Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Tested Product
2.2. Determination of MIC
2.3. Time–Kill Curve Assay
2.4. PAE and PA-SME Assays
3. Results
3.1. MIC Assay Results
3.2. Time–Kill Curve Results
3.3. PAE and PA-SME Assays Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- EFSA; European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Solarte, A.L.; Astorga, R.J.; De Aguiar, F.C.; De Frutos, C.; Barrero-Domínguez, B.; Huerta, B. Susceptibility Distribution to Essential Oils of Salmonella enterica Strains Involved in Animal and Public Health and Comparison of the Typhimurium and Enteritidis Serotypes. J. Med. Food 2018, 21, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Motta Felício, I.; Limongi de Souza, R.; de Oliveira Melo, C.; Gervázio Lima, K.Y.; Vasconcelos, U.; Olímpio de Moura, R.; Eleamen Oliveira, E. Development and characterization of a carvacrol nanoemulsion and evaluation of its antimicrobial activity against selected food-related pathogens. Lett. Appl. Microbiol. 2021, 72, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Campaniello, D.; Altieri, C.; Corbo, M.R.; Sinigaglia, M. Minimal Inhibitory Concentrations of Thymol and Carvacrol: Toward a Unified Statistical Approach to Find Common Trends. Microorganisms 2023, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Odenholt-Tornqvist, I.; Lowdin, E.; Cars, O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin, and azithromycin on respiratory tract pathogens. Antimicrob. Agents Chemother. 1995, 39, 221–226. [Google Scholar] [CrossRef]
- Majtán, V.; Majtánová, L. Postantibiotic Effects and Postantibiotic Sub-MIC Effects of Ciprofloxacin, Pefloxacin and Amikacin on the Biological Properties of Salmonella Strains. Folia Microbiol. 1997, 42, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.C.; Alfaro, L.A.; Reinoso, J.C.; Mestre, M.J.G.; Rodríguez-Gascón, A. Análisis farmacocinético-farmacodinámico en microbiología: Herramienta para evaluar el tratamiento antimicrobiano. Enferm. Infecc. Microbiol. Clin. 2015, 33, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nedbalcova, K.; Zouharova, M.; Sperling, D. Post-antibiotic effect of marbofloxacin, enrofloxacin and amoxicillin against selected respiratory pathogens of pigs. Vet. Med. 2019, 64, 67–77. [Google Scholar] [CrossRef]
- Peñalver, P.; Huerta, B.N.; Borge, C.; Astorga, R.; Romero, R.; Perea, A. Antimicrobial activity of five essential oils against origin strains of the Enterobacteriaceae family. APMIS 2005, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zorn, B.; Goñi, J.G.; Rodríguez, L.J.D. El destino de Salmonella en el interior de un ser vivo. Porci 2004, 81, 23–28. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; VET01S; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Baroni, E.; Russi, N.; Rubio, M.; Picco, E.; Formentini, E. Actividad antibacteriana in vitro de ciprofloxacina sobre Escherichia coli: Efecto del suero bovino y tamaño del inóculo. InVet 2014, 16, 7–14. [Google Scholar]
- Sidhu, P.K.; Landoni, M.F.; Aliabadi, F.S.; Lees, P. PK-PD integration and modeling of marbofloxacin in sheep. Res. Vet. Sci. 2010, 88, 134–141. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Belato, K.K.; de Oliveira, J.R.; de Oliveira, F.S.; de Oliveira, L.D.; Camargo, S.E.A. Cytotoxicity and genotoxicity of thymol verified in murine macrophages (RAW 264.7) after antimicrobial analysis in Candida albicans, Staphylococcus aureus, and Streptococcus mutans. J. Funct. Foods 2018, 40, 455–460. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Harada, K.; Shimizu, T.; Kataoka, Y.; Takahashi, T. Post-antibiotic effect of orbifloxacin against Escherichia coli and Pseudomonas aeruginosa isolates from dogs. Acta Vet. Scand. 2012, 54, 16. [Google Scholar] [CrossRef]
- Labarca, J.L. New concepts in pharmacokinetics. Must we thinj again how to use the antibiotic? Rev. Chil. Infectol. 2002, 19, S33–S37. [Google Scholar]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, F.C.; Solarte, A.L.; Tarradas, C.; Luque, I.; Maldonado, A.; Galán-Relaño, Á.; Huerta, B. Antimicrobial activity of selected essential oils against Streptococcus suis isolated from pigs. Microbiologyopen 2018, 7, e00613. [Google Scholar] [CrossRef] [PubMed]
- Wain, J.; Simpson, J.A.; Thi Diem Nga, L.; Song Diep, T.; Thanh Duy, P.; Baker, S.; Day, N.P.J.; White, N.J.; Parry, C.M. Bactericidal activities and post-antibiotic effects of ofloxacin and ceftriaxone against drug-resistant Salmonella enterica serovar Typhi. J. Antimicrob. Chemother. 2021, 76, 2606–2609. [Google Scholar] [CrossRef]
- Soren, O.; Brinch, K.S.; Patel, D.; Liu, Y.; Liu, A.; Coates, A.; Hu, Y. Antimicrobial peptide novicidin synergizes with rifampin, ceftriaxone, and ceftazidime against antibiotic-resistant Enterobacteriaceae in vitro. Antimicrob. Agents Chemother. 2015, 59, 6233–6240. [Google Scholar] [CrossRef]
- Akermi, S.; Smaoui, S.; Chaari, M.; Elhadef, K.; Gentile, R.; Hait, M.; Roymahapatra, G.; Mellouli, L. Combined in vitro/in silico approaches, molecular dynamics simulations and safety assessment of the multifunctional properties of thymol and carvacrol: A comparative insight. Chem. Biodivers. 2024, 21, e202301575. [Google Scholar] [CrossRef]
- Yan, S.; Bohach, G.A.; Stevens, D.L. Persistent acylation of high-molecular-weight penicillin-binding proteins by penicillin induces the postantibiotic effect in Streptococcus pyogenes. J. Infect. Dis. 1994, 170, 609–614. [Google Scholar] [CrossRef]
- Horváthová, E.; Sramková, M.; Lábaj, J.; Slamenová, D. Study of cytotoxic, genotoxic and DNA-protective effects of selected plant essential oils on human cells cultured in vitro. Neuro. Endocrinol. Lett. 2006, 27, 44–47. [Google Scholar] [CrossRef]
- Radha, R.; Latha, R.; Swaminathan, M.S. Chemical composition and bioactivity of essential oilfrom Syzygium travancoricum Gamble. Flavour Fragr. J. 2002, 17, 478. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of an essential oil from Origanum vulgare subsp. hirtum letsw. var. Vulkan when used as a sensory additive in feed for all animal species. EFSA J. 2017, 15, e05095. [Google Scholar] [CrossRef][Green Version]


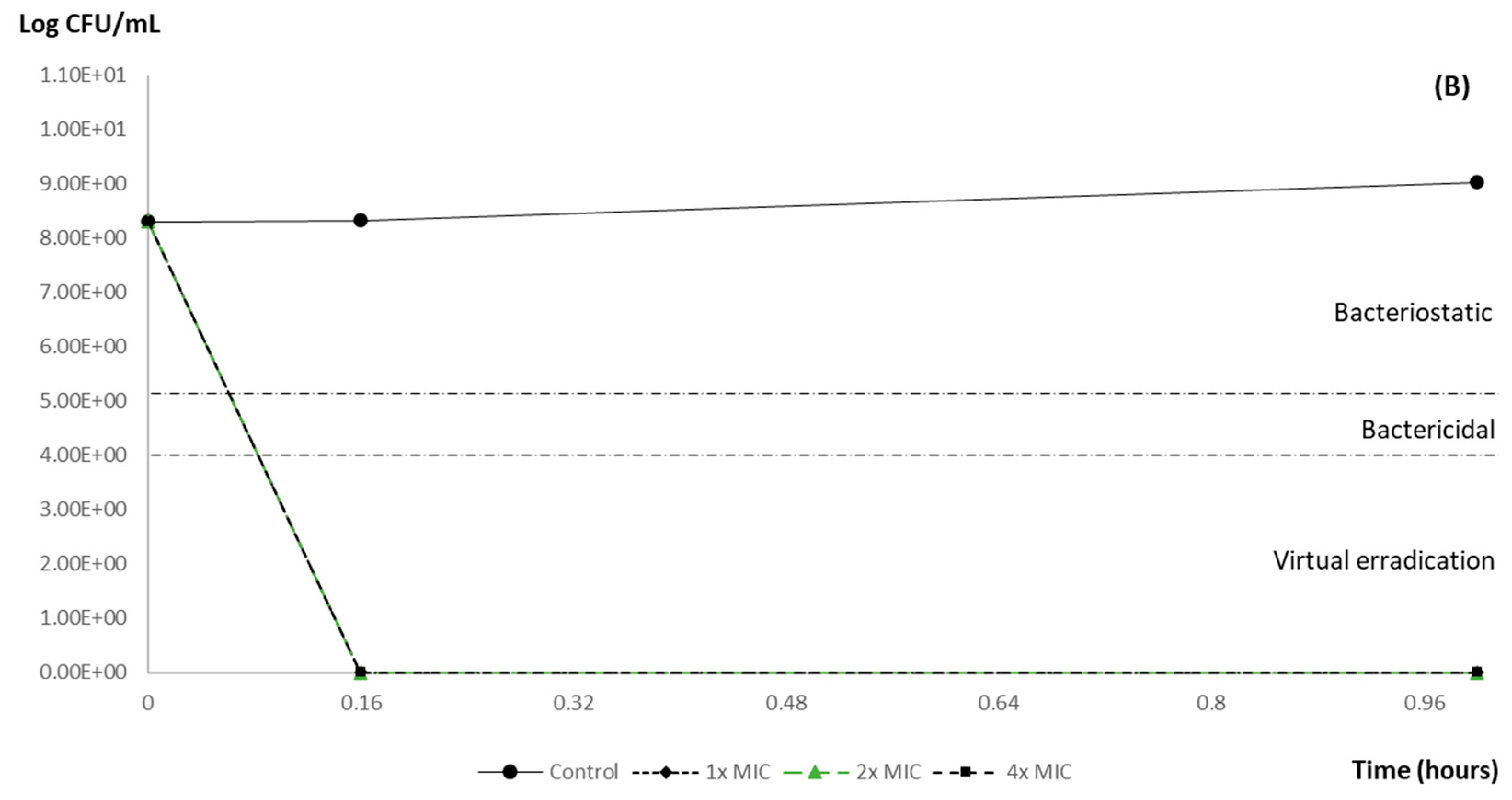
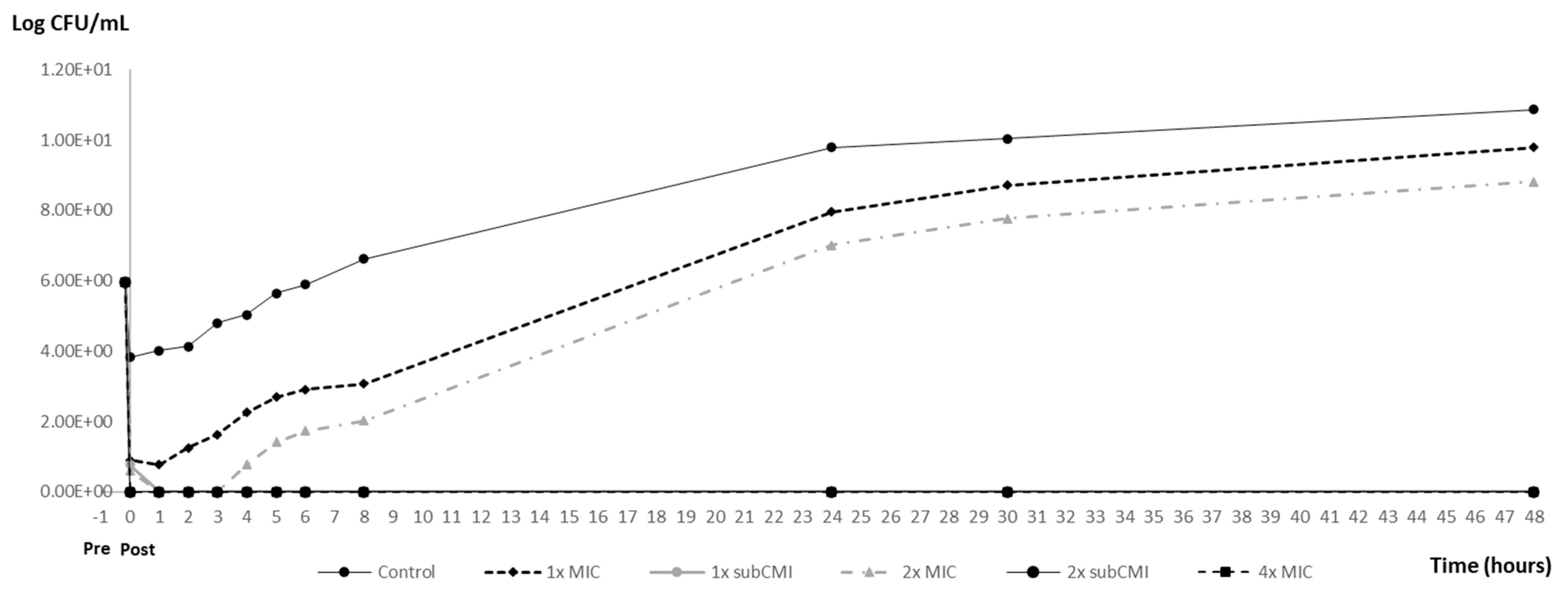
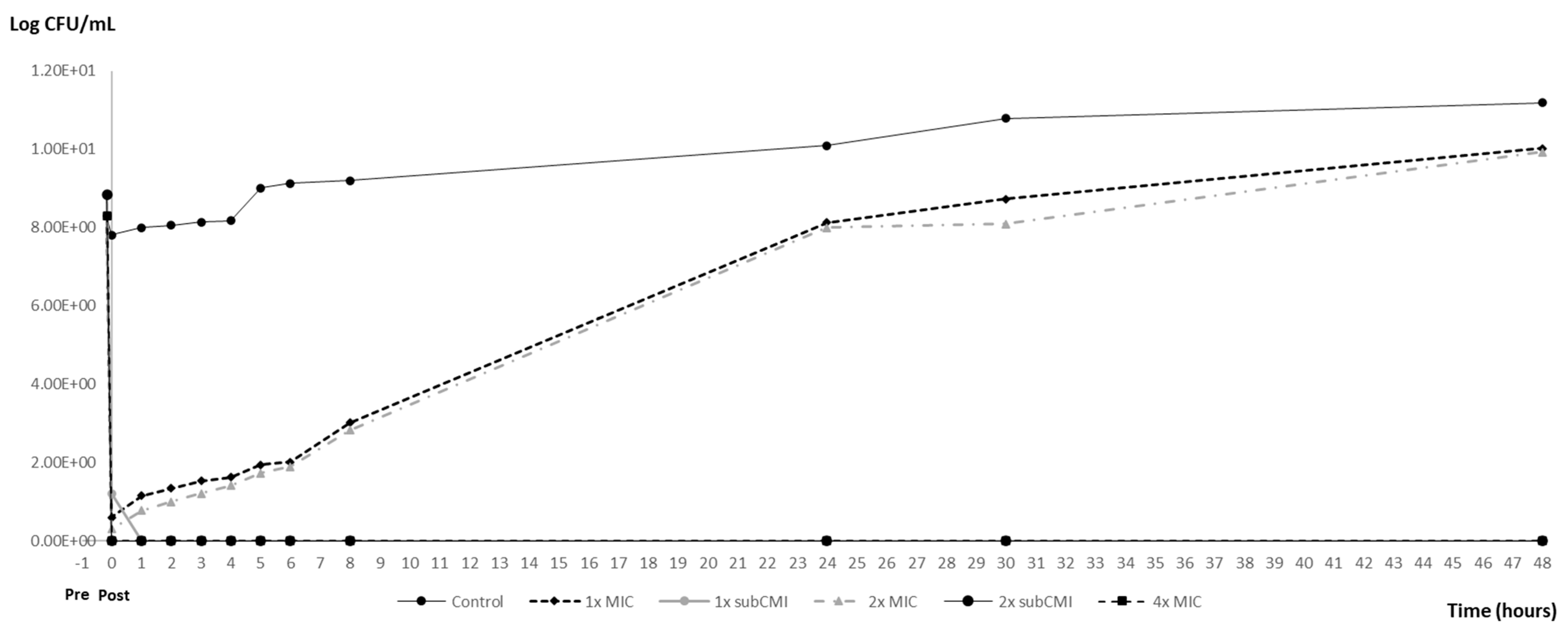
| Post-Exposition Time | Carvacrol Efficacy with Inoculum 106 | ||
| 1× CMI (0.6 mg/mL) | 2× CMI (1.2 mg/mL) | 4× CMI (2.4 mg/mL) | |
| 10 min | 100% | 100% | 100% |
| 1 h | 100% | 100% | 100% |
| 2 h | 100% | 100% | 100% |
| 4 h | 100% | 100% | 100% |
| 8 h | 100% | 100% | 100% |
| 24 h | 100% | 100% | 100% |
| Post-Exposition Time | Carvacrol Efficacy with Inoculum 108 | ||
| 1× CMI (0.6 mg/mL) | 2× CMI (1.2 mg/mL) | 4× CMI (2.4 mg/mL) | |
| 10 min | 100% | 100% | 100% |
| 1 h | 100% | 100% | 100% |
| 2 h | 100% | 100% | 100% |
| 4 h | 100% | 100% | 100% |
| 8 h | 100% | 100% | 100% |
| 24 h | 100% | 100% | 100% |
| Inoculum CFU/mL | PAEs (h) | PA-SMEs (h) | |||
|---|---|---|---|---|---|
| 1× MIC (0.6 mg/mL) | 2× MIC (1.2 mg/mL) | 4× MIC (2.4 mg/mL) | 1× + 0.25 MIC (0.6 + 0.15 mg/mL) | 2× + 0.25 MIC (1.2 + 0.15 mg/mL) | |
| 106 | 0 ± 0 | 2 ± 0 | >44.5 ± 0 | >44.5 ± 0 | >44.5 ± 0 |
| 108 | 0 ± 0 | 1 ± 0 | >43.5 ± 0 | >43.5 ± 0 | >43.5 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer, E.; Galán-Relaño, Á.; Romero-Salmoral, A.; Barraza, P.; Gómez-Gascón, L.; Tarradas, C.; Luque, I.; de Aguiar, F.C.; Huerta Lorenzo, B. Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals 2024, 14, 2631. https://doi.org/10.3390/ani14182631
Boyer E, Galán-Relaño Á, Romero-Salmoral A, Barraza P, Gómez-Gascón L, Tarradas C, Luque I, de Aguiar FC, Huerta Lorenzo B. Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals. 2024; 14(18):2631. https://doi.org/10.3390/ani14182631
Chicago/Turabian StyleBoyer, Eva, Ángela Galán-Relaño, Antonio Romero-Salmoral, Paula Barraza, Lidia Gómez-Gascón, Carmen Tarradas, Inmaculada Luque, Fabiana Carolina de Aguiar, and Belén Huerta Lorenzo. 2024. "Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium" Animals 14, no. 18: 2631. https://doi.org/10.3390/ani14182631
APA StyleBoyer, E., Galán-Relaño, Á., Romero-Salmoral, A., Barraza, P., Gómez-Gascón, L., Tarradas, C., Luque, I., de Aguiar, F. C., & Huerta Lorenzo, B. (2024). Post-Antibiotic and Post-Antibiotic Sub-Minimum Inhibitory Concentration Effects of Carvacrol against Salmonella Typhimurium. Animals, 14(18), 2631. https://doi.org/10.3390/ani14182631










