1. Introduction
Animal welfare is an essential part of performing ethical and high-quality scientific studies. Fraser et al. outlines three concepts of animal welfare: functioning, defined as the preservation of animals’ good health and normal development; natural living or replicating natural environments that promote natural behaviors; and positive affect, or the mental wellbeing of animals, characterized by the lack of fear, distress, and pain and the ability to experience normal pleasures [
1]. Light is a well-documented mediator of many aspects of rodent wellbeing, through its effects on circadian rhythm, hormone regulation, sleep, cognition, reproduction, growth, maturation, social interactions, cancer incidence, and tumor growth [
2,
3,
4,
5]. In particular, the intensity of light has been shown to induce stress and anxiety at levels as low as 60 lux and retinal deterioration of albino animals at levels as low as 80 lux [
6,
7,
8]. Light is also thought to play a role in the mental health of rodents through its impacts on the hypothalamic–pituitary–adrenal (HPA) axis and the expression of corticosterone, a hormone that has been associated with stress and depression [
9]. Lighting conditions inconsistent with natural preferences seemed to increase overall levels of corticosterone in rodents and increased depressive-like behavior [
10], and rodents exposed to 30-h blue light pulses showed significantly higher levels of blood corticosterone compared to violet, green, and dark conditions [
11]. Different lighting conditions have also been shown to impact circadian rhythms and aspects of homeostasis in rodents. Rodents housed in red cages showed an earlier onset of daily activity compared to rodents housed in regular caging [
12]. Low-contrast light/dark cycles and constant low-level lighting environments both affect the daily rhythm of rodent locomotor activity [
13]. Furthermore, the use of different-colored caging (red, yellow, and blue) had different effects on rodent physiology, with red caging in particular decreasing the circulating insulin, glucocorticoid, and glucose values [
14].
Given these impacts of light on rodent physiology, it is unknown what lighting conditions may be preferred by laboratory rats. Understanding what those preferences are is more likely to improve welfare and reduce stress. However, there is very little in the literature defining what those preferences may be. One study found that rats were less willing to explore mazes lit with white light compared to red light [
15], but they preferred white over red painted cages [
16]. Despite this preference for white caging, it is commonly accepted that rodents prefer red-tinted environments. In recent years, caging manufacturers have begun selling red-tinted cages and shelters based on the notion that mice cannot see the color red in the visual spectrum due to their lack of red-sensitive photoreceptors [
17,
18]. It is worth noting that, recently, mice have been found to detect red light at high intensities [
2]. While color is an important factor, only a single study has evaluated what intensities provoke avoidance in rats, and those thresholds depend on coat color and eye pigmentation. Pigmented animals found >65 lux to be aversive, while albino animals were more sensitive and avoided intensities above 25 lux [
19]. Piecing together all this data to define preferences for the common laboratory rat is difficult since none of these studies controlled for both the spectral make-up of light and its intensity in the same study. For instance, when light moves through red-tinted cages, the color make-up and intensity are both altered. Thus, it is difficult to know which aspects of light (intensity vs. color) rats prefer or find aversive.
The lighting environment within laboratory rodent cages can be affected by many factors, including the clarity of the cage wall or the presence of tinted thermoplastic shelters. These factors induce variation in both the wavelength and intensity of light, which, as previously stated, could both impact rodent preference. Additionally, it is not clear how much of the observed preference is innate or due to familiarity to a particular lighting environment, a phenomenon occasionally seen in animal preference research [
20]. To the best of our knowledge, no previous studies have attempted to parse the influence of light color vs. intensity on preference, nor investigated the contribution of familiarity or novelty on light preference. Therefore, this study has two primary aims. The first aim of this study is to investigate whether rodent lighting preferences are influenced by either the color or intensity of the lighting. Our initial hypothesis was that the light intensity drives rat preferences over the color make-up of the light. The second aim of our study is to determine whether home cage lighting environments influence lighting preference over time. Specifically, we hypothesized that rodents will initially prefer lighting conditions similar to those of the cage in which they were raised, but this may change over time and with experience.
2. Materials and Methods
The animal work for this study was conducted at Purdue University, in West Lafayette, Indiana, USA, in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), under the approval of the Purdue Institutional Animal Care and Use Committee (protocol 1504001229).
2.1. Animals and General Housing
Prior to the start of this study, the rats were tickled to prepare them for frequent handling. During this time, data were collected for another study to understand the rats were affected under these different lighting conditions [
21]. The specific methods for housing these animals can be found in that manuscript, but we have summarized the methods below.
Breeding pairs for this study were purchased from Charles River (Raleigh, NC, USA) at 8 weeks old. To generalize across different types of rats, both pigmented (Crl: LE, Long–Evans; LE) and albino (Crl: CD; CD) rats. Albino animals lack melanin pigment in the iris and are, therefore, more susceptible to lesions [
3] and more likely to avoid light intensities above 25 lux than pigmented rats (albino: HSD/CPB/WU; pigmented: HSD/CPB/S3) [
19].
Rats are dichromats, with two types of cones that provide color vision. One peaks at approximately 510 nm (green color; [
22]) and the other at about 358, which provides vision in the UV spectrum [
23]. Only small differences in these peak sensitivities have been identified [
24,
25]. In one study, with albino rats (type of rat not reported) showed a preference for green and blue colors [
26], while another showed that pigmented rats (Long–Evans) could successfully discriminate (80% correct) between 370 nm and a test light when wavelengths were above 420 nm [
25]. When it comes to the ability to see the color red, rat cones and non-image-forming intrinsically photosensitive retinal ganglion cells are not responsive to light above 620 nm [
27,
28]. However, activation may also depend on the intensity of the light, not just the specific wavelength. It is now believed that rats are not red-light blind [
29,
30,
31], and rat retinas respond to far-red light with great sensitivity [
32]. A study conducted by Niklaus et al. found that pigmented Brown Norway rats exposed to photopic bright red light (656 nm at approximately 92 lux) resulted in a larger response than the albino Wistar rats [
32].
The rats were housed in either a clear or red polycarbonate cage with a stainless steel wire bar lid (22″ L × 12-1/2″ W × 8″ H; Lab Products, Inc., Seaford, DE, USA, Rat/Hamster Cage 18730 or 18730M with lid 20311). The red cages were custom-made to match the tint of commonly used red intra-cage shelters (e.g., Bio Serv, Rat RetreatTM, Flemington, NJ, USA). Aspen bedding (300 g, Envigo Teklad, Madison, WI, USA, 7093), 20 g of nesting material (Crink-l’nestTM material Anderson Inc., Maumee, OH, USA), and a kiln-dried hardwood block (Bio-Serv, Flemington, NJ, USA, K3512) were provided as bedding and nesting material. An ad libitum rodent diet (Envigo Teklad, 2018, Madison, WI, USA) and reverse osmosis water were provided to all cages. To reduce clouding and preserve consistent intra-cage light conditions, the cages were washed every two weeks. On alternative weeks, when the cage bottoms were not replaced, clean bedding and nesting material were provided.
Detailed Housing Procedures
Different lighting conditions were created via the use of 16 wooden light boxes (made of medium-density fiberboard), in which the rats’ home cages could be placed. In total, four wire racks were assembled to hold the light boxes, creating unique lighting environments of 200 or 25 lux. A light box (which contained 2 rat cages) was randomly assigned to one of the following treatment groups (red 200 lux, red 25 lux, clear 200 lux, or clear 25 lux;
Figure 1). The red cages were investigated because manufacturers have started to produce red cages that are meant to mimic the tint of commonly used red shelters, which rats are thought to prefer. The light intensity levels were chosen based on the average light measurement within the front of a cage housed on the middle of a rack (in-house evaluation; 200 lux) and the low lighting conditions that a red shelter provides. This is also similar to the threshold for albino avoidance (25 lux). We were prevented from placing both red and clear cages in the same box, as red-tinted plastic significantly reduces light intensity. During breeding, each light box held a cage of CD and a cage of LE rats. At weaning, a CD and LE rat pup were paired into a single cage and each lighting box contained a cage of each sex.
Figure 1 depicts an example of this setup.
Again, refer to reference [
21] for specifics. The light intensity levels were maintained with light fixtures (premium direct wire, 36-inch fluorescent; General Electric, Boston, MA, USA, 10142) and multi-layer filters (photography-grade diffusion paper; LEE Filters, Burbank, CA, USA, 129 Heavy Frost) attached to the center of the box at the cage level. The light intensity was monitored when the animals were first housed, and twice a week afterward, using a dual-range light meter (Traceable
® Products, Webster, TX, USA, 3251CC; measuring the photopic lux). Light measurements were taken at the center of the cage. The light intensity ranges were 193.47 ± 15.16 and 25.90 lux ± 3.99 lux. Lights in the housing boxes were on a shifted 12:12 h light/dark cycle (lights on at 12:00 a.m.). The main housing room lights were kept off. In order to perform basic tasks within the main room, a light, with similar intensity to what was in the rat’s housing box, was placed on a table.
The temperature within the box was maintained at 24 ± 2 °C, and two fans (Cooler Master, Zhonghe Dist., New Taipei City, Taiwan, Silent Fan 120 SI2) were installed at the back of the light box to provide air circulation. HOBO® data loggers were used to monitor the temperature levels throughout the experiment.
2.2. Breeding Procedures
Ideally, we would have synchronized the breeding and births of the rat pups for each intensity and cage color treatment and stock. Unfortunately, we were unable to synchronize the breeding across all treatments because the clear 200 lux breeding pairs of both stock did not produce litters initially. In order to produce the pups needed, the lighting intensity was lowered to 60 lux and gradually stepped up to 200 lux during the week before birth. Therefore, no single month of testing contained all treatment combinations. As a result, months 1–2 contained the 25 lux treatments of both colors, month 4 tested the clear 200 lux, and month 5 tested the red 200 lux.
To reduce the overall number of offspring for the project, sires were removed from the breeding cages and singly housed in a different box of the same lighting conditions once the female breeders were visibly pregnant. The dams were allowed to rear their pups until weaning at 21 days ± 2 days. All rats continued to receive the same enrichment (nesting and wood block) regardless of housing arrangement. Randomly chosen offspring (using the random number generator at Random.org) were weaned into same sex pairs (1 CD and 1 LE) of the caging and lighting treatments corresponding to their rearing conditions.
2.3. Experimental Treatments
A 2 × 2 factorial design was used to compare the preferences of the rats for cage color (red or clear) and light intensity (25 or 200 lux). We calculated an a priori sample size using Mead’s resource equation (Mead, 1990). Six cages were used in each of the 4 possible combinations of cage color and light intensity, providing an overall total N of 24 cages. While the treatment was applied to the cage, behaviors could be further separated into differences between the two types of rats. Thus, the experimental unit was the rat (48 rats) with repeated measures per cage. The rats were bred in-house using the procedures outlined above in
Section 2.2.
2.4. Preference Testing Procedures
To assess the rats’ preferences for the different lighting conditions, we used a preference apparatus consisting of one light box of each lighting environment (red 200 lux, red 25 lux, clear 200 lux and clear 25 lux) connected by clear tubing. The rats were allowed to acclimate to each environment and the connecting tubing for 12 h (6 h light, 6 h dark) before having unrestricted access to all four environments for 3 consecutive days. Preference testing was conducted 3 times during each of the critical developmental periods, including the juvenile (4–6 weeks), puberty (7–9 weeks), and adulthood (10–12 weeks) stages. Videos of the test sessions and rat location were scored using instantaneous scan sampling every 15 min. The behaviors were categorized using a standard ethogram: Active behavior included all locomotive behavior, including rearing, grooming, eating, nesting, or manipulating the wood chew block; essentially, if the animal was moving around the preference apparatus, they were considered active. Inactive behavior meant the animal was motionless, and either lying curled up on its side, or curled up with its face tucked into its body and out of sight of the camera, occasionally interrupted by brief single twitches of the body. The animal may also have been sitting still or curled up with the face lifted. Unknown behavior meant the animal was out of sight or the behavior could not be determined. Video observers were blind to the cage’s original housing color and intensity conditions but not blind to the type of rat, since that could be visually determined. They were, however, blind to the hypothesis about which environment albino and pigmented rats might choose. No data were excluded from the analysis. The testing sessions were run over three days, and the data from the light periods and dark periods were summarized separately (behavior from the 3 light periods = 100% of the light budget, and behavior from the 3 dark periods = 100% of the dark budget). Once the data were summarized, any occurrences of rats being observed in the tube or doing unknown behaviors were excluded from the final dataset to assure independence of the data. Thus, the light and dark time budgets did not equal 100%. To further validate the location preference, 20 g of nesting material was provided within each testing chamber, in which the rats were able to freely move. At the end of each preference test, the amount of material in each location was weighed to determine the nest movement. The full breeding and testing procedure is summarized in
Figure 2.
2.5. Spectral Data
At the end of the study, the specific and more nuanced lighting environment was measured for each condition using a spectrometer (Ocean Optics Jaz, Winter Park, FL, USA) [
21]. The data are reported as wavelengths (nm). The measurements were taken at the center of each cage and averaged across 10 measurements (
Figure S1).
2.6. Statistical Analysis
All data were analyzed in JMP statistical software (JMP
® 16.1; SAS Institute Inc., Cary, NC, USA) using general linear mixed models (see
Table S1 for the full statistical models and output). Assumptions of the general linear mixed models were confirmed via visualization, and logit transformations were used. For the nesting material analysis, a square root transformation was used. For all tests, the level of statistical significance was set at
p < 0.05. The relationships between different factors were evaluated using three-way interactions, which were interpreted using test slices and subsequent Bonferroni corrected custom contrasts or Tukey corrections for multiple comparisons. The results from the statistical models are presented as the least square mean ± standard error of the mean (LSM ± SEM). Any data that were transformed for analysis are presented in text with back-transformed LSM values and SEM values estimated via the Delta Method.
To improve the readability of our results section, we have omitted the test statistic details from the written section but have provided the full model information in
Table S1. For the in-text stat reporting, the exact
p-values are reported unless they were smaller than 0.001. However, the results of the post hoc Tukey tests are reported as > or <0.05.
Table S2 provides the analysis script in SAS and the data for transparency.
4. Discussion
Lighting conditions can impact animal physiology and welfare, but little is known about what rats actually prefer. The primary aim of this study was to investigate whether rodent lighting preferences are influenced by either the color or intensity of the lighting. Specifically, we expected light intensity to drive rat preferences over the color make-up of light. In general, most animals preferred lower light (25 lux) locations in our preference apparatus, which was consistent across both the albino and pigmented rats. While the rats housed in clear/200 lux appeared to choose differently than the rest, there was evidence of their preference shifting to 25 lux with repeated exposure and experience. This finding is consistent with citation [
6]’s result suggesting that rodents were more averse to higher light levels.
This study not only provides further evidence for laboratory rats in a home cage preferring low lighting conditions but also what conditions they should be exposed to generally. The guides for the care and use of laboratory animals do not specify any engineering standards for lighting environment, but many reference the statement that “…325 lux (30-ft candles) approximately 1 m (3.3 ft) above the floor appear to be sufficient for animal care and do not cause clinical signs of phototoxic retinopathy in albino rats” [
33]. Instead of a maximum threshold, we might think instead about the difference in light exposure between a rat’s home cage and the room at large or environment we place them in. In a study by Barabas et al. [
34], they measured the light intensity and other environmental factors in multiple facilities across a single institution. In the study, the room light intensity ranged from approximately 16 to 1400 lux. In the same study, the average light intensity inside a cage was quite low. The measurements depended on several factors but, generally, the top of the rack was brighter (13.21 lux) than the bottom of the rack (4.93 lux). If we consider an extreme example of removing a rat cage from as low as 5 lux and opening the cage on a tabletop in the 1400 lux room, this will require a substantial adjustment that could potentially be painful. Thus, considerations of lighting outside of the cage should also be considered in order to reduce negative experiences and improve rodent welfare.
While our results support rats’ preference for lower intensity levels, we did not find as strong of evidence to support a preference for either light color. In the study by Kapogiannatou et al. [
6], the rats preferred “warmer” temperature light (2500 K) compared to “cooler” light (4000 K). In our study, only rats raised in clear, high-intensity lighting conditions preferred the clear test chamber, which was the closest comparison to the cool light in the study by Kapogiannatou et al. It should be noted that no preference or aversion for the red-tinted caging was found in this study. This observation has implications for rodents continuously housed in red-tinted cages. While not preferred, there is a low likelihood that the cages are aversive but may affect their overall color vision. Using red-tinted cages may also call into question whether this in-cage illumination meets the guide, which states “Light in animal holding rooms should provide for both adequate vision and neuroendocrine regulation…” [
33]. It is unclear whether appropriate color vision falls under this statement and unknown how the color filtering may affect eye development or perception. Thus, housing animals only in red-tinted caging is not recommended without further research.
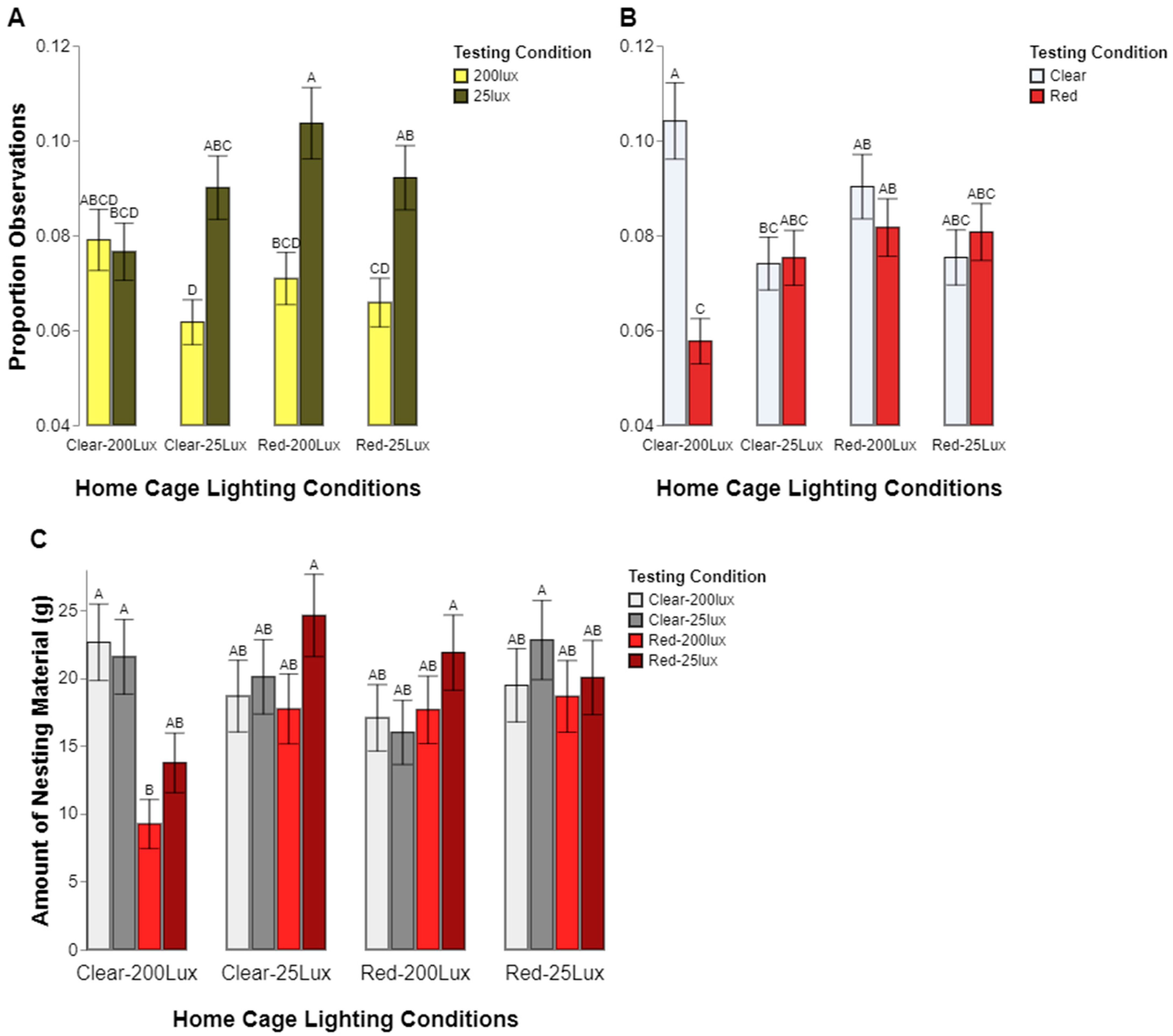
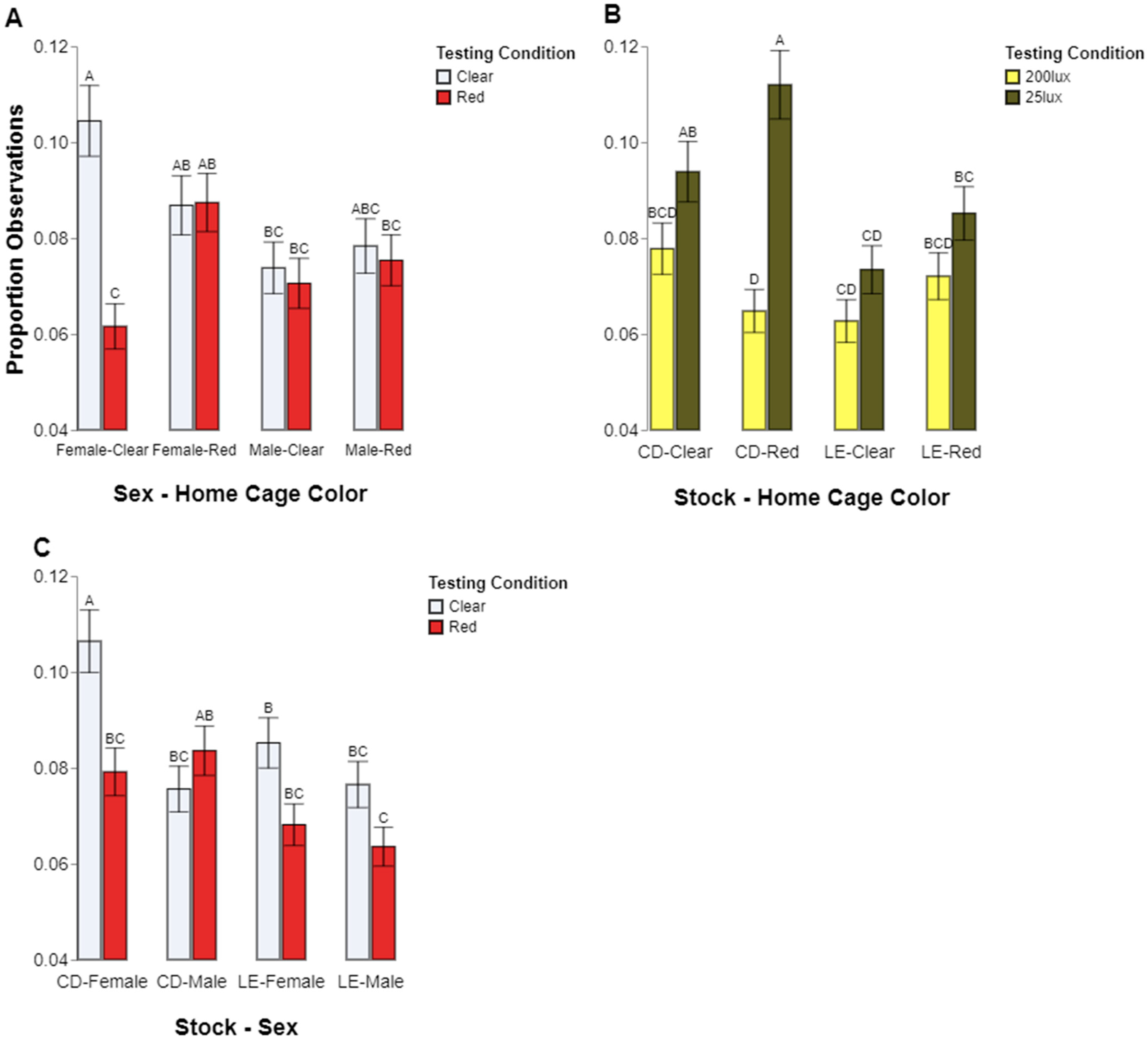
Our results also support the commonly held notion that eye pigmentation affects a rat’s perception of light. Even though we found a general preference for lower light levels across both types of rats tested, we found that the albino, CD, rats spent significantly more time in the low light condition compared to the pigmented, LE, rats. Again, the color preferences were less clear, with the CD rat preferences appearing to be contingent upon sex; the females preferred the clear chambers but the males showed no preference. The LE rats, however, did not show a significant color preference in either sex. It should be noted that the pigmented and albino stocks were genetically distinct, thus introducing the confound of other potential genetic components affecting their preferences aside from eye pigmentation.
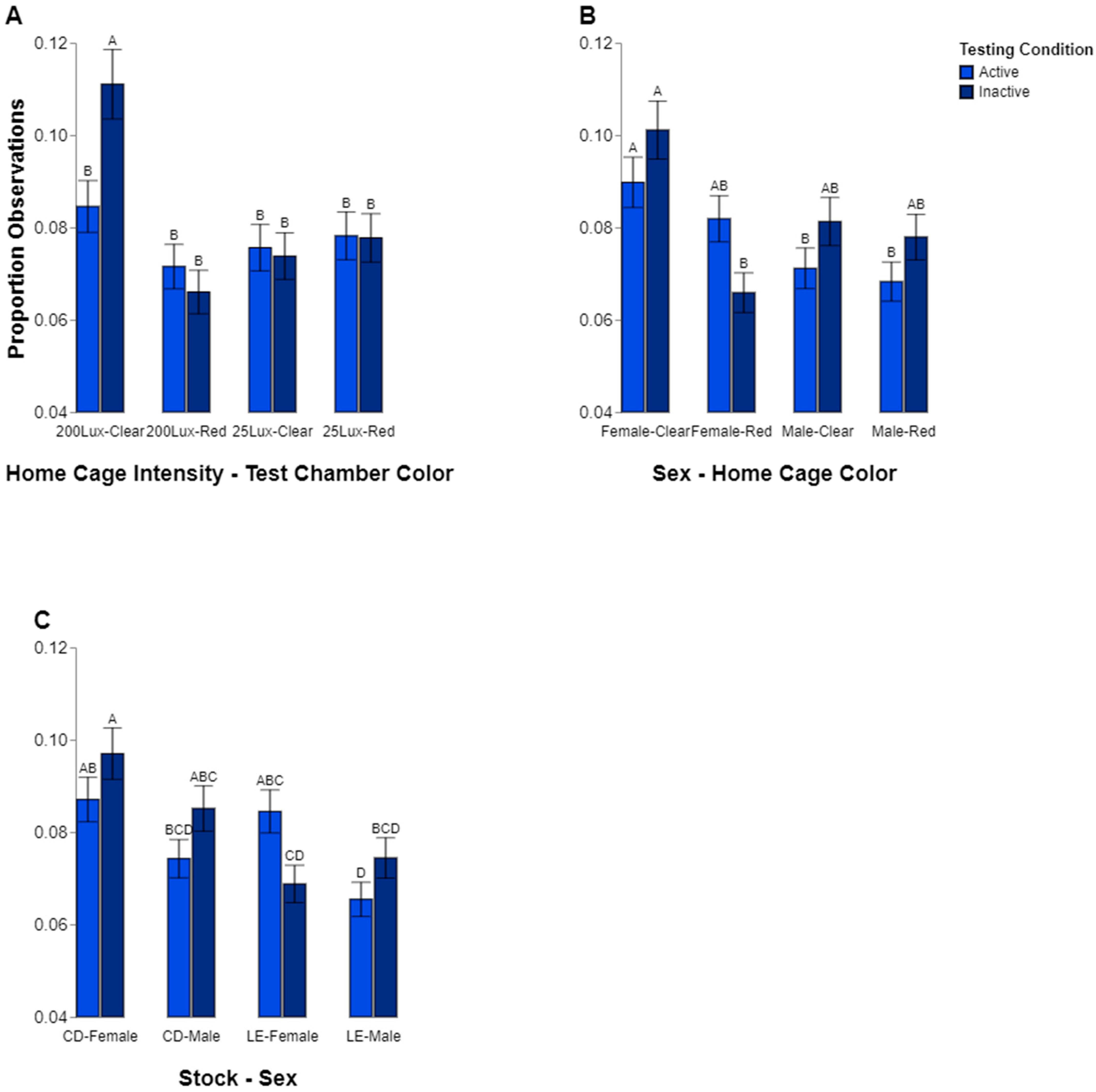
While general findings for preferences were found, it is entirely likely that the rats may have chosen a particular environment based on behavior. For instance, we wanted to see if there was a difference in preference for a location when inactive, such as sleeping, compared to when active. We found some evidence that animals may choose for aspects of cage color depending on their behavior. However, this effect seemed to exist only in the animals raised in the high-intensity home cage lighting condition. The group of animals raised in the 200 lux lighting had a higher percentage of inactive observations in the clear vs. the red chambers. They were also observed to be more inactive in the clear test chamber compared to the rats raised in low lighting (25 lux). Finally, the rats raised in 200 lux were generally more inactive when they were in the clear test chamber. This seems to suggest that the rats raised in high-intensity lighting may prefer to rest in lighting conditions with a more diverse color make-up, which is consistent with our observation that these rats spent more time in the clear testing chamber overall. These observations may suggest a link between the intensity of home cage lighting and rodent preferences for lighting color when the light is bright. It is possible that due to the long exposure in the extreme condition, their ability to explore or adjust to a different environment might be altered. However, the result illustrating a shift in time spent in the 200 vs. the 25 lux environment over the course of the study illustrates that they do change, but it may be at a slower pace than the other animals tested.
Other factors appeared to play a part in which behaviors were observed in which locations. Sex may also have played a role in where the rats spent their time. The males were observed to follow a pattern of spending about the same amount of time being active and inactive regardless of which test chamber they were in, but the females spent significantly more time inactive while in the clear test chamber than in the red. However, this effect is contextualized by the interactions among stock, sex, and behavior, which shows the same pattern independent of the test chamber color. The CD females spent significantly more time inactive than the LE females. Thus, it is not clear how much the difference in female inactivity was due to stock genetics or if the environment and color of chamber also influenced this. Taken together, our analyses of behavior fit with the overall conclusion that the cage color, and subsequently, the lighting make-up, is not a straightforward factor driving rodent lighting preferences.
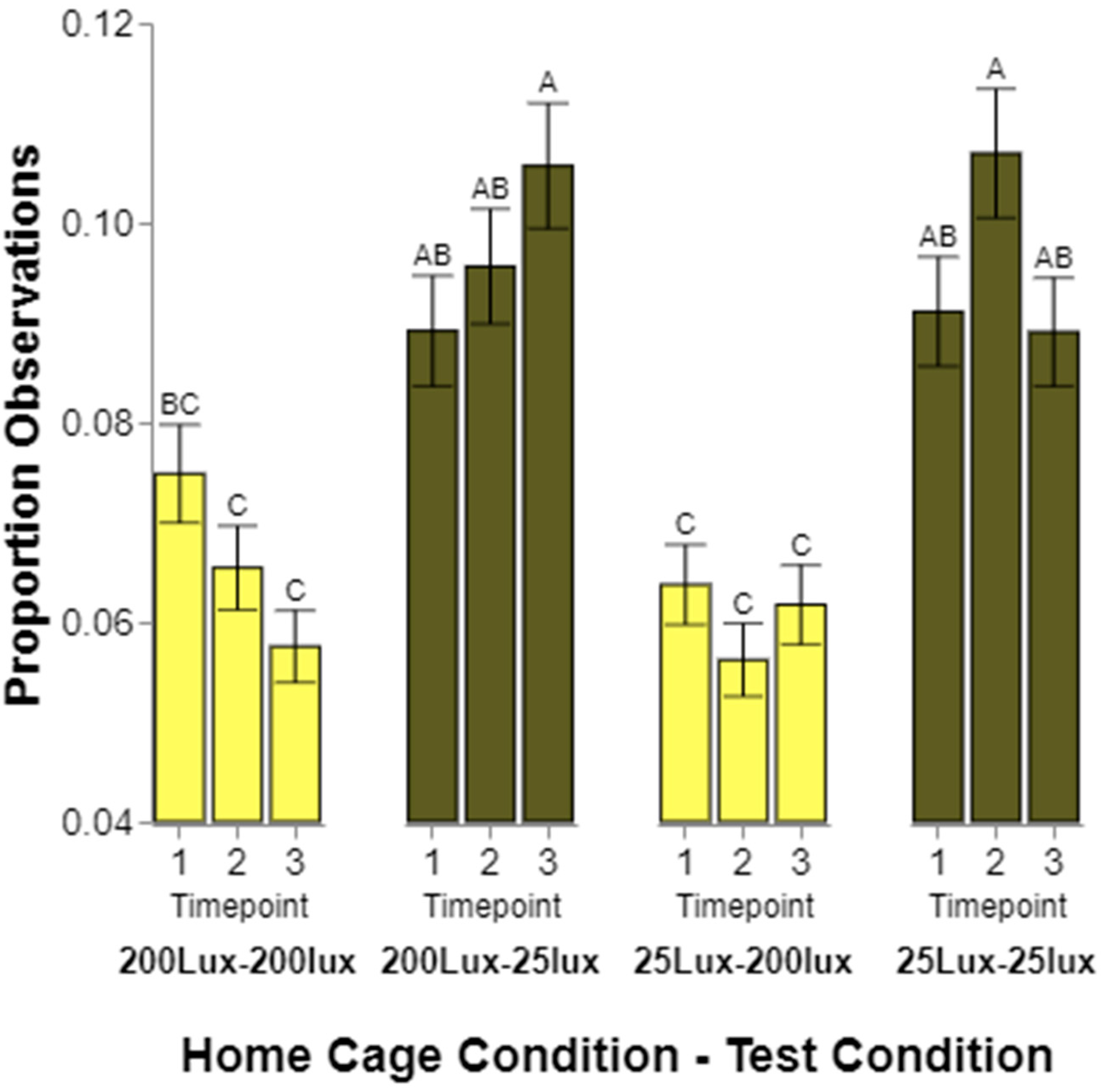
Finally, we expected the rodents to initially prefer lighting conditions similar to the cage in which they were raised, and that this would change over time and with experience. Generally, the frequency of observations for rats from one or the other home cage lighting conditions did not significantly differ from one time point to another. However, again, the rats housed in 200 lux did not fit the pattern of the other housing conditions. When first tested, the rats raised in 200 lux spent equal amounts of time in 200 and 25 lux. When tested for the second and third times, the rats spent significantly more time in 25 lux than 200. While minor, this does indicate a change from environments being perceived as equal to 25 lux being significantly preferred. The preference of the rats raised in 25 lux appeared to be relatively stable over time, showing a continued preference for 25 lux over all three tests. We considered running a linear contrast across the three time points to determine if a statistical linear slope exists, but since only three tests were conducted, we did not feel as if it was appropriate to try this analysis with so few time points.
It is important to contextualize all of these results within the known limitations of preference testing. Our experimental procedures were designed to account for many of the problems of preference testing, but there remain some considerations that were not inherently addressed by our design. While we attempted to address environmental confounds by making the testing apparatus a close approximation of housing conditions, and by examining changes in preference over time to account for familiarity and novelty, we were only able to provide four distinct lighting conditions by combining two choices for color and two choices for intensity. As such, we can only make statements on the relative preference between the choices they were presented. It is possible that a lighting environment enriched with color that activates the UV cone might be more preferred than the choices offered in this experiment. We also cannot comment on the strength or importance of these preferences as we did not illicit any operant responses from the research animals; they were simply given free access to each possible combination of lighting conditions without any functional time limitation or significant obstacles. Thus, the preferences observed in this study should be considered the rats’ behaviors elicited in response to the specific conditions described and without significant cost for any given condition. Our study should be taken as an initial assessment of the importance of light intensity and color to rats in terms of their basic preference.