Sample Collection and Parameter Determination
2.3.1. Growth Performance
2.3.2. Fish Muscle Composition and Histology Analysis of Intestine in Experiment I
2.3.3. Vibrio Culture and Challenge
2.3.4. Immune-Related Gene Expression
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Muscle Nutritional Composition
3.3. Histological Structure of Intestine
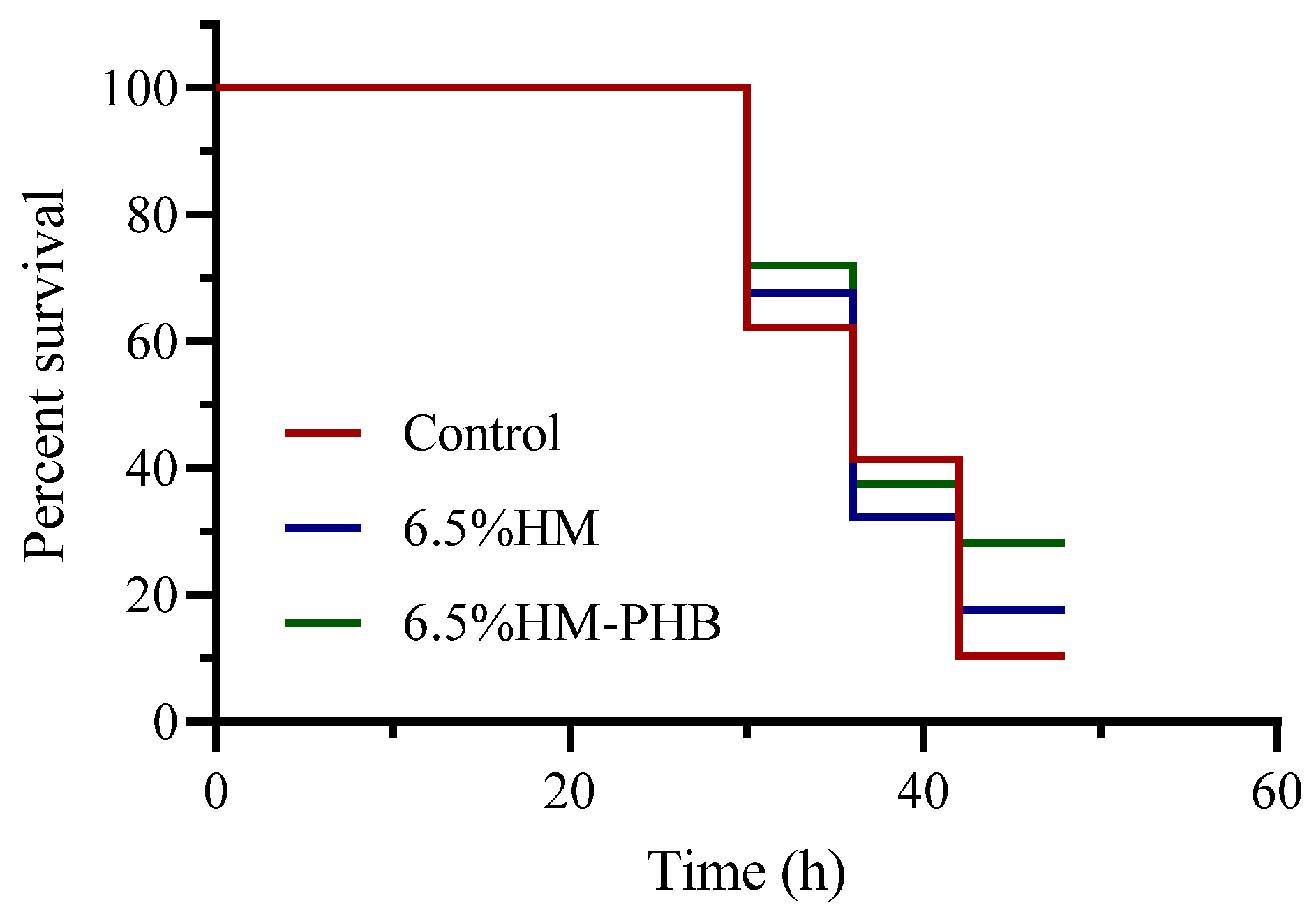
3.4. Resistance to Vibrio Challenge
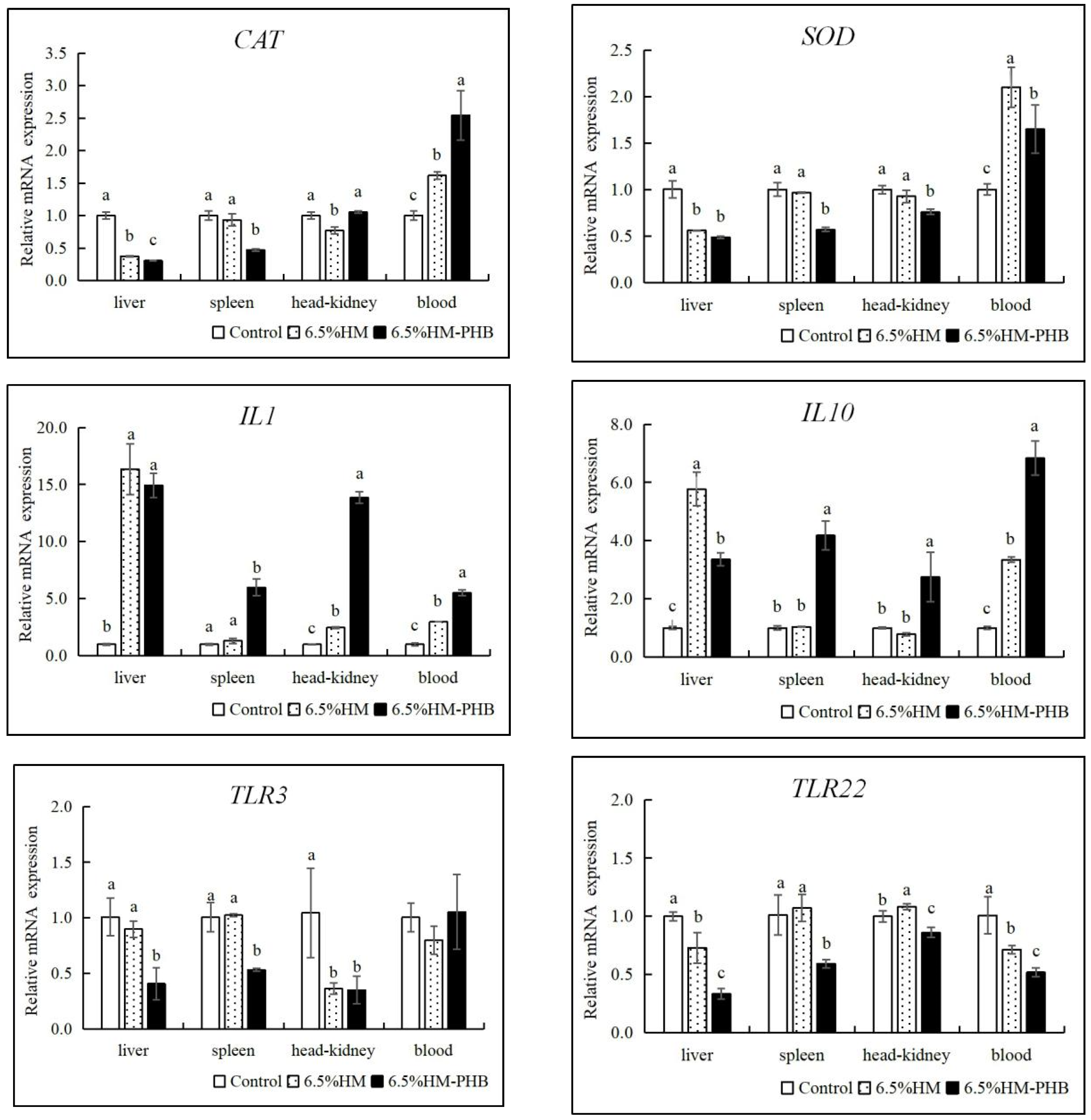
3.5. Antioxidative Enzyme and Immune-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- China Agriculture Publishing House. China Fishery Statistical Yearbook; China Agriculture Publishing House: Beijing, China, 2023. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Molecular studies, disease status and prophylactic measures in grouper aquaculture: Economic importance, diseases and immunology. Aquaculture 2010, 309, 1–14. [Google Scholar] [CrossRef]
- Furushita, M.; Okamoto, A.; Maeda, T.; Ohta, M.; Shiba, T. Isolation of Multidrug-resistant Stenotrophomonas maltophilia from cultured yellowtail (Seriola quinqueradiata) from a marine fish farm. Appl. Environ. Microbiol. 2005, 71, 5598–5600. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Pedersen, K.; Larsen, J.L. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl. Environ. Microbiol. 2000, 66, 4908–4915. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Beltrán, J.; Esteban, M. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Sun, Y.-Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, P.; Sinha, A.K.; Kunwar, P.S.; Baruah, K.; Verstraete, W.; Boon, N.; De Boeck, G.; Bossier, P. Poly-beta-hydroxybutyrate (PHB) increases growth performance and intestinal bacterial range-weighted richness in juvenile European sea bass, Dicentrarchus labrax. Appl. Microbiol. Biotechnol. 2010, 86, 1535–1541. [Google Scholar] [CrossRef]
- De Schryver, P.; Dierckens, K.; Bahn Thi, Q.Q.; Amalia, R.; Marzorati, M.; Bossier, P.; Boon, N.; Verstraete, W. Convergent dynamics of the juvenile European sea bass gut microbiota induced by poly-β-hydroxybutyrate. Environ. Microbiol. 2011, 13, 1042–1051. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Franke, A.; Clemmesen, C.; De Schryver, P.; Garcia-Gonzalez, L.; Miest, J.J.; Roth, O. Immunostimulatory effects of dietary poly-β-hydroxybutyrate in European sea bass postlarvae. Aquac. Res. 2017, 48, 5707–5717. [Google Scholar] [CrossRef]
- Franke, A.; Roth, O.; Schryver, P.D.; Bayer, T.; Garcia-Gonzalez, L.; Künzel, S.; Bossier, P.; Miest, J.J.; Clemmesen, C. Poly-β-Hydroxybutyrate administration during early life: Effects on performance, immunity and microbial community of European sea bass yolk-sac larvae. Sci. Rep. 2017, 7, 15022. [Google Scholar] [CrossRef]
- Laranja, J.L.Q.; Bossier, P. Poly-beta-hydroxybutyrate (PHB) and infection reduction in farmed aquatic animals. In Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology; Goldfine, H., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Suehs, B.A.; Yamamoto, F.Y.; Older, C.E.; Asiri, F.; Gatlin, D.M. Poly-β-hydroxybutyrate supplementation enhanced the growth and immune responses of juvenile nile Tilapia (Oreochromis niloticus). Aquaculture 2025, 594, 741384. [Google Scholar] [CrossRef]
- Quillaguamán, J.; Hashim, S.; Bento, F.; Mattiasson, B.; Hatti-Kaul, R. Poly (β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J. Appl. Microbiol. 2005, 99, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Xue, Y.S.; Aibaidula, G.; Chen, G.Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresour. Technol. 2011, 102, 8130–8136. [Google Scholar] [CrossRef]
- Yue, H.; Ling, C.; Yang, T.; Chen, X.; Chen, Y.; Deng, H.; Wu, Q.; Chen, J.; Chen, G.Q. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 2014, 7, 108. [Google Scholar] [CrossRef]
- Park, S.; Cho, J.; Choi, T.; Song, H.; Bhatia, S.; Gurav, R.; Park, S.; Park, K.; Joo, J.; Hwang, S.; et al. Improvement of polyhydroxybutyrate (PHB) plate-based screening method for PHB degrading bacteria using cell-grown amorphous PHB and recovered by sodium dodecyl sulfate (SDS). Int. J. Biol. Macromol. 2021, 177, 413–421. [Google Scholar] [CrossRef]
- Andler, R.; González-Arancibia, F.; Vilos, C.; Sepulveda-Verdugo, R.; Castro, R.; Mamani, M.; Valdés, C.; Arto-Paz, F.; Díaz-Barrera, A.; Martínez, I. Production of poly-3-hydroxybutyrate (PHB) nanoparticles using grape residues as the sole carbon source. Int. J. Biol. Macromol. 2024, 261, 129649. [Google Scholar] [CrossRef]
- Thai, T.Q.; Wille, M.; Garcia-Gonzalez, L.; Sorgeloos, P.; Bossier, P.; De Schryver, P. Poly-β-hydroxybutyrate content and dose of the bacterial carrier for Artemia enrichment determine the performance of giant freshwater prawn larvae. Appl. Microbiol. Biotechnol. 2014, 98, 5205–5215. [Google Scholar] [CrossRef]
- Gao, M.; Du, D.; Bo, Z.; Sui, L. Poly-β-hydroxybutyrate (PHB)-accumulating Halomonas improves the survival, growth, robustness and modifies the gut microbial composition of Litopenaeus vannamei postlarvae. Aquaculture 2019, 500, 607–612. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Xie, W.; Duan, H.; Du, D.; Sui, L. Effect of Halomonas storage Poly-β-hydroxybutyrates on survival, growth and vibriosis resistance of half-smooth tongue sole Cynoglossus semilaevis juveniles. Aquac. Res. 2020, 51, 4631–4637. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Official Analytical Chemists International, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2005. [Google Scholar]
- Sui, L.Y.; Sun, H.X.; Wu, X.G.; Wille, M.; Cheng, Y.X.; Sorgeloos, P. Effect of dietary HUFA on tissue fatty acid composition and reproductive performance of Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) broodstock. Aquac. Int. 2011, 19, 269–282. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, S.; Jian, J. Protection against Vibrio alginolyticus in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) immunized with an acfA-deletion live attenuated vaccine. Fish Shellfish Immunol. 2019, 86, 875–881. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zou, C.; Tan, X.; Ye, H.; Sun, Z.; Chen, S.; Liu, Q.; Xu, M.; Ye, C.; Wang, A. The hepatoprotective effects of Radix Bupleuri extracts against D-galactosamine/lipopolysaccharide induced liver injury in hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Fish Shellfish Immunol. 2018, 83, 8–17. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Dong, X.; Tan, B.; Yang, Q.; Chi, S.; Zhang, S.; Liu, H.; Yang, Y. Effects of dietary N-3 highly unsaturated fatty acids on growth, non-specific immunity, expression of some immune-related genes and resistance to Vibrio harveyi in Hybrid Grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu). Fish Shellfish Immunol. 2020, 96, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Fed High Lipid Diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Najdegerami, E.H.; Bakhshi, F.; Tokmechi, A.; Shiri Harzevili, A.; Sorgeloos, P.; Bossier, P. Dietary effects of poly-β-hydroxybutyrate on the growth performance, digestive enzyme activity, body composition, mineral uptake and bacterial challenge of rainbow trout fry (Oncorhynchus mykiss). Aquac. Nutr. 2017, 23, 246–254. [Google Scholar] [CrossRef]
- Kim, S.; Shin, J.; Medagoda, N.; Choi, S.; Park, S.Y.; Park, J.Y.; Lee, K.J. Dietary Poly-β-hydroxybutyrate improved the growth, non-specific immunity, digestive enzyme activity, intestinal morphology, phagocytic activity, and disease resistance against Vibrio parahaemolyticus of Pacific White Shrimp, Penaeus vannamei. Mar. Biotechnol. 2024, 26, 550–561. [Google Scholar] [CrossRef]
- Sui, L.; Ma, G.; Lu, W.; Deng, Y.; Bossier, P.; De Schryver, P. Effect of poly-β-hydroxybutyrate on growth, enzyme activity and intestinal microbial community of Chinese mitten crab, Eriocheir sinensis (Milne-Edwards) Juveniles. Aquac. Res. 2016, 47, 3644–3652. [Google Scholar] [CrossRef]
- Qiao, G.; Lv, T.; Zhang, M.; Chen, P.; Sun, Q.; Zhang, J.; Li, Q. β-hydroxybutyrate (β-HB) exerts anti-Inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated macrophages in Liza haematocheila. Fish Shellfish Immunol. 2020, 107, 444–451. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Zheng, X.; Wang, Y.; Li, H.; Liu, Q.; Zhang, J. Effect of dietary poly-β-hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of Pacific White Shrimp Litopenaeus vannamei (Boone, 1931). Fish Shellfish Immunol. 2017, 60, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Najdegerami, E.H.; Tran, T.N.; Defoirdt, T.; Marzorati, M.; Sorgeloos, P.; Boon, N.; Bossier, P. Effects of poly-β-hydroxybutyrate (PHB) on Siberian sturgeon (Acipenser baerii) fingerlings performance and its gastrointestinal tract microbial community. FEMS Microbiol. Ecol. 2012, 79, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.F.A.; Hassan, H.U.; Yones, A.-M.; Abdel-Tawwab, Y.A.; Metwalli, A.A.A.-T. Assessing the Effect of Different Feeding Frequencies Combined with Stocking Density, Initial Weight, and Dietary Protein Ratio on the Growth Performance of Tilapia, Catfish and Carp. Sci. Afr. 2021, 12, e00806. [Google Scholar] [CrossRef]
- Shao, T.; Chen, X.; Zhai, D.; Wang, T.; Long, X.; Liu, Z. Evaluation of the Effects of Different Stocking Densities on Growth and Stress Responses of Juvenile Hybrid Grouper ♀ Epinephelus Fuscoguttatus × ♂ Epinephelus Lanceolatus in Recirculating Aquaculture Systems. J. Fish Biol. 2019, 95, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Li, G.; Wu, H.-B.; Liu, X.-G.; Yao, Y.-H.; Tao, L.; Liu, H. An Integrated Recirculating Aquaculture System (RAS) for Land-Based Fish Farming: The Effects on Water Quality and Fish Production. Aquacult. Eng. 2011, 45, 93–102. [Google Scholar] [CrossRef]
- Najdegerami, E.H.; Tokmachi, A.; Bakhshi, F. Evaluating the effects of dietary prebiotic mixture of mannan oligosaccharide and poly--hydroxybutyrate on the growth performance, immunity, and survival of rainbow trout, Oncorhynchus mykiss (Walbaum 1792), fingerlings. J. World Aquacult. Soc. 2017, 48, 415–425. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Wang, Z.; Lv, T.; Li, X.; Tian, H.; Zhang, Y.; Cang, P.; Chi, S.; Sun, Y.; et al. Dietary poly-β-hydroxybutyrate supplementation can effectively improve growth, digestive enzyme activities, immune-related gene expression, disease resistance, and intestinal mucosal barrier of gibel carp, Carassius auratus gibelio. J. World Aquacult. Soc. 2022, 53, 816–835. [Google Scholar] [CrossRef]
- Yang, P.; Hu, H.; Li, Y.; Ai, Q.; Zhang, W.; Zhang, Y.; Mai, K. Effect of dietary Xylan on immune response, tight junction protein expression and bacterial community in the intestine of Juvenile turbot (Scophthalmus maximus L.). Aquaculture 2019, 512, 734361. [Google Scholar] [CrossRef]
- Situmorang, M.L.; De Schryver, P.; Dierckens, K.; Bossier, P. Effect of poly-β-hydroxybutyrate on growth and disease resistance of Nile tilapia Oreochromis niloticus juveniles. Vet. Microbiol. 2016, 182, 44–49. [Google Scholar] [CrossRef]
- Halet, D.; Defoirdt, T.; van Damme, P.; Vervaeren, H.; Forrez, I.; van de Wiele, T.; Boon, N.; Sorgeloos, P.; Bossier, P.; Verstraete, W. Poly-β-hydroxybutyrate-accumulating bacteria protect gnotobiotic Artemia franciscana from pathogenic Vibrio campbellii. FEMS Microbiol. Ecol. 2007, 60, 363–369. [Google Scholar] [CrossRef]
- Liu, Y.; De Schryver, P.; Van Delsen, B.; Maignien, L.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P.; Defoirdt, T. PHB-degrading bacteria isolated from the gastrointestinal tract of aquatic animals as protective actors against luminescent vibriosis. FEMS Microbiol. Ecol. 2010, 74, 196–204. [Google Scholar] [CrossRef]
- Kumari, J.; Sahoo, P.K. High dietary vitamin C affects growth, non-specific immune responses and disease resistance in Asian catfish, Clarias batrachus. Mol. Cell. Biochem. 2005, 280, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Qiao, G.; Sun, Q.; Zhang, M.; Xu, C.; Lv, T.; Qi, Z.; Yang, W.; Li, Q. Antioxidant system of soiny mullet (Liza haematocheila) is responsive to dietary poly-β-hydroxybutyrate (PHB) supplementation based on immune-related enzyme activity and de novo transcriptome analysis. Fish Shellfish Immunol. 2019, 95, 314–327. [Google Scholar] [CrossRef]
- Bilodeau, A.L.; Waldbieser, G.C. Activation of TLR3 and TLR5 in channel catfish exposed to virulent Edwardsiella ictaluri. Dev. Comp. Immunol. 2005, 29, 713–721. [Google Scholar] [CrossRef]
- Phelan, P.E.; Mellon, M.T.; Kim, C.H. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio). Mol. Immunol. 2005, 42, 1057–1071. [Google Scholar] [CrossRef]
- Duan, H.; Zuo, J.; Pan, N.; Cui, X.; Guo, J.; Sui, L. 3-hydroxybutyrate helps crayfish resistant to Vibrio parahaemolyticus infection in versatile ways. Fish Shellfish Immunol. 2023, 132, 108444. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.F.; Yang, Z.Y.; Zhu, R.; Li, D.L.; Shang, G.J.; Wang, H.T.; Meng, S.T.; Wang, Y.T.; Liu, S.Y.; et al. Alleviative effect of poly-β-hydroxybutyrate on lipopolysaccharide-induced oxidative stress, inflammation and cell apoptosis in Cyprinus carpio. Int. J. Biol. Macromol. 2023, 253, 126784. [Google Scholar] [CrossRef]
- Le Xuan, C.; Linh, N.V.; Wannavijit, S.; Outama, P.; Fontana, C.M.; Meepowpan, P.; Van Doan, H. Influences of makiang (Syzygium nervosum) seed powder on growth performance, immunological response, antioxidant and immune related gene expression in Juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2024, 588, 740943. [Google Scholar] [CrossRef]
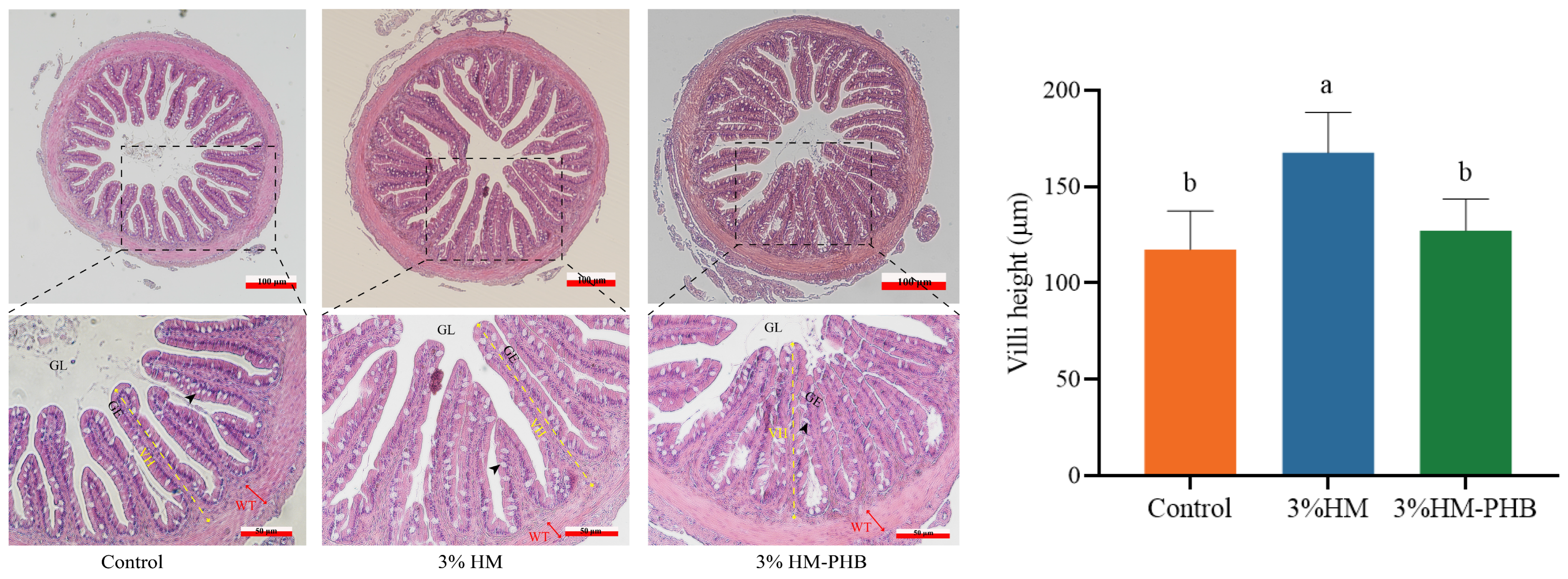
| Control | 3% HM | 3% HM-PHB | ||
|---|---|---|---|---|
| Crude fat (% dw) | 10.11 ± 0.09 | 9.93 ± 0.03 | 10.07 ± 0.10 | |
| Crude protein (% dw) | 55.73 ± 0.15 | 55. 40 ± 0.16 | 55.31 ± 0.22 | |
| Fatty acid (mg/g dw) | C14:0 | 6.35 | 6.24 | 5.79 |
| C15:0 | 0.31 | 0.32 | 0.30 | |
| C16:0 | 28.97 | 29.04 | 27.51 | |
| C16:1n7 | 3.87 | 3.95 | 3.71 | |
| C17:0 | 0.18 | 0.18 | 0.19 | |
| C18:0 | 20.78 | 20.30 | 19.78 | |
| C18:1n9 | 10.54 | 10.48 | 10.12 | |
| C18:1n7 | 5.18 | 5.43 | 5.17 | |
| C18:2n6 | 6.49 | 6.36 | 6.30 | |
| C18:3n3 | 3.06 | 3.05 | 3.08 | |
| C20:1n9 | 2.28 | 2.24 | 2.43 | |
| C20:5n3 (EPA) | 14.72 | 14.40 | 14.32 | |
| C22:6n3 (DHA) | 12.12 | 11.76 | 11.50 | |
| Primers | Primers Forward/Reverse (5′ to 3′) | Amplification Efficiency | Annealing Temperature | References |
|---|---|---|---|---|
| Catalase (CAT) | F: GCGTTTGGTTACTTTGAGGTGA R: GAGAAGCGGACAGCAATAGGT | 97.9% | 60 | [27] |
| Superoxide dismutase (SOD) | F: TACGAGAAGGAGAGCGGAAGA R: ATACCGAGGAGGGGGATGA | 90.7% | 60 | [27] |
| Interleukin 1 (IL1) | F: CGACATGGTGCGGTTTC R: TCTGTAGCGGCTGGTGG | 109.2% | 60 | [28] |
| Interleukin 10 (IL10) | F: TTCGACGAGCTCAAGAGTGAG R: TGCCGTTTAGAAGCCAGATACA | 109.6% | 64 | [29] |
| Toll-like receptor 3 (TLR3) | F: TCTCCATTCCGTCACCTTCC R: TCATCCAGCCCGTTACTATCC | 103.3% | 64 | [29] |
| Toll-like receptor 22 (TLR22) | F: CGAGCCAGGTAAACCCATCA R: CTCATCAAACAGGCGGAAGC | 92.1% | 60 | [29] |
| Treatment | Control | 3% HM | 3% HM-PHB |
|---|---|---|---|
| SR (%) | 97.5 ± 3.19 | 100 | 100 |
| IBW (g) | 2.93 ± 0.03 | 2.97 ± 0.04 | 2.85 ± 0.09 |
| FBW (g) | 32.71 ± 0.86 b | 37.8 ± 2.40 a | 35.23 ± 1.96 ab |
| WG (%) | 1015.41 ± 83.16 | 1172.28 ± 84.91 | 1140.85 ± 116.50 |
| SGR (%/day) | 4.93 ± 0.13 | 5.22 ± 0.15 | 5.08 ± 0.13 |
| FCR | 0.76 ± 0.02 | 0.78 ± 0.01 | 0.80 ± 0.02 |
| HSI (%) | 3.31 ± 0.36 | 3.17 ± 0.34 | 3.02 ± 0.22 |
| Control | 3% HM | 3% HM-PHB | ||
|---|---|---|---|---|
| Moisture (%) | 73.50 ± 0.46 a | 74.41 ± 0.72 ab | 72.81 ± 1.08 b | |
| Crude fat (% dw) | 6.92 ± 1.03 | 8.59 ± 1.58 | 7.62 ± 1.32 | |
| Crude protein (% dw) | 84.22 ± 2.00 a | 82.77 ± 2.89 a | 77.87 ± 3.21 b | |
| Fatty acid (mg/g dw) | C14:0 | 1.96 ± 0.98 b | 3.05 ± 0.32 ab | 3.46 ± 0.93 a |
| C15:0 | 0.21 ± 0.08 | 0.28 ± 0.03 | 0.31 ± 0.05 | |
| C16:0 | 10.75 ± 4.41 b | 16.63 ± 2.10 ab | 19.06 ± 4.53 a | |
| C16:1n7 | 2.42 ± 1.08 b | 3.82 ± 0.39 ab | 4.45 ± 1.14 a | |
| C17:0 | 0.16 ± 0.06 b | 0.22 ± 0.02 ab | 0.24 ± 0.04 a | |
| C18:0 | 3.46 ± 1.14 | 4.40 ± 0.53 | 4.93 ± 0.92 | |
| C18:1n9 | 6.65 ± 2.71 b | 10.18 ± 1.44 ab | 11.84 ± 2.84 a | |
| C18:1n7 | 1.58 ± 0.59 b | 2.17 ± 0.09 ab | 2.47 ± 0.56 a | |
| C18:2n6 | 2.85 ± 1.10 b | 3.96 ± 0.33 ab | 4.39 ± 0.86 a | |
| C18:3n3 | 0.88 ± 0.34 | 1.13 ± 0.15 | 1.14 ± 0.24 | |
| C20:1n9 | 0.81 ± 0.27 b | 1.19 ± 0.13 ab | 1.33 ± 0.31 a | |
| C20:5n3 (EPA) | 4.28 ± 1.85 | 5.76 ± 0.56 | 6.38 ± 1.13 | |
| C22:6n3 (DHA) | 8.73 ± 3.33 | 10.82 ± 0.94 | 11.78 ± 1.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Ma, H.; Gao, M.; Du, D.; Liu, L.; Sui, L. Effect of Amorphous Halomonas-PHB on Growth, Body Composition, Immune-Related Gene Expression and Vibrio anguillarum Resistance of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatu ♂) Juveniles. Animals 2024, 14, 2649. https://doi.org/10.3390/ani14182649
Xie W, Ma H, Gao M, Du D, Liu L, Sui L. Effect of Amorphous Halomonas-PHB on Growth, Body Composition, Immune-Related Gene Expression and Vibrio anguillarum Resistance of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatu ♂) Juveniles. Animals. 2024; 14(18):2649. https://doi.org/10.3390/ani14182649
Chicago/Turabian StyleXie, Wei, Haoran Ma, Meirong Gao, Dongdong Du, Liangsen Liu, and Liying Sui. 2024. "Effect of Amorphous Halomonas-PHB on Growth, Body Composition, Immune-Related Gene Expression and Vibrio anguillarum Resistance of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatu ♂) Juveniles" Animals 14, no. 18: 2649. https://doi.org/10.3390/ani14182649
APA StyleXie, W., Ma, H., Gao, M., Du, D., Liu, L., & Sui, L. (2024). Effect of Amorphous Halomonas-PHB on Growth, Body Composition, Immune-Related Gene Expression and Vibrio anguillarum Resistance of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatu ♂) Juveniles. Animals, 14(18), 2649. https://doi.org/10.3390/ani14182649






