New Advances in Attention-Deficit/Hyperactivity Disorder-like Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Pathogenesis
2.1. Human ADHD
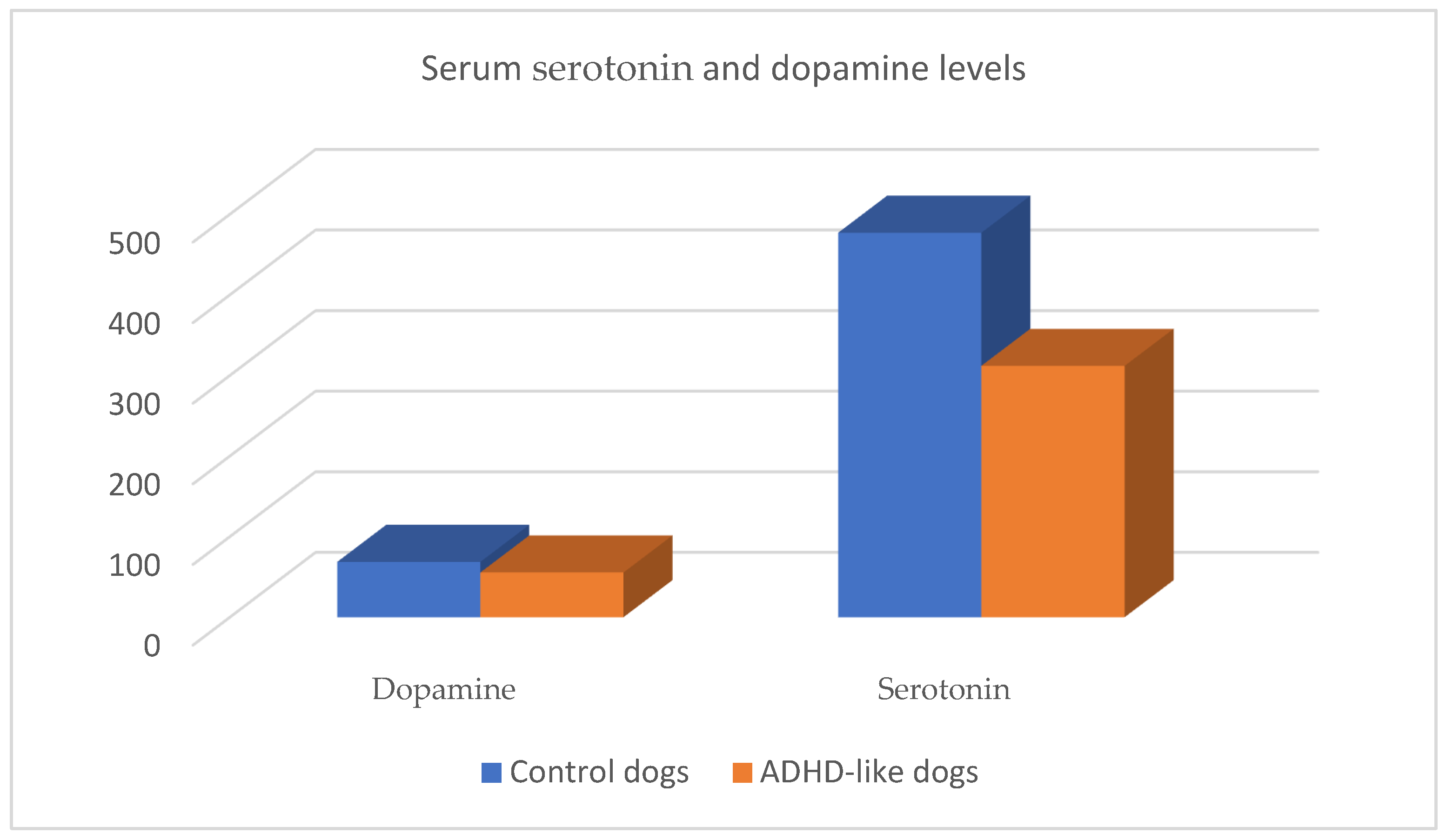
2.2. The Role of Dopamine
2.3. The Role of Serotonin
2.4. The Role of Norepineprhyne
2.5. Risk Factors Related to ADHD-like
2.5.1. The Role of Genetics
2.5.2. The Role of the Environment
2.5.3. Demographic Risk Factors
| Risk Factors Related to ADHD-like |
|---|
| Genetic factors |
|
| Environmental factors |
|
| Demographic factors |
|
3. Symptomatology
- Concurrent hyperactivity/impulsivity and attention deficit.
- Predominantly inattentive presentation.
- Predominantly hyperactive/impulsive presentation.
| Signs Related to ADHD-like in Dogs | |
|---|---|
| Hyperactivity–Impulsivity Signs | Attention Deficit Signs |
|
|
| Somatic signs of ADHD-like [1] | |
| Gastrointestinal signs, elevated heart and respiratory rates, dilated pupils, and elevated temperature. | |
| Comorbidities related to ADHD-like [18,21,22,43,79,81] | |
| |
4. Diagnosis
- Age at weaning and management.
- Triggering moments, locations, and times of the day when the behavior occurs.
- Relationship of the behavior with the presence or absence of the owner.
- Daily play and exercise activities of the animal.
- Times, duration, and contexts in which the dog is left alone or confined and social interactions.
- Daily resting time. Sleep behavior.
- Dog’s ability to learn commands and maintain interest in certain stimuli.
- Strategies used by the owner to curb or control the behavior.
- Behavioral Regulation: a group of items reflecting excitability and ability to control both initiation and termination of behavior.
- Response to Novelty and Aggression: items indicating active responding when faced with aversion and uncertainty.
- Responsiveness: items describing behavioral sensitivity to environmental cues.
5. Prognosis
6. Treatment
6.1. Behavior Modification Guidelines
- Avoiding situations that overstimulate the dog and trigger the behavior. It may be advisable for the owner to compile a list of stimuli or situations triggering the problematic behavior.
- Withholding reinforcement from the owner, such as administering rewards at inappropriate times that may increase attention demands [90].
- Interacting with the dog when it is relaxed, ignoring exaggerated attention demands. If it becomes overly excited, it is advisable to use a signal to end the interaction, and if this fails, employ the “time out” technique (interrupting positive interaction) [90].
- Maintaining routines regarding activities involving the animal to make its environment more predictable.
- Use of interactive and chew toys. A recent study suggests that chew toys provide relaxation during moments of stress for dogs [92]. Nevertheless, it should be monitored by pet parents because some dogs could become excessively excited or frustrated.
- Positive reinforcement training exercises, where quick rewards are recommended to maintain the animal’s interest, increasing dopamine in CNS.
6.2. Biological Therapies and Other Tools
7. Prevention
- Avoid breeding animals exhibiting signs of attention problems, hyperactivity, impulsivity, or aggression.
- Avoid early weaning of puppies to ensure adequate social experiences with their mother and littermates.
- Minimize prolonged periods of separation after the animal’s adoption.
- Fulfill the social needs of the animal through social play, petting, and other enjoyable interactions.
- Educate the dog in a positive manner.
- Provide appropriate exercise based on the age and breed of the animal.
- Provide conditions for the animal to sleep for enough hours.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luescher, U.A. Hyperkinesis in Dogs: Six Case Reports. Can. Vet. J. 1993, 34, 368–370. [Google Scholar] [PubMed]
- Bleuer-Elsner, S.; Muller, G.; Beata, C.; Zamansky, A.; Marlois, N. Effect of Fluoxetine at a Dosage of 2–4 mg/kg Daily in Dogs Exhibiting Hypersensitivity-Hyperactivity Syndrome, a Retrospective Study. J. Vet. Behav. 2021, 44, 25–31. [Google Scholar] [CrossRef]
- Marlois, N.; Groux, D.; Mege, C.; Béata, C.; Sarcey, G.; Massal, N.; Masson, S.; Subtil, F.; Marion, M. Assessment of a Canine Hypersensitivity-Hyperactivity Syndrome Rating Scale. Dog Behav. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Hoppe, N.; Bininda-Emonds, O.R.P.; Gansloßer, U. Correlates of Attention Deficit Hyperactivity Disorder (ADHD)-Like Behavior in Domestic Dogs: First Results from a Questionnaire-Based Study. Vet. Med. Open J. 2017, 2, 95–131. [Google Scholar] [CrossRef]
- Bleuer-Elsner, S.; Zamansky, A.; Fux, A.; Kaplun, D.; Romanov, S.; Sinitca, A.; Masson, S.; van der Linden, D. Computational Analysis of Movement Patterns of Dogs with ADHD-like Behavior. Animals 2019, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Sulkama, S.; Puurunen, J.; Salonen, M.; Mikkola, S.; Hakanen, E.; Araujo, C.; Lohi, H. Canine Hyperactivity, Impulsivity, and Inattention Share Similar Demographic Risk Factors and Behavioural Comorbidities with Human ADHD. Transl. Psychiatry 2021, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Topál, J.; Péch, É.; Miklósi, Á. Measuring Attention Deficit and Activity in Dogs: A New Application and Validation of a Human ADHD Questionnaire. Appl. Anim. Behav. Sci. 2007, 103, 105–117. [Google Scholar] [CrossRef]
- Lit, L.; Schweitzer, J.B.; Iosif, A.M.; Oberbauer, A.M. Owner Reports of Attention, Activity, and Impulsivity in Dogs: A Replication Study. Behav. Brain Funct. 2010, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, Á.; Muñiz de Miguel, S.; Graña, N.; Costas, X.; Diéguez, F.J. Serotonin and Dopamine Blood Levels in ADHD-Like Dogs. Animals 2023, 13, 1037. [Google Scholar] [CrossRef]
- Piotti, P.; Satchell, L.P.; Lockhart, T.S. Impulsivity and Behaviour Problems in Dogs: A Reinforcement Sensitivity Theory Perspective. Behav. Process. 2018, 151, 104–110. [Google Scholar] [CrossRef]
- Tsakiris, A. A One Health Approach to Neurodiversity and Attention-Deficit/Hyperactivity Disorder in People and Dogs: Implications for Research, Treatments, and Acceptance. J. Am. Holist. Vet. Med. Assoc. 2023, 73, 11–17. [Google Scholar]
- Paclt, I.; Koudelová, J.; Křepelová, A.; Uhlíková, P.; Gazdíková, M.; Bauer, P. Biochemical Markers and Genetic Research of ADHD. Neuroendocrinol. Lett. 2005, 26, 423–430. [Google Scholar] [PubMed]
- Faraone, S.V.; Biederman, J.; Spencer, T.J.; Aleardi, M. Comparing the Efficacy of Medications for ADHD Using Meta-Analysis. MedGenMed Medscape Gen. Med. 2006, 8, 4. [Google Scholar]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical Brain Volume Differences in Participants with Attention Deficit Hyperactivity Disorder in Children and Adults: A Cross-Sectional Mega-Analysis. Artic. Lancet Psychiatry 2017, 4, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Csibra, B.; Bunford, N.; Gácsi, M. Evaluating ADHD Assessment for Dogs: A Replication Study. Animals 2022, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Piturru, P. Methylphenidate Use in Dogs with Attention Deficit Hyperactivity Disorder (ADHD). A Case Report of a Weimaraner Bitch. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014, 42, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Gaultier, E. Retrospective Study on Hypersensitivity-Hyperactivity Syndrome in Dogs: Long-Term Outcome of High Dose Fluoxetine Treatment and Proposal of a Clinical Score. Dog Behav. 2018, 4, 15–32. [Google Scholar] [CrossRef]
- Bunford, N.; Csibra, B.; Peták, C.; Ferdinandy, B.; Miklósi, Á.; Gácsi, M. Associations among Behavioral Inhibition and Owner-Rated Attention, Hyperactivity/Impulsivity, and Personality in the Domestic Dog (Canis Familiaris). J. Comp. Psychol. 2019, 133, 233–243. [Google Scholar] [CrossRef]
- Sulkama, S.; Salonen, M.; Mikkola, S.; Hakanen, E.; Puurunen, J.; Araujo, C.; Lohi, H. Aggressiveness, ADHD-like Behaviour, and Environment Influence Repetitive Behaviour in Dogs. Sci. Rep. 2022, 12, 3520. [Google Scholar] [CrossRef]
- Csibra, B.; Bunford, N.; Gácsi, M. Development of a Human-Analogue, 3-Symptom Domain Dog ADHD and Functionality Rating Scale (DAFRS). Sci. Rep. 2024, 14, 1808. [Google Scholar] [CrossRef]
- Dinwoodie, I.R.; Dwyer, B.; Zottola, V.; Gleason, D.; Dodman, N.H. Demographics and Comorbidity of Behavior Problems in Dogs. J. Vet. Behav. 2019, 32, 62–71. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, Comorbidity, and Breed Differences in Canine Anxiety in 13,700 Finnish Pet Dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef] [PubMed]
- Didehban, N.; Pourmahdi Borujeni, M.; Avizeh, R.; Mosallanejad, B. Problematic Behaviors in Companion Dogs: A Survey of Their Prevalence and Associated Factors. J. Vet. Behav. 2020, 39, 6–13. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S. Causal Models of Attention-Deficit/Hyperactivity Disorder: From Common Simple Deficits to Multiple Developmental Pathways. Biol. Psychiatry 2005, 57, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, X.F.; Lee, P.; Sharp, W.; Jeffries, N.O.; Greenstein, D.K.; Clasen, L.S.; Blumenthal, J.D.; James, R.S.; Ebens, C.L.; Walter, J.M.; et al. Developmental Trajectories of Brain Volume Abnormalities in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. JAMA 2022, 288, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Greven, C.U.; Bralten, J.; Mennes, M.; O’Dwyer, L.; van Hulzen, K.E.; Rommelse, N.; Schweren, L.J.S. Developmentally Stable Whole-Brain Volume Reductions and Developmentally Sensitive Caudate and Putamen Volume Alterations in Those with Attention-Deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry 2015, 72, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K. Neuro-Anatomic Evidence for the Maturational Delay Hypothesis of ADHD. Proc. Natl. Acad. Sci. USA 2007, 104, 19663–19664. [Google Scholar] [CrossRef] [PubMed]
- Quist, J.F.; Barr, C.L.; Schachar, R.; Roberts, W.; Malone, M.; Tannock, R.; Basile, V.S.; Beitchman, J.; Kennedy, J.L. The Serotonin 5-HT1B Receptor Gene and Attention Deficit Hyperactivity Disorder. Mol. Psychiatry 2003, 8, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A.; Coquerel, A. Dopamine: A Key Regulator to Adapt Action, Emotion, Motivation and Cognition. Curr. Opin. Neurol. 2003, 16, S3–S9. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Kollins, S.H.; Wigal, T.L.; Newcorn, J.H.; Telang, F.; Fowler, J.S.; Zhu, W.; Logan, J.; Ma, Y.; et al. Evaluating Dopamine Reward Pathway in ADHD: Clinical Implications. JAMA J. Am. Med. Assoc. 2009, 302, 1084–1091. [Google Scholar] [CrossRef]
- Kubinyi, E.; Wallis, L.J. Dominance in Dogs as Rated by Owners Corresponds to Ethologically Valid Markers of Dominance. PeerJ 2019, 2019, e6838. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, E.; Vas, J.; Hejjas, K.; Ronai, Z.; Brúder, I.; Turcsán, B.; Sasvari-Szekely, M.; Miklósi, Á. Polymorphism in the Tyrosine Hydroxylase (TH) Gene Is Associated with Activity-Impulsivity in German Shepherd Dogs. PLoS ONE 2012, 7, e30271. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Nara, H.; Inoue-Murayama, M.; Shimada, M.K.; Koshimura, A.; Ueda, Y.; Kitagawa, H.; Takeuchi, Y.; Mori, Y.; Murayama, Y.; et al. Allele Frequency Distribution of the Canine Dopamine Receptor D4 Gene Exon III and I in 23 Breeds. J. Vet. Med. Sci. 2004, 66, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Hejjas, K.; Vas, J.; Kubinyi, E.; Sasvari-Szekely, M.; Miklosi, A.; Ronai, Z. Novel Repeat Polymorphisms of the Dopaminergic Neurotransmitter Genes among Dogs and Wolves. Mamm. Genome 2007, 18, 871–879. [Google Scholar] [CrossRef]
- Hejjas, K.; Kubinyi, E.; Ronai, Z.; Szekely, A.; Vas, J.; Miklósi, Á.; Sasvari-Szekely, M.; Kereszturi, E. Molecular and Behavioral Analysis of the Intron 2 Repeat Polymorphism in the Canine Dopamine D4 Receptor Gene. Genes Brain Behav. 2009, 8, 330–336. [Google Scholar] [CrossRef]
- Banerjee, E.; Nandagopal, K. Does Serotonin Deficit Mediate Susceptibility to ADHD? Neurochem. Int. 2015, 82, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Rosado, B.; García-Belenguer, S.; Palacio, J.; Chacón, G.; Villegas, A.; Alcalde, A.I. Serotonin Transporter Activity in Platelets and Canine Aggression. Vet. J. 2010, 186, 104–105. [Google Scholar] [CrossRef]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacón, G.; Villegas, A.; Palacio, J. Assessment of Serotonin in Serum, Plasma, and Platelets of Aggressive Dogs. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Blood Concentrations of Serotonin, Cortisol and Dehydroepiandrosterone in Aggressive Dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Amat, M.; Le Brech, S.; Camps, T.; Torrente, C.; Mariotti, V.M.; Ruiz, J.L.; Manteca, X. Differences in Serotonin Serum Concentration between Aggressive English Cocker Spaniels and Aggressive Dogs of Other Breeds. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 19–25. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Behavioural and Physiological Correlates of Impulsivity in the Domestic Dog (Canis Familiaris). Physiol. Behav. 2012, 105, 676–682. [Google Scholar] [CrossRef]
- Del Campo, N.; Chamberlain, S.R.; Sahakian, B.J.; Robbins, T.W. The Roles of Dopamine and Noradrenaline in the Pathophysiology and Treatment of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2011, 69, e145–e157. [Google Scholar] [CrossRef]
- Elia, J.; Glessner, J.T.; Wang, K.; Takahashi, N.; Shtir, C.J.; Hadley, D.; Sleiman, P.M.A.; Zhang, H.; Kim, C.E.; Robison, R.; et al. Genome-Wide Copy Number Variation Study Associates Metabotropic Glutamate Receptor Gene Networks with Attention Deficit Hyperactivity Disorder. Nat. Genet. 2012, 44, 78–84. [Google Scholar] [CrossRef]
- Naaijen, J.; Bralten, J.; Poelmans, G.; Glennon, J.C.; Franke, B.; Buitelaar, J.K. Glutamatergic and GABAergic Gene Sets in Attention-Deficit/Hyperactivity Disorder: Association to Overlapping Traits in ADHD and Autism. Transl. Psychiatry 2017, 7, e999. [Google Scholar] [CrossRef]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The Physiology of Brain Histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Miyazaki, C.; Koyama, M.; Ota, E.; Swa, T.; Mlunde, L.B.; Amiya, R.M.; Tachibana, Y.; Yamamoto-Hanada, K.; Mori, R. Allergic Diseases in Children with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. BMC Psychiatry 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Newcorn, J.H.; Kollins, S.H.; Wigal, T.L.; Telang, F.; Fowler, J.S.; Goldstein, R.Z.; Klein, N.; Logan, J.; et al. Motivation Deficit in ADHD Is Associated with Dysfunction of the Dopamine Reward Pathway. Mol. Psychiatry 2011, 16, 1147–1154. [Google Scholar] [CrossRef]
- Lowe, N.; Kirley, A.; Hawi, Z.; Sham, P.; Wickham, H.; Kratochvil, C.J.; Smith, S.D.; Lee, S.Y.; Levy, F.; Kent, L.; et al. Joint Analysis of the DRD5 Marker Concludes Association with Attention-Deficit/Hyperactivity Disorder Confined to the Predominantly Inattentive and Combined Subtypes. Am. J. Hum. Genet. 2004, 74, 348–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hejjas, K.; Vas, J.; Topal, J.; Szantai, E.; Ronai, Z.; Szekely, A.; Kubinyi, E.; Horvath, Z.; Sasvari-Szekely, M.; Miklosi, A. Association of Polymorphisms in the Dopamine D4 Receptor Gene and the Activity-Impulsivity Endophenotype in Dogs. Anim. Genet. 2007, 38, 629–633. [Google Scholar] [CrossRef]
- Mü ller Smith, K.; Daly, M.; Fischer, M.; Yiannoutsos, C.T.; Bauer, L.; Barkley, R.; Navia, B.A. Association of the Dopamine Beta Hydroxylase Gene with Attention Deficit Hyperactivity Disorder: Genetic Analysis of the Milwaukee Longitudinal Study. Am. J. Med. Genet. 2003, 119, 77–85. [Google Scholar] [CrossRef]
- Lunõ, I.; Rosado, B.; Palacio, J.; Villegas, A.; González-Martínez, Á.; Garciá-Belenguer, S. Hyperactivity in a Weimaraner Dog. Dog Behav. 2015, 1, 32–40. [Google Scholar] [CrossRef]
- Carlson, N.R. Physiology of Behavior; Golobar, Ed.; Pearson Education: Essex, UK, 2022. [Google Scholar]
- Blier, P.; El Mansari, M. The Importance of Serotonin and Noradrenaline in Anxiety. Int. J. Psychiatry Clin. Pract. 2007, 11, 16–23. [Google Scholar] [CrossRef]
- Stahl, S.M. Stahl’s Essential Psychopharmacology, 5th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Mogavero, F.; Jager, A.; Glennon, J.C. Clock Genes, ADHD and Aggression. Neurosci. Biobehav. Rev. 2018, 91, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M. Serotonin Concentrations in Whole Blood of Hyperactive Children. J. Pediatr. 1971, 78, 985–990. [Google Scholar] [CrossRef]
- Spivak, B.; Vered, Y.; Yoran-Hegesh, R.; Averbuch, E.; Mester, R.; Graf, E.; Weizman, A. Circulatory Levels of Catecholamines, Serotonin and Lipids in Attention Deficit Hyperactivity Disorder. Acta Psychiatr. Scand. 1999, 99, 300–304. [Google Scholar] [CrossRef]
- Lombroso, P.J.; Quist, J.F.; Kennedy, J.L. Genetics of Childhood Disorders: XXIII. ADHD, Part 7: The Serotonin System. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 253–256. [Google Scholar]
- Barkley, R.A. Behavioral Inhibition, Sustained Attention, and Executive Functions: Constructing a Unifying Theory of ADHD. Psychol. Bull. 1997, 121, 65–94. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T.; Pliszka, S.R. Catecholamine Influences on Prefrontal Cortical Function: Relevance to Treatment of Attention Deficit Hyperactivity Disorder and Related Disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef]
- Rohde, L.A.; Buitelaar, J.K.; Gerlach, M.; Faraone, S.V. La Federación Mundial de Guía TDAH; Sociedad Española de Medicina de la Adolescencia (SEMA): Madrid, Spain, 2019; ISBN 9788582715789. [Google Scholar]
- Sanders, A.R.; Bhongir, N.; vonHoldt, B.; Pellegrini, M. Association of DNA Methylation with Energy and Fear-Related Behaviors in Canines. Front. Psychol. 2022, 13, 1025494. [Google Scholar] [CrossRef]
- Harvey, N.D.; Craigon, P.J.; Blythe, S.A.; England, G.C.W.; Asher, L. Social Rearing Environment Influences Dog Behavioral Development. J. Vet. Behav. Clin. Appl. Res. 2016, 16, 13–21. [Google Scholar] [CrossRef]
- Lindsay, S. Handbook of Applied Dog Behavior and Training, Vol 2: Etiology and Assessment of Behavior Problems; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Fox, M.W.; Stelzner, D. The Effects of Early Experience on the Development of Inter and Intraspecies Social Relationships in the Dog. Anim. Behav. 1967, 15, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The Role of Early Experience in Emotional Arousal. Ann. N. Y. Acad. Sci. 1969, 159, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J. DSM-5® Guía Para El Diagnóstico Clínico; Manual Moderno: Mexico City, Mexico, 2015. [Google Scholar]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-Deficit/Hyperactivity Disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Development and Validation of a Psychometric Tool for Assessing Impulsivity in the Domestic Dog (Canis Familiaris). Int. J. Comp. Psychol. 2011, 24, 210–225. [Google Scholar] [CrossRef]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the Risk and Age of Onset of Cancer and Behavioral Disorders in Gonadectomized Vizslas. J. Am. Vet. Med. Assoc. 2014, 244, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Fadel, F.R.; Driscoll, P.; Pilot, M.; Wright, H.; Zulch, H.; Mills, D. Differences in Trait Impulsivity Indicate Diversification of Dog Breeds into Working and Show Lines. Sci. Rep. 2016, 6, 22162. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.A.; Forndran, S.; Stauber, C.; Woerner, K.; Gansloßer, U. The Social Behaviour of Neutered Male Dogs Compared to Intact Dogs (Canis Lupus Familiaris): Video Analyses, Questionnaires and Case Studies. Vet. Med. Open J. 2017, 2, 22–37. [Google Scholar] [CrossRef]
- Carreiro, C.; Reicher, V.; Kis, A.; Gácsi, M. Owner—Rated Hyperactivity/Impulsivity Is Associated with Sleep Efficiency in Family Dogs: A Non - Invasive EEG Study. Sci. Rep. 2023, 13, 1291. [Google Scholar] [CrossRef]
- Landsberg, G.; Radosta, L.A.L. Behavior Problems of the Dog and Cat, 4th ed.; Elsevier Inc.: St. Louis, MI, USA, 2024; ISBN 978-0-702-08214-6. [Google Scholar]
- Biederman, J.; Faraone, S.V.; Spencer, T.; Wilens, T.; Norman, D.; Lapey, K.A.; Mick, E.; Lehman, B.K.; Doyle, A. Patterns of Psychiatric Comorbidity, Cognition, and Psychosocial Functioning in Adults with Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 1993, 150, 1792–1798. [Google Scholar] [CrossRef]
- Cook, E.H.; Stein, M.A.; Ellison, T.; Unis, A.S.; Leventhal, B.L. Attention Deficit Hyperactivity Disorder and Whole-Blood Serotonin Levels: Effects of Comorbidity. Psychiatry Res. 1995, 57, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Von Gontard, A.; Equit, M. Comorbidity of ADHD and Incontinence in Children. Eur. Child Adolesc. Psychiatry 2015, 24, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Peek, S.I.; Meller, S.; Twele, F.; Packer, R.M.A.; Volk, H.A. Epilepsy Is More than a Simple Seizure Disorder: Parallels between Human and Canine Cognitive and Behavioural Comorbidities. Vet. J. 2024, 303, 106060. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Guitaut, N.; Medam, T.; Béata, C. Link between Foreign Body Ingestion and Behavioural Disorder in Dogs. J. Vet. Behav. 2021, 45, 25–32. [Google Scholar] [CrossRef]
- Harvey, N.; Craigon, P.; Shaw, S.; Blott, S.; England, G. Behavioural Differences in Dogs with Atopic Dermatitis Suggest Stress Could Be a Significant Problem Associated with Chronic Pruritus. Animals 2019, 9, 813. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, J.W.; Wang, J.H.; Loh, C.H.; Chen, T.L. Associations of Atopic Dermatitis with Attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Dermatology 2024, 240, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.; Su, J.C. The Psychology of Atopic Dermatitis. J. Clin. Med. 2024, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, J.; Tiira, K.; Lehtonen, M.; Hanhineva, K.; Lohi, H. Non-Targeted Metabolite Profiling Reveals Changes in Oxidative Stress, Tryptophan and Lipid Metabolisms in Fearful Dogs. Behav. Brain Funct. 2016, 12, 7. [Google Scholar] [CrossRef]
- Riemer, S.; Mills, D.S.; Wright, H. Impulsive for Life? The Nature of Long-Term Impulsivity in Domestic Dogs. Anim. Cogn. 2014, 17, 815–819. [Google Scholar] [CrossRef]
- Csibra, B.; Reicher, V.; Csepregi, M. Towards an Objective Measurement Tool for ADHD-like Traits in Family Dogs: A Comprehensive Test Battery. Animals 2024, 14, 1841. [Google Scholar] [CrossRef]
- Stiles, E.K.; Palestrini, C.; Beauchamp, G.; Frank, D. Physiological and Behavioral Effects of Dextroamphetamine on Beagle Dogs. J. Vet. Behav. Clin. Appl. Res. 2011, 6, 328–336. [Google Scholar] [CrossRef]
- Vieira de Castro, A.C.; Barrett, J.; de Sousa, L.; Olsson, I.A.S. Carrots versus Sticks: The Relationship between Training Methods and Dog-Owner Attachment. Appl. Anim. Behav. Sci. 2019, 219, 104831. [Google Scholar] [CrossRef]
- Vieira de Castro, A.C.; Fuchs, D.; Morello, G.M.; Pastur, S.; de Sousa, L.; Olsson, I.A.S. Does Training Method Matter? Evidence for the Negative Impact of Aversive-Based Methods on Companion Dog Welfare. PLoS ONE 2020, 15, e0225023. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.F. Canine and Feline Behavior, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Overall, K. Manual of Clinical Behavioral Medicine for Dogs and Cats-E-Book; Mosby: Maryland Heights, MI, USA, 2013. [Google Scholar]
- Flint, H.E.; Atkinson, M.; Lush, J.; Hunt, A.B.G.; King, T. Long-Lasting Chews Elicit Positive Emotional States in Dogs during Short Periods of Social Isolation. Animals 2023, 13, 552. [Google Scholar] [CrossRef] [PubMed]
- Rossano, F.; Caiazza, C.; Sobrino, A.; Solini, N.; Vellucci, A.; Zotti, N.; Fornaro, M.; Gillman, K.; Cattaneo, C.I.; Van den Eynde, V.; et al. Efficacy and Safety of Selegiline across Different Psychiatric Disorders: A Systematic Review and Meta-Analysis of Oral and Transdermal Formulations. Eur. Neuropsychopharmacol. 2023, 72, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, D.R.; Sallee, F.R.; Pelham, W.E.; Bukstein, O.G.; Daviss, W.B.; McDermott, M.P.; Burrows-MacLean, L.; Como, P.; Hoffman, M.T.; Lock, T.M.; et al. Clonidine for Attention-Deficit/Hyperactivity Disorder: I. Efficacy and Tolerability Outcomes. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
- DiFonzo, N.; Bordia, P. Reproduced with Permission of the Copyright Owner. Further Reproduction Prohibited without. J. Allergy Clin. Immunol. 1998, 130, 556. [Google Scholar]
- Packer, R.M.A.; Law, T.H.; Davies, E.; Zanghi, B.; Pan, Y.; Volk, H.A. Effects of a Ketogenic Diet on ADHD-like Behavior in Dogs with Idiopathic Epilepsy. Epilepsy Behav. 2016, 55, 62–68. [Google Scholar] [CrossRef]
- Maffeo, N.N.; Springer, C.M.; Albright, J.D. A Retrospective Study on the Clinical Use and Owner Perception of Venlafaxine Efficacy as Part of a Multimodal Treatment for Canine Fear, Anxiety, and Aggression. J. Vet. Behav. 2023, 64–65, 54–59. [Google Scholar] [CrossRef]
| Neurotransmitter | Role in ADHD |
|---|---|
| Dopamine | Participates in motor activity regulation and limbic functions, playing an essential role in attention and cognition, especially in executive functions [30] and reward processing [31]. In dogs, dopamine is associated with problems of aggression, impulsivity, attention deficits, and/or general nervousness [32,33,34,35,36]. |
| Serotonin | Involved in mood and emotion regulation, and plays an important role in behavioral inhibition, which is affected in ADHD [37]. Altered serotonin transmission in patients with ADHD could be related to comorbidities with obsessive–compulsive disorders, aggression, and affective disorders (major depression and/or anxiety) [38]. In dogs, it is associated with aggression, impulsiveness, fear, and ADHD-like [9,39,40,41,42,43]. |
| Norepinephrine | Norepinephrine neurotransmission plays an important role in regulating higher cognitive functions such as working memory and inhibitory control [44]. |
| Glutamate | The most abundant excitatory neurotransmitter in the central nervous system. Involved in many neuronal functions such as synaptic transmission, neuronal migration, excitability, plasticity, impulsivity, and compulsivity [38]. Different genetic variations in the glutamatergic system have been associated with the presentation of ADHD symptoms [45,46]. |
| Histamine | Regulates arousal and attention. Histaminergic neurons increase alertness and prevent sleep [47]. Plays an important role in neuro-immunological reactions. Children with ADHD are more likely to develop asthma, allergic rhinitis, atopic dermatitis, and allergic conjunctivitis than non-ADHD individuals [48]. |
| Signs Related to ADHD in Humans | |
|---|---|
| Inattention Signs | Hyperactivity and Impulsivity Signs |
| Hyperactivity |
| |
| Impulsivity | |
| |
| Several hyperactive–impulsive or inattentive symptoms that caused impairment were present prior to age 12 years | |
| Several impairments from the symptoms are present in two or more settings (e.g., at home, school, or work; with friends or relatives; in other activities). | |
| There is clear evidence that the symptoms interfere with, or reduce, the quality of social, academic, or occupational functioning. | |
| The symptoms do not occur exclusively during the course of schizophrenia or another psychotic disorder and are not better accounted for by another mental disorder (e.g., mood disorder, anxiety disorder, dissociative disorder, or a personality disorder). | |
| Diagnosis Criteria for ADHD-like Dogs |
|---|
| Compulsory criteria |
|
| Suspected criteria |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, Á.; Muñiz de Miguel, S.; Diéguez, F.J. New Advances in Attention-Deficit/Hyperactivity Disorder-like Dogs. Animals 2024, 14, 2067. https://doi.org/10.3390/ani14142067
González-Martínez Á, Muñiz de Miguel S, Diéguez FJ. New Advances in Attention-Deficit/Hyperactivity Disorder-like Dogs. Animals. 2024; 14(14):2067. https://doi.org/10.3390/ani14142067
Chicago/Turabian StyleGonzález-Martínez, Ángela, Susana Muñiz de Miguel, and Francisco Javier Diéguez. 2024. "New Advances in Attention-Deficit/Hyperactivity Disorder-like Dogs" Animals 14, no. 14: 2067. https://doi.org/10.3390/ani14142067
APA StyleGonzález-Martínez, Á., Muñiz de Miguel, S., & Diéguez, F. J. (2024). New Advances in Attention-Deficit/Hyperactivity Disorder-like Dogs. Animals, 14(14), 2067. https://doi.org/10.3390/ani14142067





