Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact and Colorimetry Management
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Farms
2.2. Experimental Design
2.3. Sample Size Justification
2.4. Ethical Approval
2.5. Clinical and Laboratory Procedures
2.5.1. Rumenocentesis and Rumen pH Measurement
2.5.2. Milk Analyses
2.5.3. Rumen Wall Sampling and Image Acquisition
2.5.4. Rumen Wall Image Color Analysis
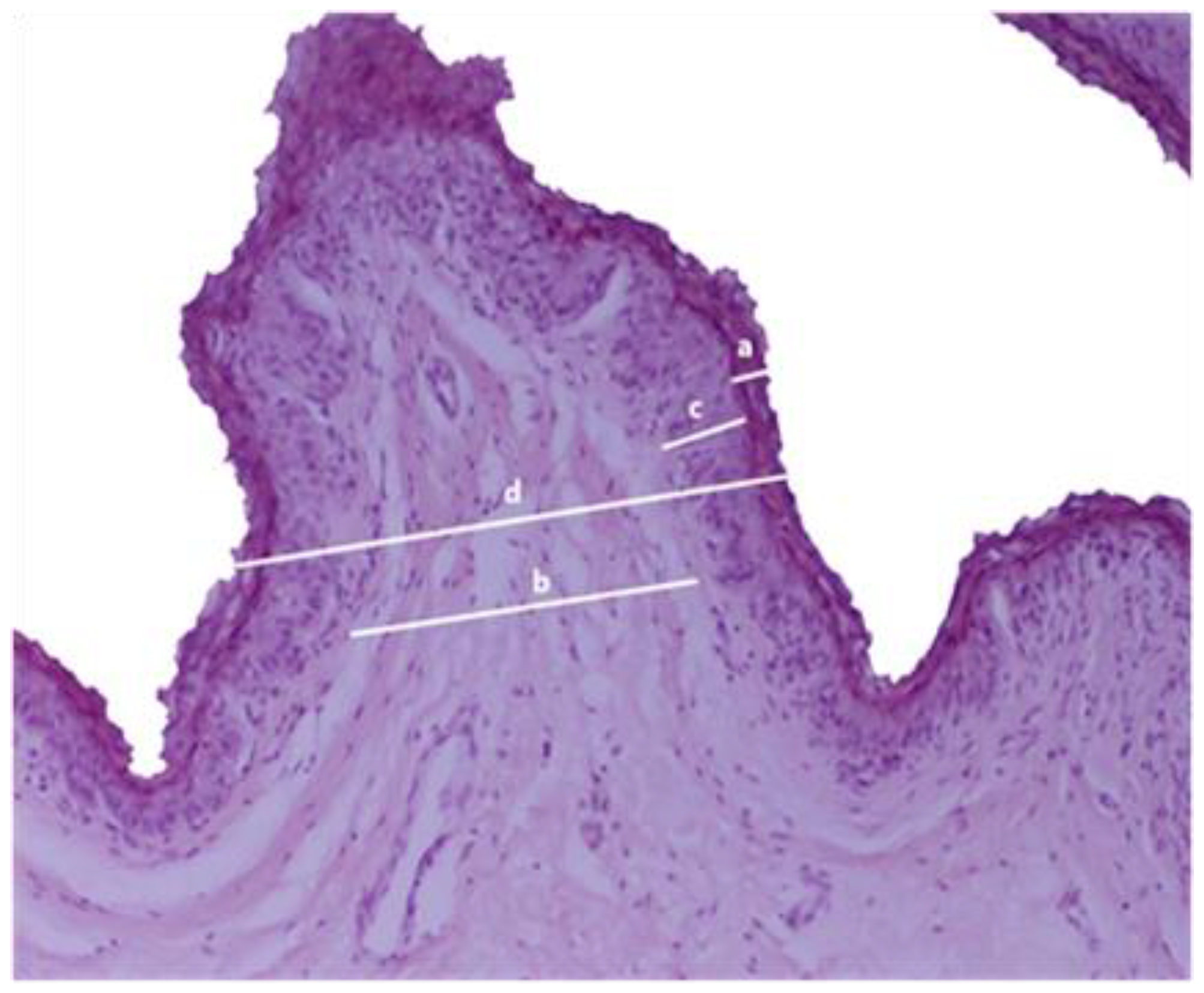
2.5.5. Histological Examination
2.6. Statistical Analysis
3. Results
- Category 1: Farms with a low prevalence of SARA, including 17 farms (41% of the total), with each farm exhibiting only 0–2 cases of pH < 5.8 in the 30 measurements.
- Category 2: Farms with a moderate prevalence of SARA, comprising 19 farms (45% of the total), with each farm showing 3–9 cases of pH < 5.8 in the 30 measurements.
- Category 3: Farms with a high prevalence of SARA, consisting of 6 farms (14% of the total), with each farm having 10–14 cases of pH < 5.8 in the 30 measurements.
3.1. Milk Analysis
3.2. Color Measurement
3.3. Ruminal Epithelium Histopathology and Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Rackwitz, R.; Oba, M. Epithelial Capacity for Apical Uptake of Short Chain Fatty Acids Is a Key Determinant for Intraruminal pH and the Susceptibility to Subacute Ruminal Acidosis in Sheep. J. Nutr. 2009, 139, 1714–1720. [Google Scholar] [PubMed]
- Xia, G.; Sun, J.; Fan, Y.; Zhao, F.; Ahmed, G.; Jin, Y.; Zhang, Y.; Wang, H. β-Sitosterol Attenuates High Grain Diet-Induced Inflammatory Stress and Modifies Rumen Fermentation and Microbiota in Sheep. Animals 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Church, D.C. Livestock Feeds and Feeding, 3rd ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 1991; p. 349. [Google Scholar]
- Commun, L.; Mialon, M.M.; Martin, C.; Baumont, R.; Veissier, I. Risk of subacute ruminal acidosis in sheep with separate access to forage and concentrate. J. Anim. Sci. 2009, 87, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.S.; Khan, M.A.; Khan, M.S.; Ashraf, K.; Saleem, M.H.; Khan, N.U.; Usman, T.; Ahmad, I. Effect of different level of concentrate feeding on hematobiochemical parameters in an experimentally induced sub-acute ruminal acidosis (SARA) in sheep and its management with different concentration of antacids. J. Anim. Plant Sci. 2018, 28, 80–84. [Google Scholar]
- Voulgarakis, N.; Gougoulis, D.; Psalla, D.; Papakonstantinou, G.; Angelidou-Tsifida, M.; Papatsiros, V.; Athanasiou, L.; Christodoulopoulos, G. Ruminal Acidosis Part I: Clinical manifestations, epidemiology and impact of the disease. J. Hell. Vet. Med. Soc. 2023, 74, 5883–5891. [Google Scholar] [CrossRef]
- Desnoyers, M.; Duvaux-Ponter, C.; Rigalma, K.; Roussel, S.; Martin, O.; Giger-Reverdin, S. Effect of concentrate percentage on ruminal pH and time-budget in dairy goats. Animal 2008, 2, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, Z. Physiological, biochemical and histopathological effects of fermentative acidosis in ruminant production: A minimal review. Span. J. Agric. Res. 2011, 9, 414–422. [Google Scholar] [CrossRef]
- Xu, T.; Tao, H.; Chang, G.; Zhang, K.; Xu, L.; Shen, X. Lipopolysaccharide derived from the rumen down-regulates stearoyl-CoA desaturase 1 expression and alters fatty acid composition in the liver of dairy cows fed a high-concentrate diet. BMC Vet. Res. 2015, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gozho, G.N.; Gakhar, N.; Khafipour, E.; Krause, D.O.; Plaizier, J.C. Evaluation of diagnostic measures for subacute ruminal acidosis in dairy cows. Can. J. Anim. Sci. 2012, 92, 353–364. [Google Scholar] [CrossRef]
- Kitkas, G.; Valergakis, G.; Kritsepi-Konstantinou, M.; Gelasakis, A.; Arsenos, G.; Kalaitzakis, E.; Panousis, N. Effects of ruminal pH and subacute ruminal acidosis on milk yield and composition of Holstein cows in different stages of lactation. J. Hell. Vet. Med. Soc. 2019, 70, 1551–1560. [Google Scholar] [CrossRef]
- Voulgarakis, N.; Athanasiou, L.; Psalla, D.; Gougoulis, D.; Papatsiros, V.; Christodoulopoulos, G. Ruminal Acidosis Part II: Diagnosis, Prevention and Treatment. J. Hell. Vet. Med. Soc. 2024, 74, 6329–6336. [Google Scholar] [CrossRef]
- Enemark, J.M.D. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef]
- Lopez-Campos, Ó.; Bodas, R.; Prieto, N.; Giraldez, F.J.; Perez, V.; Andres, S. Naringin dietary supplementation at 0.15% rates does not provide protection against sub-clinical acidosis and does not affect the responses of fattening lambs to road transportation. Animal 2010, 4, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Andrés, S.; Jaramillo, E.; Bodas, R.; Blanco, C.; Benavides, J.; Fernández, P.; González, E.P.; Frutos, J.; Belenguera, A.; Lópeza, S.; et al. Grain grinding size of cereals in complete pelleted diets for growing lambs: Effects on ruminal microbiota and fermentation. Small Rumin. Res. 2018, 159, 38–44. [Google Scholar] [CrossRef]
- Fanning, J.P.; Hynd, P.I.; Cockcroft, P.D. The relative roles of the ruminal fluid and epithelium in the aetiology of ruminal acidosis. Small Rumin. Res. 2018, 162, 57–62. [Google Scholar] [CrossRef]
- Chmiel, M.; Slowinski, M.; Dasiewicz, K.; Florowski, T. Application of a computer vision system to classify beef as normal or dark, firm, and dry. J. Anim. Sci. 2012, 90, 4126–4130. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.P.E.; Moraes, E.P.; Anaya, K.; Silva, Y.M.O.; Lopes, H.A.P.; Andrade Neto, J.C.; Oliveira, J.P.F.; Oliveira, J.B.; Rangel, A.H.N. Lactoperoxidase potential in diagnosing subclinical mastitis in cows via image processing. PLoS ONE 2022, 17, e0263714. [Google Scholar] [CrossRef]
- Voulgarakis, N.; Gougoulis, D.A.; Psalla, D.; Papakonstantinou, G.I.; Katsoulos, P.D.; Katsoulis, K.; Angelidou-Tsifida, M.; Athanasiou, L.V.; Papatsiros, V.G.; Christodoulopoulos, G. Can Computerized Rumen Mucosal Colorimetry Serve as an Effective Field Test for Managing Subacute Ruminal Acidosis in Feedlot Cattle? Vet. Res. Commun. 2024, 48, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Eurostat 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20220517-2 (accessed on 7 July 2024).
- Papanikolopoulou, V.; Vouraki, S.; Priskas, S.; Theodoridis, A.; Dimitriou, S.; Arsenos, G. Economic Performance of Dairy Sheep Farms in Less-Favoured Areas of Greece: A Comparative Analysis Based on Flock Size and Farming System. Sustainability 2023, 15, 1681. [Google Scholar] [CrossRef]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2017, 101, 6715–6729. [Google Scholar] [CrossRef]
- Atkinson, O. Prevalence of subacute ruminal acidosis (SARA) on UK dairy farms. Cattle Pract. 2014, 22, 1–9. [Google Scholar]
- Lira, M.A.A.; Simões, S.V.D.; Riet-Correa, F.; Pessoa, C.M.R.; Dantas, A.F.M.; Miranda Neto, E.G. Diseases of the digestive system of sheep and goats in the semiarid region of Brazil. Pesqui. Vet. Bras. 2013, 33, 193–198. [Google Scholar] [CrossRef]
- Mialon, M.M.; Deiss, V.; Andanson, S.; Anglard, F.; Doreau, M.; Veissier, I. An assessment of the impact of rumenocentesis on pain and stress in cattle and the effect of local anaesthesia. Vet. J. 2012, 194, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Stelletta, C.; Berzaghi, P.; Gianesella, M.; Andrighetto, I. Subacute rumen acidosis in lactating cows: An investigation in intensive Italian dairy herds. J. Anim. Physiol. Anim. Nutr. 2007, 91, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.R.; Kuby, P.J. Elementary Statistics, 11th ed.; Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- RGB Color Detector. 2022. Available online: https://www.the-programmer.com/ (accessed on 24 September 2021).
- CIE. Colourimetry: Official Recommendations of the International Commission on Illumination; CIE Publ. No. 15.2; CIE Central Bureau: Vienna, Austria, 1986. [Google Scholar]
- Povey, G.; Stubbings, L.; Phillips, K. Feeding the Ewe A Manual for Consultants, Vets and Producers, 2nd ed.; AHDB Agriculture and Horticulture Development Board Middlemarch Business Park Siskin Parkway East: Coventry, UK, 2018; pp. 1–64. [Google Scholar]
- Tao, H.; Chang, G.; Xu, T.; Zhao, H.; Zhang, K.; Shen, X. Feeding a High Concentrate Diet Down-Regulates Expression of ACACA, LPL and SCD and Modifies Milk Composition in Lactating Goats. PLoS ONE 2015, 10, e0130525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, A.L.; Zhao, F.F.; Yu, L.H.; Wang, M.Z.; Osorio, J.S.; Wang, H.R. Thiamine ameliorates inflammation of the ruminal epithelium of Saanen goats suffering from subacute ruminal acidosis. J. Dairy Sci. 2020, 103, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Aschenbach, J.R.; Neubauer, V.; Kröger, I.; Khiaosa-Ard, R.; Baumgartner, W.; Zebeli, Q. Signals for identifying cows at risk of subacute ruminal acidosis in dairy veterinary practice. J. Anim. Physiol. Anim. Nutr. 2018, 102, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, V.; Humer, E.; Kröger, I.; Braid, T.; Wagner, M.; Zebeli, Q. Differences between pH of indwelling sensors and the pH of fluid and solid phase in the rumen of dairy cows fed varying concentrate levels. J. Anim. Physiol. Anim. Nutr. 2017, 102, 343–349. [Google Scholar] [CrossRef]
- Villot, C.; Meunier, B.; Bodin, J.; Martin, C.; Silberberg, M. Relative reticulo-rumen pH indicators for subacute ruminal acidosis detection in dairy cows. Animal 2017, 12, 481–490. [Google Scholar] [CrossRef]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J.P. Subacute ruminal acidosis (SARA): A review. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.B.; Oetzel, G.R.; Nordlund, K.V. Clinical forum–subacute ruminal acidosis–a problem in UK dairy herds? UK Vet. 2005, 10, 40–46. [Google Scholar]
- Lean, I.J.; Annison, F.; Bramley, E.; Browning, G.; Cusack, P.; Farquharson, B.; Little, S.; Nandapi, D. Ruminal Acidosis- Aetiopahtogenesis, Prevention and Treatment: A Review for Veterinarians and Nutritional Professionals; Reference Advisory Group on Fermentative Acidosis of Ruminants (RAGFAR): Melbourne, Australia, 2007; pp. 1–60. [Google Scholar]
- Giger-Reverdin, S.; Rigalma, K.; Desnoyers, M.; Sauvant, D.; Duvaux-Ponter, C. Effect of concentrate level on feeding behavior and rumen and blood parameters in dairy goats: Relationships between behavioral and physiological parameters and effect of between-animal variability. J. Dairy Sci. 2014, 97, 4367–4378. [Google Scholar] [CrossRef] [PubMed]
- Christodoulopoulos, G. Maedi–Visna: Clinical review and short reference on the disease status in Mediterranean countries. Small Rumin. Res. 2006, 62, 47–53. [Google Scholar] [CrossRef]
- McKenna, S.L.; Keefe, G.P.; Tiwari, A.; VanLeeuwen, J.; Barkema, H.W. Johne’s disease in Canada part II: Disease impacts, risk factors, and control programs for dairy producers. Can. Vet. J. 2006, 47, 1089–1099. [Google Scholar]
- Alhidary, I.; Abdelrahman, M.M.; Alyemni, A.H.; Khan, R.U.; Al-Mubarak, A.H.; Albaadani, H.H. Characteristics of rumen in Naemi lamb: Morphological changes in response to altered feeding regimen. Acta Histochem. 2016, 118, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Alhidary, I.; Alyemni, A.H.; Khan, R.U.; Bello, A.R.; Al-Saiady, M.Y.; Amran, R.A. Effect of alfalfa hay on rumen fermentation patterns and serum biochemical profile of growing Naemi lambs with ad libitum access to total mixed rations. Pak. J. Zool. 2005, 49, 15–19. [Google Scholar] [CrossRef]
- Hamada, T.; Maeda, S.; Kameoka, K. Effects of minerals on formation of colour in the rumen epithelium of kids. J. Dairy Sci. 1969, 53, 588–591. [Google Scholar] [CrossRef]
- Steele, M.A.; Greenwood, S.L.; Croom, J.; McBride, B.W. An increase in dietary non-structural carbohydrates alters the structure and metabolism of the rumen epithelium in lambs. Can. J. Anim. Sci. 2012, 92, 123–130. [Google Scholar] [CrossRef]
- Bo Trabi, E.; Seddik, H.E.; Xie, F.; Wang, X.; Liu, J.; Mao, S. Effect of pelleted high-grain total mixed ration on rumen morphology, epithelium-associated microbiota and gene expression of proinflammatory cytokines and tight junction proteins in Hu sheep. Anim. Feed Sci. Technol. 2020, 263, 114453. [Google Scholar] [CrossRef]
- Wang, L.; Qi, W.; Mao, S.; Zhu, W.; Liu, J. Effects of whole corn high-grain diet feeding on ruminal bacterial community and epithelial gene expression related to VFA absorption and metabolism in fattening lambs. J. Anim. Sci. 2022, 100, skac056. [Google Scholar] [CrossRef] [PubMed]
- Garcia Diaz, T.; Ferriani Branco, A.; Jacovaci, F.A.; Cabreira Jobim, C.; Bolson, D.C.; Pratti Daniel, J.L. Inclusion of live yeast and mannanoligosaccharides in high grain-based diets for sheep: Ruminal parameters, inflammatory response and rumen morphology. PLoS ONE 2018, 13, e0193313. [Google Scholar]
| Chemical Composition | Category 1 i (n = 17) | Category 2 ii (n = 19) | Category 3 iii (n = 6) | p-Value |
|---|---|---|---|---|
| ME (Mcal/kg DM) | 2.65 ± 0.07 | 2.66 ± 0.07 | 2.66 ± 0.08 | 0.96 |
| CP (% of DM) | 16.06 ± 0.97 | 16.05 ± 0.97 | 16.50 ± 1.38 | 0.72 |
| NDF (% of DM) | 14.47 ± 0.80 | 14.42 ± 0.77 | 14.33 ± 1.21 | 0.95 |
| ADF (% of DM) | 5.94 ± 0.83 | 6.11 ± 0.81 | 6.17 ± 0.75 | 0.77 |
| Feeding Amount (g/day) | 1,682 ± 107 | 1,705 ± 118 | 1,758 ± 150 | 0.43 |
| Farm Sampling Period | Category 1 i (n = 17) | Category 2 ii (n = 19) | Category 3 iii (n = 6) | p-Value |
|---|---|---|---|---|
| Rumen pH | 5.89 ± 0.01 | 5.81 ± 0.02 | 5.74 ± 0.01 | <0.001 |
| Milk yield (liters/ewe/milking period) | 412.0 ± 1.1 | 414.5 ± 2.1 | 415.1 ± 0.6 | 0.143 |
| Milk fat content (%) | 5.92 ± 0.14 | 5.69 ± 0.11 | 5.65 ± 0.06 | <0.001 |
| Milk protein content (%) | 5.72 ± 0.10 | 5.72 ± 0.12 | 5.83 ± 0.05 | 0.064 |
| Milk lactose content (%) | 4.71 ± 0.09 | 4.81 ± 0.14 | 4.95 ± 0.16 | 0.001 |
| Milk total solids’ content (%) | 10.87 ± 0.23 | 11.06 ± 0.31 | 11.73 ± 0.40 | <0.001 |
| Milk fat/milk protein ratio | 1.04 ± 0.02 | 0.99 ± 0.02 | 0.96 ± 0.01 | <0.001 |
| Color Components | Category 1 i (n = 111) | Category 2 ii (n = 124) | Category 3 iii (n = 45) | p-Value |
|---|---|---|---|---|
| L | 39.93 ± 5.24 | 30.59 ± 4.92 | 21.98 ± 6.92 | <0.001 |
| a | 0.66 ± 4.03 | 1.01 ± 4.34 | 2.28 ± 4.04 | 0.091 |
| b | 9.54 ± 5.80 | 9.53 ± 4.67 | 4.49 ± 6.92 | <0.001 |
| Red | 100.94 ± 14.33 | 78.38 ± 9.98 | 58.41 ± 12.34 | <0.001 |
| Green | 93.38 ± 12.69 | 71.16 ± 13.14 | 51.60 ± 16.13 | <0.001 |
| Blue | 78.92 ± 15.15 | 57.53 ± 14.52 | 46.33 ± 17.81 | <0.001 |
| RGB-SUM | 273.23 ± 36.99 | 207.08 ± 32.36 | 156.34 ± 43.06 | <0.001 |
| HSV | 0.40 ± 0.06 | 0.33 ± 0.06 | 0.24 ± 0.05 | <0.001 |
| HSL | 35.47 ± 4.85 | 26.82 ± 4.08 | 20.44 ± 5.57 | <0.001 |
| Ruminal Epithelium | Category 1 i (n = 15) | Category 2 ii (n = 15) | Category 3 iii (n = 15) | p-Value |
|---|---|---|---|---|
| Stratum corneum (μm) | 33.67 ± 12.83 | 43.07 ± 24.97 | 41.67 ± 16.25 | 0.342 |
| Connective tissue (μm) | 103.27 ± 39.35 | 135.00 ± 61.32 | 148.13 ± 63.83 | 0.090 |
| Non-keratinized epithelium (μm) | 80.13 ± 14.46 | 116.47 ± 28.64 | 166.67 ± 21.41 | <0.001 |
| Total epithelium (μm) | 217.07 ± 51.69 | 294.53 ± 61.26 | 356.47 ± 74.08 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voulgarakis, N.; Gougoulis, D.A.; Psalla, D.; Papakonstantinou, G.I.; Katsoulis, K.; Angelidou-Tsifida, M.; Athanasiou, L.V.; Papatsiros, V.G.; Christodoulopoulos, G. Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact and Colorimetry Management. Animals 2024, 14, 2061. https://doi.org/10.3390/ani14142061
Voulgarakis N, Gougoulis DA, Psalla D, Papakonstantinou GI, Katsoulis K, Angelidou-Tsifida M, Athanasiou LV, Papatsiros VG, Christodoulopoulos G. Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact and Colorimetry Management. Animals. 2024; 14(14):2061. https://doi.org/10.3390/ani14142061
Chicago/Turabian StyleVoulgarakis, Nikolaos, Dimitrios A. Gougoulis, Dimitra Psalla, Georgios I. Papakonstantinou, Konstantinos Katsoulis, Mariana Angelidou-Tsifida, Labrini V. Athanasiou, Vasileios G. Papatsiros, and Georgios Christodoulopoulos. 2024. "Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact and Colorimetry Management" Animals 14, no. 14: 2061. https://doi.org/10.3390/ani14142061
APA StyleVoulgarakis, N., Gougoulis, D. A., Psalla, D., Papakonstantinou, G. I., Katsoulis, K., Angelidou-Tsifida, M., Athanasiou, L. V., Papatsiros, V. G., & Christodoulopoulos, G. (2024). Subacute Rumen Acidosis in Greek Dairy Sheep: Prevalence, Impact and Colorimetry Management. Animals, 14(14), 2061. https://doi.org/10.3390/ani14142061










