Reversible Sterilization of Channel Catfish via Overexpression of Glutamic Acid Decarboxylase Gene
Abstract
Simple Summary
Abstract
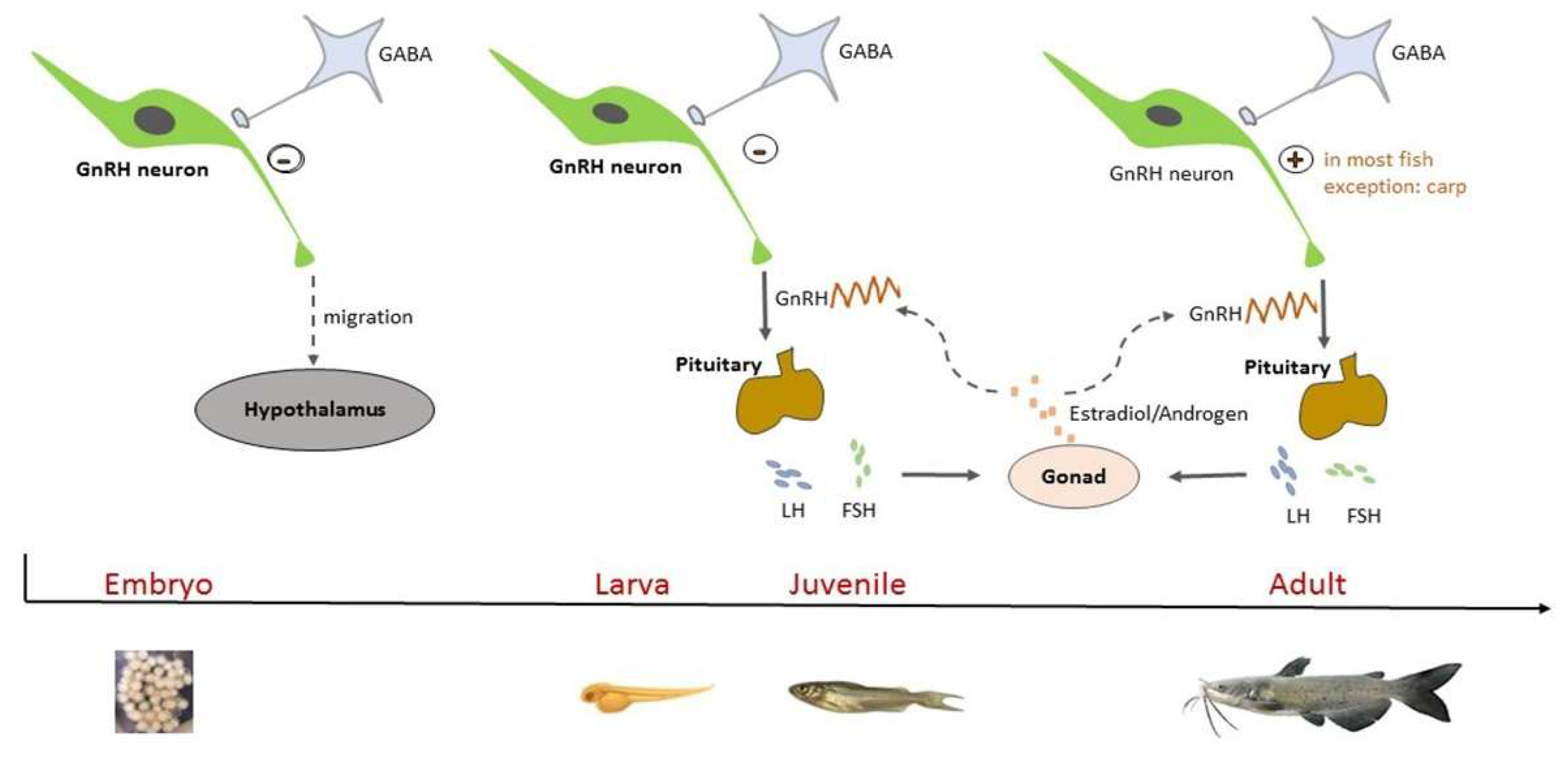
1. Introduction
2. Materials and Methods
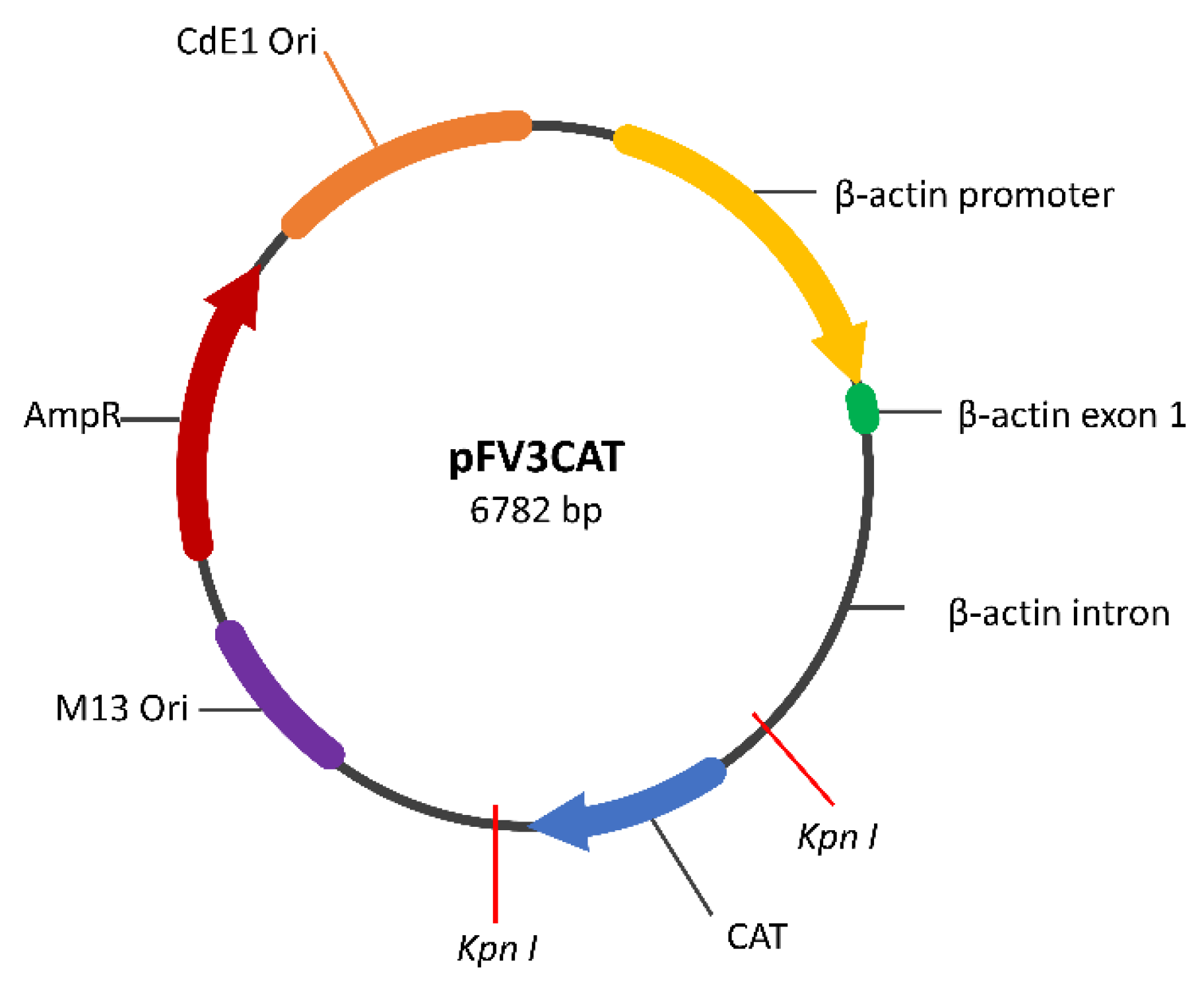
2.1. Construction of the GAD65 Transgene Construct
2.2. Plasmid Preparation
2.3. Introduction of GAD65 Construct through Electroporation
2.4. Embryo Incubation
2.5. Confirmation of Transgene Integration by PCR and Sequencing
2.6. Sexual Maturation, Fertility Evaluation, and Hormone Therapy of F1 and F2 Fish
2.7. Measuring of Serum GnRH, Estradiol, and Testosterone Levels by ELISA
2.8. Statistical Analysis
3. Results
3.1. Mortality of the P1 Embryos
3.2. Identification of Transgenic F2 Progenies and Growth Evaluation
3.3. Gravidity, Fertility Assessment, and Hormone Therapy of the F1 and F2 Generations
3.4. Hormone Levels in the F2 Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wierman, M.E.; Kiseljak-Vassiliades, K.; Tobet, S. Gonadotropin-releasing hormone (GnRH) neuron migration: Initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front. Neuroendocrinol. 2011, 32, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vastagh, C.; Farkas, I.; Csillag, V.; Watanabe, M.; Kalló, I.; Liposits, Z. Cholinergic Control of GnRH Neuron Physiology and Luteinizing Hormone Secretion in Male Mice: Involvement of ACh/GABA Cotransmission. J. Neurosci. 2024, 44, e1780232024. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fukuda, A.; Nabekura, J. The role of GABA in the regulation of GnRH neurons. J. Neurosci. 2014, 8, 117165. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.; Tobet, S. Hypothalamic Development: Role of GABA. In Developmental Neuroendocrinology; Wary, S., Blackshaw, S., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 181–205. [Google Scholar]
- Martin, S.C.; Heinrich, G.; Sandell, J.H. Sequence and expression of glutamic acid decarboxylase isoforms in the developing zebrafish. J. Comp. Neurol. 1998, 396, 253–266. [Google Scholar] [CrossRef]
- Li, K.; Xu, E. The role and the mechanism of γ-aminobutyric acid during central nervous system development. Neurosci. Bull. 2008, 24, 195. [Google Scholar] [CrossRef] [PubMed]
- Casoni, F.; Hutchins, B.I.; Donohue, D.; Fornaro, M.; Condie, B.G.; Wray, S. SDF and GABA interact to regulate axophilic migration of GnRH neurons. J. Cell Sci. 2012, 125, 5015–5025. [Google Scholar] [CrossRef] [PubMed]
- Bless, E.P.; Westaway, W.A.; Schwarting, G.A.; Tobet, S.A. Effects of γ-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology 2000, 141, 1254–1262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salari, A.-A.; Amani, M. Neonatal blockade of GABA-A receptors alters behavioral and physiological phenotypes in adult mice. Int. J. Dev. Neurosci. 2017, 57, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Heger, S.; Seney, M.; Bless, E.; Schwarting, G.A.; Bilger, M.; Mungenast, A.; Ojeda, S.R.; Tobet, S.A. Overexpression of glutamic acid decarboxylase-67 (GAD-67) in gonadotropin-releasing hormone neurons disrupts migratory fate and female reproductive function in mice. Endocrinology 2003, 144, 2566–2579. [Google Scholar] [CrossRef]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.-G.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; Obata, K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 1997, 94, 6496–6499. [Google Scholar] [CrossRef]
- Lee, J.; Tiong, J.; Maddox, D.; Condie, B.; Wray, S. Temporal migration of gonadotrophin-releasing hormone-1 neurones is modified in GAD67 knockout mice. J. Neuroendocrinol. 2008, 20, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, G.A.; Wierman, M.E.; Tobet, S.A. Gonadotropin-releasing hormone neuronal migration. Semin. Reprod. Med. 2007, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Shan, Y.; Whittington, N.C.; Wray, S. Nasal placode development, GnRH neuronal migration and Kallmann syndrome. Front. Cell Dev. Biol. 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Quinton, R.; Maggi, R. Recent advances in understanding and managing Kallmann syndrome. Fac. Rev. 2021, 10, 37. [Google Scholar] [CrossRef]
- Herbison, A.E.; Porteous, R.; Pape, J.-R.; Mora, J.M.; Hurst, P.R. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 2008, 149, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. J. Neurosci. 2022, 16, 953252. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.; Spanswick, D.; Fraser, E.; Lariviere, K.; Crump, D.; Chiu, S.; MacMillan, M.; Schulz, R. The role of amino acid neurotransmitters in the regulation of pituitary gonadotropin release in fish. Biochem. Cell Biol. 2000, 78, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tao, B.; Chen, J.; Jia, S.; Zhu, Z.; Trudeau, V.L.; Hu, W. GABAergic neurons and their modulatory effects on GnRH3 in zebrafish. Endocrinology 2017, 158, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Root, A.R.; Sanford, J.D.; Kavanaugh, S.I.; Sower, S.A. In vitro and in vivo effects of GABA, muscimol, and bicuculline on lamprey GnRH concentration in the brain of the sea lamprey (Petromyzon marinus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 493–501. [Google Scholar] [CrossRef]
- Nakane, R.; Oka, Y. Excitatory action of GABA in the terminal nerve gonadotropin-releasing hormone neurons. J. Neurophysiol. 2010, 103, 1375–1384. [Google Scholar] [CrossRef]
- Khan, I.; Thomas, P. GABA exerts stimulatory and inhibitory influences on gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Neuroendocrinology 1999, 69, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kah, O.; Trudeau, V.L.; Sloley, B.D.; Chang, J.P.; Dubourg, P.; Yu, K.L.; Peter, R.E. Influence of GABA on gonadotrophin release in the goldfish. Neuroendocrinology 1992, 55, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.; Sloley, B.; Peter, R. GABA stimulation of gonadotropin-II release in goldfish: Involvement of GABAA receptors, dopamine, and sex steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993, 265, R348–R355. [Google Scholar] [CrossRef] [PubMed]
- Mañanos, E.L.; Anglade, I.; Chyb, J.; Saligaut, C.; Breton, B.; Kah, O. Involvement of γ-aminobutyric acid in the control of GtH-1 and GtH-2 secretion in male and female rainbow trout. Neuroendocrinology 1999, 69, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kalarani, A.; Vinodha, V.; Moses, I. Inter-relations of brain neurosteroids and monoamines towards reproduction in fish. Reprod. Breed. 2021, 1, 137–148. [Google Scholar] [CrossRef]
- Chyb, J.; Sokołowska-Mikołajczyk, M.; Mikołajczyk, T.; Socha, M.; Epler, P. The influence of gabaergic drugs upon LH secretion from the pituitary of the common carp: An in vitro study. Reprod. Biol. 2001, 1, 51–61. [Google Scholar] [PubMed]
- Trudeau, V.L. Neuroendocrine control of reproduction in teleost fish: Concepts and controversies. Annu. Rev. Anim. Biosci. 2022, 10, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Christian, C.A.; Moenter, S.M. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J. Neurosci. 2007, 27, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Kauffman, A.S. Estrogen regulation of the molecular phenotype and active translatome of AVPV kisspeptin neurons. Endocrinology 2021, 162, bqab080. [Google Scholar] [CrossRef]
- Linard, B.; Anglade, I.; Corio, M.; Navas, J.M.; Pakdel, F.; Saligaut, C.; Kah, O. Estrogen receptors are expressed in a subset of tyrosine hydroxylase-positive neurons of the anterior preoptic region in the rainbow trout. Neuroendocrinology 1996, 63, 156–165. [Google Scholar] [CrossRef]
- Herbison, A.E.; Robinson, J.E.; Skinner, D.C. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: Co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 1993, 57, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Anglade, I.; Douard, V.; Le Jossic-Corcos, C.; Mananos, E.; Mazurais, D.; Michel, D.; Kah, O. The GABAergic system: A possible component of estrogenic feedback on gonadotropin secretion in rainbow trout (Oncorhynchus mykiss). Bull. Fr. Pêche Piscic. 1998, 350–351, 647–654. [Google Scholar] [CrossRef][Green Version]
- Lariviere, K.; MacEachern, L.; Greco, V.; Majchrzak, G.; Chiu, S.; Drouin, G.; Trudeau, V. GAD65 and GAD67 isoforms of the glutamic acid decarboxylase gene originated before the divergence of cartilaginous fishes. Mol. Biol. Evol. 2002, 19, 2325–2329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trabucchi, M.; Trudeau, V.L.; Drouin, G.; Tostivint, H.; Ihrmann, I.; Vallarino, M.; Vaudry, H. Molecular characterization and comparative localization of the mRNAs encoding two glutamic acid decarboxylases (GAD65 and GAD67) in the brain of the African lungfish, Protopterus annectens. J. Comp. Neurol. 2008, 506, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, K.; Huang, J.; Tan, Z.; Ruan, J. Effects of two kinds of fishery drugs on the expressions of GAD and GABA-T mRNA in crucian carp (Carassius auratus gibelio). Fish Physiol. Biochem. 2020, 46, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, C.; Konno, A.; Hosoi, N.; Kaneko, R.; Mukai, R.; Nakai, J.; Hirai, H. GABAergic neuron-specific whole-brain transduction by AAV-PHP. B incorporated with a new GAD65 promoter. Mol. Brain 2021, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lei, D.; Lu, Y.; Huang, Q.; Wu, Y.; Yang, S.; Wu, Y. Parvalbumin in the metabolic pathway of glutamate and γ-aminobutyric acid: Influence on expression of GAD65 and GAD67. Arch. Biochem. Biophys. 2023, 734, 109499. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Qin, Z.; Lu, C.; Ye, Z.; Elaswad, A.; Bangs, M.; Li, H.; Zhang, Y.; Huang, Y.; Shi, H. Gene editing of the catfish gonadotropin-releasing hormone gene and hormone therapy to control the reproduction in channel catfish, Ictalurus punctatus. Biology 2022, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, A.; Khalil, K.; Cline, D.; Page-McCaw, P.; Chen, W.; Michel, M.; Cone, R.; Dunham, R. Microinjection of CRISPR/Cas9 protein into channel catfish, Ictalurus punctatus, embryos for gene editing. J. Vis. Exp. 2018, 131, e56275. [Google Scholar] [CrossRef]
- Su, B.; Perera, D.A.; Zohar, Y.; Abraham, E.; Stubblefield, J.; Fobes, M.; Beam, R.; Argue, B.; Ligeon, C.; Padi, J. Relative effectiveness of carp pituitary extract, luteininzing hormone releasing hormone analog (LHRHa) injections and LHRHa implants for producing hybrid catfish fry. Aquaculture 2013, 372, 133–136. [Google Scholar] [CrossRef]
- Dunham, R.A.; Elaswad, A.; Qin, Z. Gene editing in channel catfish via double electroporation of zinc-finger nucleases. In Zinc Finger Proteins; Springer Science+Business Media, LLC.: New York, NY, USA, 2018; pp. 201–214. [Google Scholar]
- Elaswad, A.; Khalil, K.; Ye, Z.; Liu, Z.; Liu, S.; Peatman, E.; Odin, R.; Vo, K.; Drescher, D.; Gosh, K. Effects of CRISPR/Cas9 dosage on TICAM1 and RBL gene mutation rate, embryonic development, hatchability and fry survival in channel catfish. Sci. Rep. 2018, 8, 16499. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, A.; Khalil, K.; Ye, Z.; Alsaqufi, A.; Abdelrahman, H.; Su, B.; Perera, D.A.; Dong, S.; Abass, N.; Dunham, R. Effects of cecropin transgenesis and interspecific hybridization on the resistance to Ichthyophthirius multifiliis in channel catfish and female channel catfish× male blue catfish hybrids. N. Am. J. Aquac. 2019, 81, 242–252. [Google Scholar] [CrossRef]
- Ye, Z. Alternative Approaches for Repressible Transgenic Sterilization of Channel Catfish (Ictalurus punctatus). Ph.D. Dissertation, Auburn University, Auburn, AL, USA, 2017. [Google Scholar]
- Su, B.; Shang, M.; Li, C.; Perera, D.A.; Pinkert, C.A.; Irwin, M.H.; Peatman, E.; Grewe, P.; Patil, J.G.; Dunham, R.A. Effects of transgenic sterilization constructs and their repressor compounds on hatch, developmental rate and early survival of electroporated channel catfish embryos and fry. Transgenic Res. 2015, 24, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Waagepetersen, H.S.; Sonnewald, U.; Schousboe, A. The GABA paradox: Multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J. Neurochem. 1999, 73, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Zafra, F.; Castren, E.; Thoenen, H.; Lindholm, D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl. Acad. Sci. USA 1991, 88, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Lee, Y.; Lee, G.H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res. 2019, 42, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A. Metabolic signaling in the brain and the role of astrocytes in control of glutamate and GABA neurotransmission. Neurosci. Lett. 2019, 689, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Bajić, S.S.; Đokić, J.; Dinić, M.; Tomić, S.; Popović, N.; Brdarić, E.; Golić, N.; Tolinački, M. GABA potentiate the immunoregulatory effects of Lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro. Sci. Rep. 2020, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Yarrow, J.F.; McCoy, S.C.; Borst, S.E. Growth hormone isoform responses to GABA ingestion at rest and after exercise. Med. Sci. Sports Exerc. 2008, 40, 104. [Google Scholar] [CrossRef]
- Athapaththu, A.M.G.K.; Molagoda, I.M.N.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Jeon, Y.-J.; Park, J.-H.; Lee, B.-J.; Kim, G.-Y. Gamma-aminobutyric acid (GABA) promotes growth in zebrafish larvae by inducing IGF-1 expression via GABAA and GABAB receptors. Int. J. Mol. Sci. 2021, 22, 11254. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gazi, S.K. Neurotransmitters; Overview. In Encyclopedia of the Neurological Sciences, 2nd ed.; Michael, J., Aminoff, R.B.D., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 565–572. [Google Scholar]
- Xie, W.y.; Hou, X.y.; Yan, F.b.; Sun, G.r.; Han, R.l.; Kang, X.t. Effect of γ-aminobutyric acid on growth performance and immune function in chicks under beak trimming stress. Anim. Sci. 2013, 84, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.B.; Kim, Y.B.; Lee, J.W.; Kim, D.H.; Moon, B.H.; Chang, H.H.; Choi, Y.H.; Lee, K.W. Role of dietary gamma-aminobutyric acid in broiler chickens raised under high stocking density. Anim. Nutr. 2020, 6, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.S.; Hargreaves, J.A. Biology and Culture of Channel Catfish; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Lee, K.; Porteous, R.; Campbell, R.; Lüscher, B.; Herbison, A.E. Knockdown of GABAA receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology 2010, 151, 4428–4436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spicer, O.S.; Wong, T.-T.; Zmora, N.; Zohar, Y. Targeted mutagenesis of the hypophysiotropic Gnrh3 in zebrafish (Danio rerio) reveals no effects on reproductive performance. PLoS ONE 2016, 11, e0158141. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Palevitch, O.; Gothilf, Y.; Zohar, Y. Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: Effects on neurogenesis, neuronal migration, and reproduction. Endocrinology 2010, 151, 332–340. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, V.; Murgas, L.; Andrade, E.; López, P.; Freitas, R.; Ferreira, M. Effect of timing of hormonal induction on reproductive activity in lambari (Astyanax bimaculatus). Theriogenology 2012, 77, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Gothilf, Y.; Meiri, I.; King, J.; Okuzawa, K.; Elizur, A.; Zohar, Y. Levels of the native forms of GnRH in the pituitary of the gilthead seabream, Sparus aurata, at several characteristic stages of the gonadal cycle. Gen. Comp. Endocrinol. 1998, 112, 394–405. [Google Scholar] [CrossRef]
- Maruska, K.P.; Fernald, R.D. Reproductive status regulates expression of sex steroid and GnRH receptors in the olfactory bulb. Behav. Brain Res. 2010, 213, 208–217. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z.; Gur, G.; Melamed, P.; Rosenfeld, H.; Elizur, A.; Levavi-Sivan, B. Regulation of fish gonadotropins. Int. Rev. Cytol. 2003, 225, 131–185. [Google Scholar] [CrossRef] [PubMed]

| Sex | Genotype | Total Number | Reproductive Score * | ||
|---|---|---|---|---|---|
| 5 | 4 | 4 & 5 | |||
| Female | Control | 227 | 22 (9.7%) | 25 (11%) | 47 (20.7%) |
| GAD | 14 | 0 | 1 (7.1%) | 1 (7.1%) | |
| Male | Control | 232 | 10 (4.3%) | 20 (8.6%) | 30 (12.5%) |
| GAD | 23 | 0 | 1 (4.3%) | 1 (4.3%) | |
| Both sexes | Control | 459 | 32 (7.0%) | 45 (9.8%) | 77 (16.8%) a |
| GAD | 37 | 0 | 2(5.4%) | 2 (5.4%) b | |
| Trial | Genotype | Without Implant * | With Implant | ||||
|---|---|---|---|---|---|---|---|
| N | Pairs Spawned | % | N | Pairs Spawned | % | ||
| Six-year-old F1 | Control | 12 | 10 | 83.3 | - | - | - |
| GAD | 11 | 5 | 45.5 | 6 | 4 | 66.7 | |
| Nine-year-old F1 | Control | 6 | 4 | 66.7 | |||
| GAD | 10 | 2 | 20.0 | 3 | 0 | 0.0 | |
| Three-year-old F2 | Control | 6 | 4 | 66.7 | - | - | - |
| GAD | 15 | 3 | 20.0 | 5 | 0 | 0.0 | |
| Combined generations | Control | 24 | 18 | 75.0 a | - | - | - |
| GAD | 36 | 10 | 27.8 b | 14 | 4 | 28.6 | |
| Genotype | Hormone * | |||
|---|---|---|---|---|
| GnRH (ng/mL) | Estradiol (pg/mL) | Testosterone (ng/mL) | ||
| Female | Male | Female | Male | |
| GAD | 9.23 ± 2.49 (n = 24) | 8.14 ± 2.21 (n = 20) | 501.00 ± 69.96 (n = 24) | ND |
| Control | 11.04 ± 4.06 (n = 10) | 9.03 ± 2.36 (n = 11) | 520.48 ± 148.31 (n = 10) | ND |
| Genotype | Hormone * | |||
|---|---|---|---|---|
| GnRH (ng/mL) | Estradiol (pg/mL) | Testosterone (pg/mL) | ||
| Female | Male | Female | Male | |
| GAD | 3.05 ± 1.22 a (n = 6) | 1.02 ± 0.31 b (n = 6) | 262.17 ± 82.25 (n = 6) | 288.84 ± 62.62 A (n = 6) |
| Control | 3.07 ± 0.66 a (n = 6) | 2.34 ± 1.22 a (n = 4) | 254.14 ± 71.73 (n = 6) | 699.12 ± 211.90 B (n = 4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Elaswad, A.; Su, B.; Alsaqufi, A.; Shang, M.; Bugg, W.S.; Qin, G.; Drescher, D.; Li, H.; Qin, Z.; et al. Reversible Sterilization of Channel Catfish via Overexpression of Glutamic Acid Decarboxylase Gene. Animals 2024, 14, 1899. https://doi.org/10.3390/ani14131899
Ye Z, Elaswad A, Su B, Alsaqufi A, Shang M, Bugg WS, Qin G, Drescher D, Li H, Qin Z, et al. Reversible Sterilization of Channel Catfish via Overexpression of Glutamic Acid Decarboxylase Gene. Animals. 2024; 14(13):1899. https://doi.org/10.3390/ani14131899
Chicago/Turabian StyleYe, Zhi, Ahmed Elaswad, Baofeng Su, Ahmed Alsaqufi, Mei Shang, William S. Bugg, Guyu Qin, David Drescher, Hanbo Li, Zhenkui Qin, and et al. 2024. "Reversible Sterilization of Channel Catfish via Overexpression of Glutamic Acid Decarboxylase Gene" Animals 14, no. 13: 1899. https://doi.org/10.3390/ani14131899
APA StyleYe, Z., Elaswad, A., Su, B., Alsaqufi, A., Shang, M., Bugg, W. S., Qin, G., Drescher, D., Li, H., Qin, Z., Odin, R., Makhubu, N., Abass, N., Dong, S., & Dunham, R. (2024). Reversible Sterilization of Channel Catfish via Overexpression of Glutamic Acid Decarboxylase Gene. Animals, 14(13), 1899. https://doi.org/10.3390/ani14131899







