In Vitro Antibacterial Activities of Fosfomycin against Escherichia coli Isolates from Canine Urinary Tract Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Identification
2.2. Detection of Escherichia coli Strains Producing Extended-Spectrum Beta-Lactamase (ESBL)
2.3. Determination MIC of Other Antibacterial Drugs
2.4. Determination MIC of Fosfomycin
2.5. Determination MPC of Fosfomycin
2.6. Estimation PK/PD of Fosfomycin
2.7. Statistical Analysis
3. Results
3.1. Escherichia coli Isolates
3.2. Escherichia coli Strains Producing Extended-Spectrum Beta-Lactamase (ESBL)
3.3. MIC of Other Antibacterial Drugs
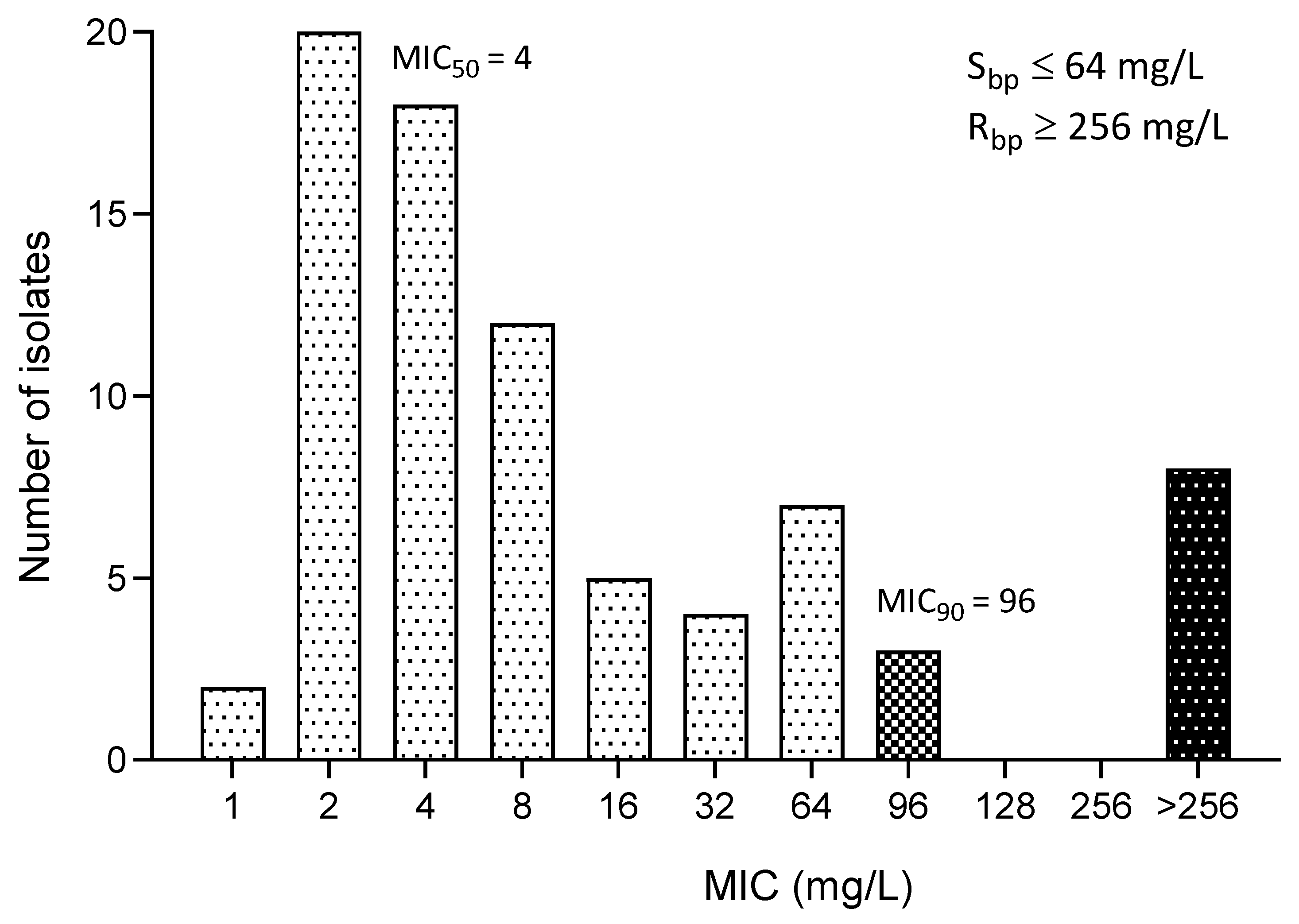
3.4. MIC of Fosfomycin
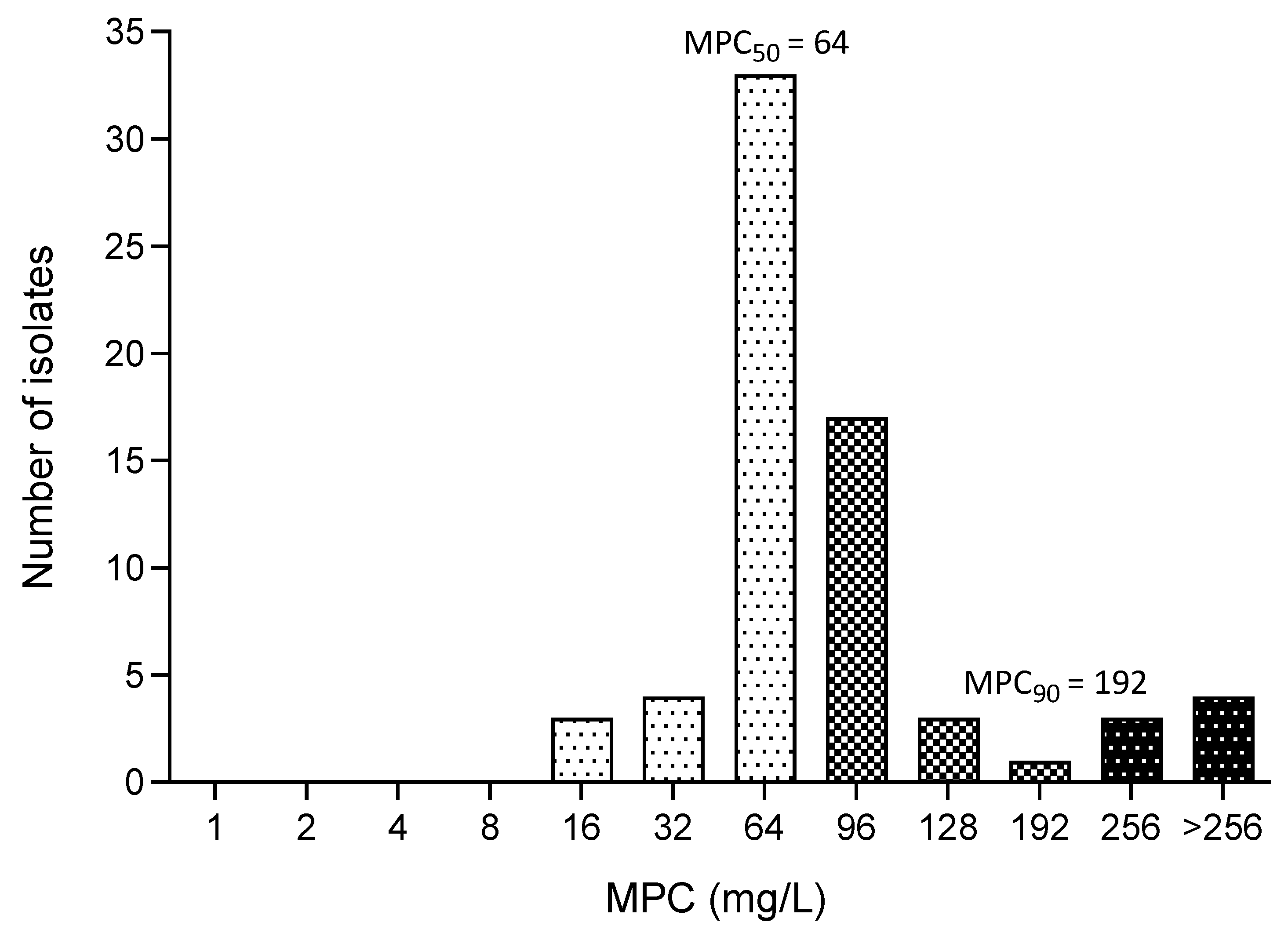
3.5. MPC of Fosfomycin
3.6. Estimation PK/PD of Fosfomycin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theuretzbacher, U.; Van Bambeke, F.; Cantón, R.; Giske, C.G.; Mouton, J.W.; Nation, R.L.; Paul, M.; Turnidge, J.D.; Kahlmeter, G. Reviving old antibiotics. J. Antimicrob. Chemother. 2015, 70, 2177–2181. [Google Scholar] [CrossRef]
- Cassir, N.; Rolain, J.-M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T. New ways of using old antibiotics in pediatrics: Focus on fosfomycin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.R.; Rubin, J.E.; Chirino-Trejo, M.; Dowling, P.M. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can. Vet. J. 2008, 49, 985–990. [Google Scholar] [PubMed]
- Wong, C.; Epstein, S.E.; Westropp, J.L. Antimicrobial Susceptibility Patterns in Urinary Tract Infections in Dogs (2010–2013). J. Vet. Intern. Med. 2015, 29, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.V.; Norris, C.R.; Franti, C.E.; Eisele, P.H.; Johnson, D.L.; Ruby, A.L.; Jang, S.S. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995). J. Vet. Intern. Med. 2001, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Rampacci, E.; Bottinelli, M.; Stefanetti, V.; Hyatt, D.R.; Sgariglia, E.; Coletti, M.; Passamonti, F. Antimicrobial susceptibility survey on bacterial agents of canine and feline urinary tract infections: Weight of the empirical treatment. J. Glob. Antimicrob. Resist. 2018, 13, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Gualco, L.; Debbia, E.A.; Schito, G.C.; Schito, A.M. In vitro activity of fosfomycin against gram-negative urinary pathogens and the biological cost of fosfomycin resistance. Int. J. Antimicrob. Agents 2003, 22 (Suppl. S2), 53–59. [Google Scholar] [CrossRef]
- Bergan, T.; Thorsteinsson, S.B.; Albini, E. Pharmacokinetic profile of fosfomycin trometamol. Chemotherapy 1993, 39, 297–301. [Google Scholar] [CrossRef]
- Boothe, D.M. Small Animal Clinical Pharmacology & Therapeutics, 2nd ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2012; pp. 339–340. [Google Scholar]
- USFDA. MONUROL (Fosfomycin Tromethamine) Sachet. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf (accessed on 19 May 2024).
- WHO. Critically Important Antimicrobials for Human Medicine. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 19 May 2024).
- EMA. Categorisation of Antibiotics in the European Union. Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf (accessed on 19 May 2024).
- Plumb, D.C. Plumb’s Veterinary Drug Handbook, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 722–724. [Google Scholar]
- Papich, M.G. Saunders Handbook of Veterinary Drugs: Small and Large Animal, 4th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2016; pp. 344–345. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; CLSI: Berwyn, PA, USA, 2021; pp. 45–79. [Google Scholar]
- Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 2003, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, M.; Kunishima, Y.; Takahashi, S.; Takeyama, K.; Tsukamoto, T. Time courses of bacterial density in urine during antibacterial chemotherapy and influential factors in patients having positive bacteriuria with a complicated urinary tract. J. Infect. Chemother. 2007, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.C.; Haake, D.A.; Churchill, B.M.; Justice, S.S.; Kim, J.H. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology 2015, 86, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M. New concepts in antimicrobial susceptibility testing: The mutant prevention concentration and mutant selection window approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, X.; Domagala, J.; Drlica, K. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Zhao, X.; Hansen, G.; Drlica, K. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Hansen, G.; Metzler, K.; Hedlin, P. The Role of PK/PD Parameters to Avoid Selection and Increase of Resistance: Mutant Prevention Concentration. J. Chemother. 2004, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 7th ed.; CLSI Supplement VET01S; CLSI: Berwyn, PA, USA, 2024; pp. 24–41. [Google Scholar]
- Falagas, M.E.; Maraki, S.; Karageorgopoulos, D.E.; Kastoris, A.C.; Mavromanolakis, E.; Samonis, G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int. J. Antimicrob. Agents 2010, 35, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Karageorgopoulos, D.E. Pandrug Resistance (PDR), Extensive Drug Resistance (XDR), and Multidrug Resistance (MDR) among Gram-Negative Bacilli: Need for International Harmonization in Terminology. Clin. Infect. Dis. 2008, 46, 1121–1122. [Google Scholar] [CrossRef] [PubMed]
- Marcusson, L.L.; Olofsson, S.K.; Lindgren, P.K.; Cars, O.; Hughes, D. Mutant prevention concentrations of ciprofloxacin for urinary tract infection isolates of Escherichia coli. J. Antimicrob. Chemother. 2005, 55, 938–943. [Google Scholar] [CrossRef]
- Jariyapamornkoon, N.; Patthanachai, K.; Suanpairintr, N. Plasma and Urine Pharmacokinetics of Oral Fosfomycin Tromethamine in Dogs. Vet. Sci. 2023, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, H.; Wang, Y.; Wang, H.; Hu, J.; Zhang, G. Pharmacodynamic Parameters of Pharmacokinetic/Pharmacodynamic (PK/PD) Integration Models. Front. Vet. Sci. 2022, 9, 860472. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Papich, M.G. Pharmacokinetic-pharmacodynamic (PK-PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet. Microbiol. 2014, 171, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; del Castillo, J.R.; Bousquet-Melou, A. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Leesombun, A.; Boonmasawai, S. Categorization of antimicrobial agents prescribed inthe Veterinary Teaching Hospital in Thailand. J. Appl. Anim. Sci. 2019, 12, 25–28. [Google Scholar]
- Hubka, P.; Boothe, D.M. In vitro susceptibility of canine and feline Escherichia coli to fosfomycin. Vet. Microbiol. 2011, 149, 277–282. [Google Scholar] [CrossRef]
- Zdzieblo, M.; Biernasiuk, A.; Helon, P.; Malm, A. Fosfomycin activity against strains isolated from urine specimens. Curr. Issues Pharm. Med. Sci. 2023, 36, 217–220. [Google Scholar] [CrossRef]
- Sojo-Dorado, J.; López-Hernández, I.; Hernández-Torres, A.; Retamar-Gentil, P.; Merino de Lucas, E.; Escolà-Vergé, L.; Bereciartua, E.; García-Vázquez, E.; Pintado, V.; Boix-Palop, L.; et al. Effectiveness of fosfomycin trometamol as oral step-down therapy for bacteraemic urinary tract infections due to MDR Escherichia coli: A post hoc analysis of the FOREST randomized trial. J. Antimicrob. Chemother. 2023, 78, 1658–1666. [Google Scholar] [CrossRef]
- Abbott, I.J.; Dekker, J.; van Gorp, E.; Wijma, R.A.; Raaphorst, M.N.; Klaassen, C.H.W.; Meletiadis, J.; Mouton, J.W.; Peleg, A.Y. Impact of bacterial species and baseline resistance on fosfomycin efficacy in urinary tract infections. J. Antimicrob. Chemother. 2020, 75, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.J.; Mei, Q.; Ye, Y.; Li, H.R.; Liu, B.; Li, J.B. Validation of the mutant selection window hypothesis with fosfomycin against Escherichia coli and Pseudomonas aeruginosa: An in vitro and in vivo comparative study. J. Antibiot. 2017, 70, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X.; Blondeau, J.M.; Hesje, C. Low correlation between MIC and mutant prevention concentration. Antimicrob. Agents Chemother. 2006, 50, 403–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gianvecchio, C.; Lozano, N.A.; Henderson, C.; Kalhori, P.; Bullivant, A.; Valencia, A.; Su, L.; Bello, G.; Wong, M.; Cook, E.; et al. Variation in Mutant Prevention Concentrations. Front. Microbiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- MacLeod, D.L.; Barker, L.M.; Sutherland, J.L.; Moss, S.C.; Gurgel, J.L.; Kenney, T.F.; Burns, J.L.; Baker, W.R. Antibacterial activities of a fosfomycin/tobramycin combination: A novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 2009, 64, 829–836. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.L.; Velayudhan, J.; Kenney, T.F.; Therrien, J.H.; Sutherland, J.L.; Barker, L.M.; Baker, W.R. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 1529–1538. [Google Scholar] [CrossRef][Green Version]
- Mazzei, T.; Cassetta, M.I.; Fallani, S.; Arrigucci, S.; Novelli, A. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 2006, 28 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef]
- Descourouez, J.L.; Jorgenson, M.R.; Wergin, J.E.; Rose, W.E. Fosfomycin synergy in vitro with amoxicillin, daptomycin, and linezolid against vancomycin-resistant Enterococcus faecium from renal transplant patients with infected urinary stents. Antimicrob. Agents Chemother. 2013, 57, 1518–1520. [Google Scholar] [CrossRef][Green Version]
- Petek, M.; Baebler, S.; Kuzman, D.; Rotter, A.; Podlesek, Z.; Gruden, K.; Ravnikar, M.; Urleb, U. Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: Modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiol. 2010, 10, 159. [Google Scholar] [CrossRef]
- Chavan, R.; Naphade, B.; Waykar, B.; Bhagwat, S. Investigations on In Vivo Pharmacokinetic/Pharmacodynamic Determinants of Fosfomycin in Murine Thigh and Kidney Infection Models. Microb. Drug Resist. 2022, 29, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Zhao, M.; VanScoy, B.; Taylor, D.S.; Ellis-Grosse, E.; Ambrose, P.G.; Andes, D.R. In Vivo Pharmacokinetics and Pharmacodynamics of ZTI-01 (Fosfomycin for Injection) in the Neutropenic Murine Thigh Infection Model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Docobo-Perez, F.; Drusano, G.L.; Johnson, A.; Goodwin, J.; Whalley, S.; Ramos-Martin, V.; Ballestero-Tellez, M.; Rodriguez-Martinez, J.M.; Conejo, M.C.; van Guilder, M.; et al. Pharmacodynamics of fosfomycin: Insights into clinical use for antimicrobial resistance. Antimicrob. Agents Chemother. 2015, 59, 5602–5610. [Google Scholar] [CrossRef] [PubMed]
| Antibacterial Drugs | %S | %I | %R |
|---|---|---|---|
| Ampicillin | 5.26 | NA | 94.74 |
| Amoxicillin | 47.44 | 12.82 | 39.74 |
| Piperacillin | 24.36 | 2.56 | 73.08 |
| Cephalexin | 28.21 | 7.69 | 64.10 |
| Cefpodoxime | 50.00 | NA | 50.00 |
| Cefovecin | 53.85 | 3.85 | 42.31 |
| Ceftiofur | 50.00 | 2.56 | 47.44 |
| Imipenem | 94.87 | 1.28 | 3.85 |
| Amikacin | 88.46 | NA | 11.54 |
| Gentamicin | 56.41 | 2.56 | 41.03 |
| Tobramycin | 55.13 | 30.77 | 14.10 |
| Enrofloxacin | 15.38 | 5.13 | 79.49 |
| Marbofloxacin | 20.51 | 1.28 | 78.21 |
| Tetracycline | 30.77 | 2.56 | 66.67 |
| Nitrofurantoin | 91.03 | 8.97 | 3.85 |
| Chloramphenicol | 50.00 | 20.51 | 29.49 |
| Sulfamethoxazole/trimethoprim | 47.44 | NA | 52.56 |
| MIC Parameters | All E. coli | ESBL-Producing E. coli | MDR E. coli |
|---|---|---|---|
| (n = 79) | (n = 25) | (n = 44) | |
| Range of MIC (mg/L) | 1—≥256 | 2—≥256 | 2—≥256 |
| MIC50 (mg/L) | 4 | 4 | 4 |
| MIC90 (mg/L) | 96 | 96 | 256 |
| Susceptibility (%) | 86.06 | 88.89 | 79.55 |
| MPC Parameters | All E. coli | ESBL-Producing E. coli | MDR E. coli |
|---|---|---|---|
| (n = 68) | (n = 22) | (n = 35) | |
| Range of MPC (mg/L) | 16—≥256 | 48—≥256 | 16—≥256 |
| MPC50 (mg/L) | 64 | 64 | 64 |
| MPC90 (mg/L) | 192 | 192 | 256 |
| MPC50/MIC50 | 16 | 16 | 16 |
| MPC90/MIC90 | 2 | 2 | 1 |
| PK/PD | Parameters | 40 mg/kg PO | 80 mg/kg PO |
|---|---|---|---|
| PK (plasma) [29] | AUC0–24 (mg*h/L) | 145.47 | 343.16 |
| Cmax (mg/L) | 34.46 | 66.40 | |
| T (h) | 24 | 24 | |
| PD | MIC50 (mg/L) | 4 | |
| MIC90 (mg/L) | 96 | ||
| MPC50 (mg/L) | 64 | ||
| MPC90 (mg/L) | 192 | ||
| PK/PD (MIC50) | AUC/MIC50 | 36.37 | 85.79 |
| Cmax/MIC50 | 8.62 | 16.60 | |
| T > MIC50 | <30% | >50% | |
| PK/PD (MIC90) | AUC/MIC90 | 1.52 | 3.57 |
| Cmax/MIC90 | 0.36 | 0.69 | |
| T > MIC90 | 0% | 0% | |
| PK/PD (MPC50) | AUC/MPC50 | 2.27 | 5.36 |
| Cmax/MPC50 | 0.54 | 1.04 | |
| T > MPC50 | 0% | <10% | |
| PK/PD (MPC90) | AUC/MPC90 | 0.76 | 1.79 |
| Cmax/MPC90 | 0.18 | 0.35 | |
| T > MPC90 | 0% | 0% | |
| PK/PD | Parameters | 40 mg/kg PO | 80 mg/kg PO |
|---|---|---|---|
| PK (urine) [29] | AUC0–24 (mg × h/L) | 15,390.22 | 42,779.13 |
| Cmax (mg/L) | 4463.07 | 8784.93 | |
| T (h) | 24 | 24 | |
| PD | MIC50 (mg/L) | 4 | |
| MIC90 (mg/L) | 96 | ||
| MPC50 (mg/L) | 64 | ||
| MPC90 (mg/L) | 192 | ||
| PK/PD (MIC50) | AUC/MIC50 | 3847.56 | 10,694.78 |
| Cmax/MIC50 | 1115.77 | 2196.23 | |
| T > MIC50 | 100% | 100% | |
| PK/PD (MIC90) | AUC/MIC90 | 160.31 | 445.62 |
| Cmax/MIC90 | 46.49 | 91.51 | |
| T > MIC90 | >50% | 100% | |
| PK/PD (MPC50) | AUC/MPC50 | 240.47 | 668.42 |
| Cmax/MPC50 | 69.74 | 137.26 | |
| T > MPC50 | >50% | 100% | |
| PK/PD (MPC90) | AUC/MPC90 | 80.16 | 222.81 |
| Cmax/MPC90 | 23.25 | 45.75 | |
| T > MPC90 | <50% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jariyapamornkoon, N.; Nuanualsuwan, S.; Suanpairintr, N. In Vitro Antibacterial Activities of Fosfomycin against Escherichia coli Isolates from Canine Urinary Tract Infection. Animals 2024, 14, 1916. https://doi.org/10.3390/ani14131916
Jariyapamornkoon N, Nuanualsuwan S, Suanpairintr N. In Vitro Antibacterial Activities of Fosfomycin against Escherichia coli Isolates from Canine Urinary Tract Infection. Animals. 2024; 14(13):1916. https://doi.org/10.3390/ani14131916
Chicago/Turabian StyleJariyapamornkoon, Nattha, Suphachai Nuanualsuwan, and Nipattra Suanpairintr. 2024. "In Vitro Antibacterial Activities of Fosfomycin against Escherichia coli Isolates from Canine Urinary Tract Infection" Animals 14, no. 13: 1916. https://doi.org/10.3390/ani14131916
APA StyleJariyapamornkoon, N., Nuanualsuwan, S., & Suanpairintr, N. (2024). In Vitro Antibacterial Activities of Fosfomycin against Escherichia coli Isolates from Canine Urinary Tract Infection. Animals, 14(13), 1916. https://doi.org/10.3390/ani14131916






