Changes in Parameters Registered by Innovative Technologies in Cows with Subclinical Acidosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm and Animals
2.2. Allocation to Groups for Analysis
2.3. Measurement of Variables
2.3.1. Registration of In-Line Milk Fat-to-Protein Ratio
2.3.2. Recticulorumen Data Collection
2.4. Duration of Experimental Observation
2.5. Statistical Analyses
3. Results
3.1. Statistical Overview of the Examined Indicators or Descriptive Distribution of Variables
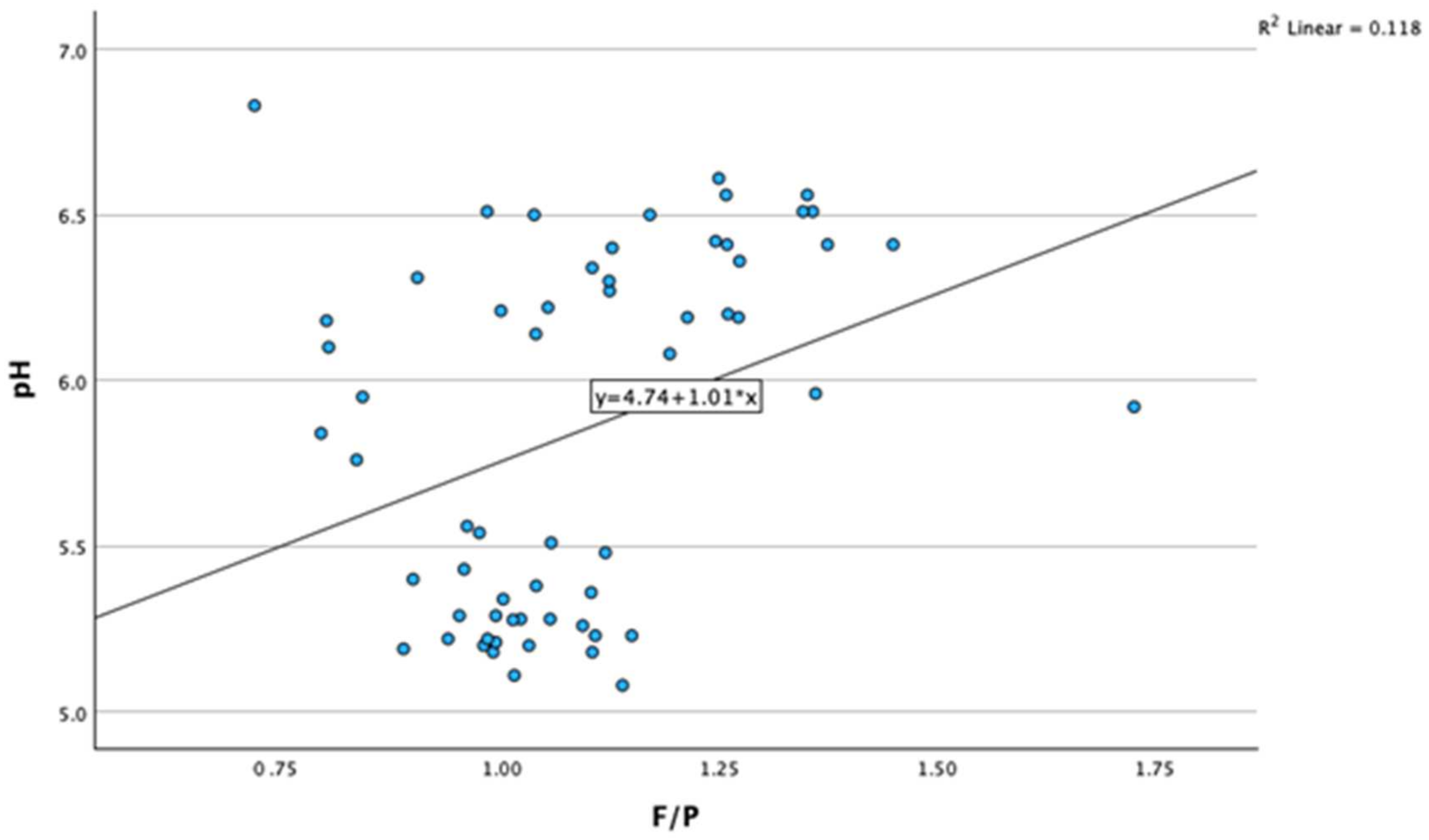
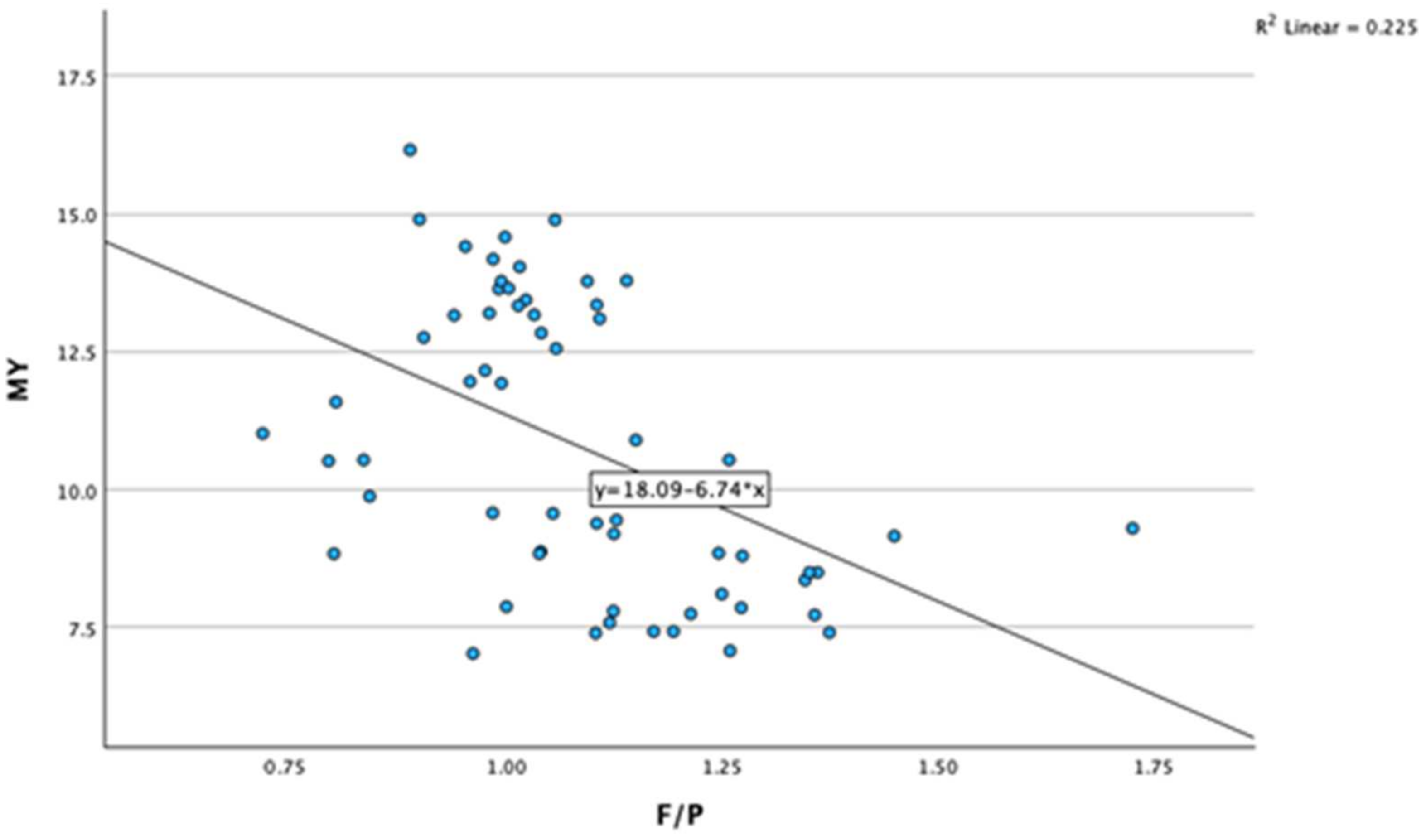
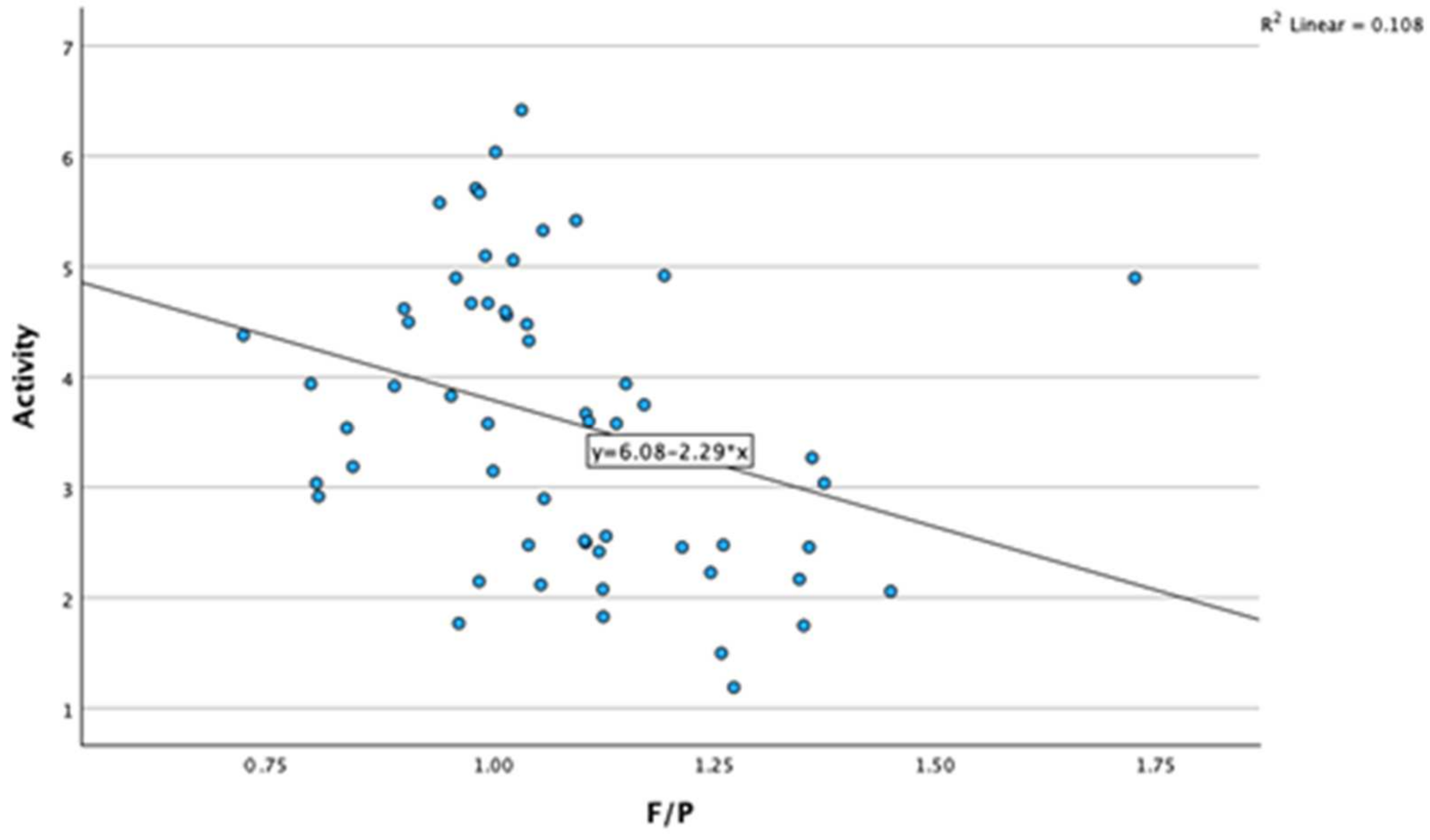
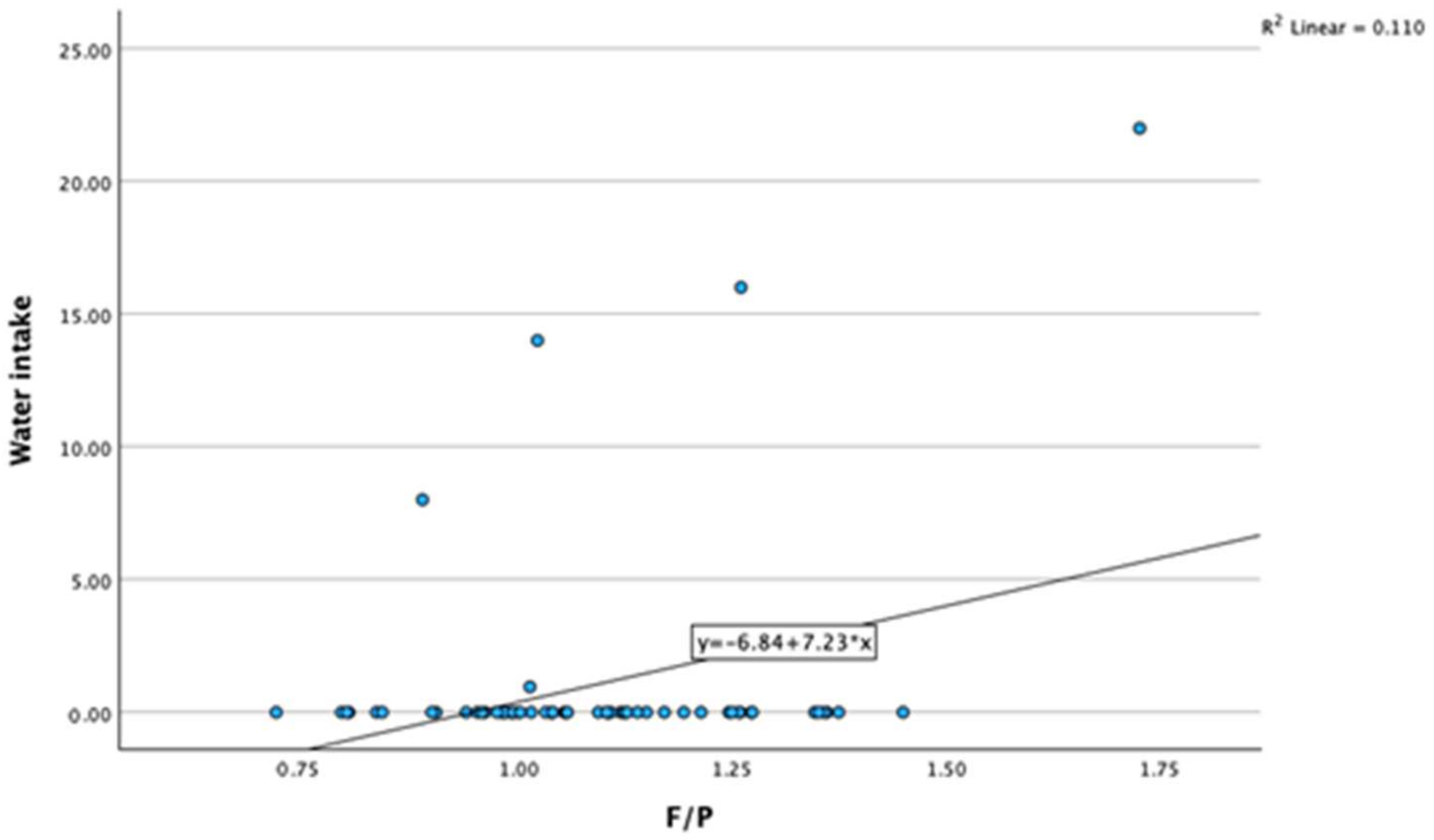
3.2. Correlations between Milk Fat-to-Protein Ratio and Other Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reist, M.; Erdin, D.; von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Chilliard, Y.; Hammon, H.M.; Morel, C.; Philipona, C.; Zbinden, Y.; et al. Estimation of Energy Balance at the Individual and Herd Level Using Blood and Milk Traits in High-Yielding Dairy Cows1,2. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Erickson, P.S.; Kalscheur, K.F. Chapter 9—Nutrition and Feeding of Dairy Cattle. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 157–180. ISBN 978-0-12-817052-6. [Google Scholar]
- Albornoz, R.I.; Harvatine, K.J.; Allen, M.S. Diet Starch Concentration and Starch Fermentability Affect Energy Intake and Energy Balance of Cows in the Early Postpartum Period. J. Dairy Sci. 2019, 102, 5161–5171. [Google Scholar] [CrossRef] [PubMed]
- Oetzel, G.R. Herd-Level Ketosis—Diagnosis and Risk Factors. In Proceedings of the Preconference Seminar 7C: Dairy Herd Problem Investigation Strategies: Transition Cow Troubleshooting, Vancouver, BC, Canada, 19 September 2007; pp. 67–91. [Google Scholar]
- Krause, K.M. To Buffer or Not? Supplemental Bicarb and Subacute Ruminal Acidosis. In Proceedings of the Southwest Animal Nutrition Conference, Tempe, AZ, USA, 11 August 2008. [Google Scholar]
- Krause, K.M.; Oetzel, G.R. Understanding and Preventing Subacute Ruminal Acidosis in Dairy Herds: A Review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute Ruminal Acidosis in Dairy Cows: The Physiological Causes, Incidence and Consequences. Vet. J. Lond. Eng. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Enemark, J.M.D. The Monitoring, Prevention and Treatment of Sub-Acute Ruminal Acidosis (SARA): A Review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Zilaitis, V.; Juozaitiene, V.; Stoskus, R.; Televicius, M. Changes in Reticulorumen Content Temperature and pH According to Time of Day and Yearly Seasons. Pol. J. Vet. Sci. 2016, 19, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.L.; Cannizzo, C. Incidence, Prevalence and Impact of SARA in Dairy Herds. Anim. Feed Sci. Technol. 2012, 172, 4–8. [Google Scholar] [CrossRef]
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute Ruminal Acidosis in Dairy Herds: Microbiological and Nutritional Causes, Consequences, and Prevention Strategies. Anim. Nutr. 2022, 10, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gozho, G.N.; Gakhar, N.; Khafipour, E.; Krause, D.O.; Plaizier, J.C. Evaluation of Diagnostic Measures for Subacute Ruminal Acidosis in Dairy Cows. Can. J. Anim. Sci. 2012, 92, 353–364. [Google Scholar] [CrossRef]
- Francesio, A.; Viora, L.; Denwood, M.J.; Tulley, W.; Brady, N.; Hastie, P.; Hamilton, A.; Davison, C.; Michie, C.; Jonsson, N.N. Contrasting Effects of High-Starch and High-Sugar Diets on Ruminal Function in Cattle. J. Dairy Res. 2020, 87, 175–183. [Google Scholar] [CrossRef]
- Capuzzello, G.; Viora, L.; Borelli, E.; Jonsson, N.N. Evaluation of an Indwelling Bolus Equipped with a Triaxial Accelerometer for the Characterisation of the Diurnal Pattern of Bovine Reticuloruminal Contractions. J. Dairy Res. 2023, 90, 9–15. [Google Scholar] [CrossRef]
- Mutsvangwa, T.; Walton, J.P.; Plaizier, J.C.; Duffield, T.F.; Bagg, R.; Dick, P.; Vessie, G.; McBride, B.W. Effects of a Monensin Controlled-Release Capsule or Premix on Attenuation of Subacute Ruminal Acidosis in Dairy Cows. J. Dairy Sci. 2002, 85, 3454–3461. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, C.; Confessore, A.; Bozzi, R.; Sirtori, F.; Pugliese, C. Review: Precision Livestock Farming Technologies in Pasture-Based Livestock Systems. Animal 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Cantor, M.C.; Costa, J.H.C.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- AlZahal, O.; AlZahal, H.; Steele, M.A.; Van Schaik, M.; Kyriazakis, I.; Duffield, T.F.; McBride, B.W. The Use of a Radiotelemetric Ruminal Bolus to Detect Body Temperature Changes in Lactating Dairy Cattle. J. Dairy Sci. 2011, 94, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Prado, M.J.; Long, N.M.; Wright, E.C.; Goad, C.L.; Wettemann, R.P. Relationship of Ruminal Temperature with Parturition and Estrus of Beef Cows1. J. Dairy Sci. 2011, 89, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.T.; Peña, G.; Risco, C.; Barbosa, C.C.; Vieira-Neto, A.; Galvão, K.N. Utility of Inline Milk Fat and Protein Ratio to Diagnose Subclinical Ketosis and to Assign Propylene Glycol Treatment in Lactating Dairy Cows. Can. Vet. J. 2015, 56, 850–854. [Google Scholar] [PubMed]
- Caja, G.; Castro-Costa, A.; Knight, C.H. Engineering to Support Wellbeing of Dairy Animals. J. Dairy Res. 2016, 83, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Džermeikaitė, K.; Januškevičius, V.; Šimonytė, I.; Baumgartner, W. In-Line Registered Milk Fat-to-Protein Ratio for the Assessment of Metabolic Status in Dairy Cows. Animals 2023, 13, 3293. [Google Scholar] [CrossRef] [PubMed]
- Djokovic, R.; Cincovic, M.; Ilic, Z.; Kurcubic, V.; Andjelic, B.; Petrovic, M.; Lalic, N.; Jasovic, B. Relationships Between Contents of Biochemical Metabolites in Blood and Milk in Dairy Cows During Transition and Mid Lactation. Int. J. Appl. Res. Vet. Med. 2019, 17, 771–776. [Google Scholar]
- Duffield, T. Subclinical Ketosis in Lactating Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B. Invited Review: Corrected Milk: Reconsideration of Common Equations and Milk Energy Estimates. J. Dairy Sci. 2023, 106, 2230–2246. [Google Scholar] [CrossRef] [PubMed]
- Zschiesche, M.; Mensching, A.; Sharifi, A.R.; Hummel, J. The Milk Fat-to-Protein Ratio as Indicator for Ruminal pH Parameters in Dairy Cows: A Meta-Analysis. Dairy 2020, 1, 259–268. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Inline Reticulorumen pH as an Indicator of Cows Reproduction and Health Status. Sensors 2020, 20, 1022. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.K. Determination of Subclinical Metabolic Disorders in Transition Dairy Cows. Ph.D. Dissertation, University of Kassel, Witzenhausen, Germany, 2017. [Google Scholar]
- Sutton, J.D. Altering Milk Composition by Feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Boisclair, Y.R.; Bauman, D.E. Recent Advances in the Regulation of Milk Fat Synthesis. Animal 2009, 3, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Meneses, J.A.M.; de Sá, O.A.A.L.; Coelho, C.F.; Pereira, R.N.; Batista, E.D.; Ladeira, M.M.; Casagrande, D.R.; Gionbelli, M.P. Effect of Heat Stress on Ingestive, Digestive, Ruminal and Physiological Parameters of Nellore Cattle Feeding Low- or High-Energy Diets. Livest. Sci. 2021, 252, 104676. [Google Scholar] [CrossRef]
- Mensching, A.; Hummel, J.; Sharifi, A.R. Statistical Modeling of Ruminal pH Parameters from Dairy Cows Based on a Meta-Analysis. J. Dairy Sci. 2020, 103, 750–767. [Google Scholar] [CrossRef]
- Humer, E.; Petri, R.M.; Aschenbach, J.R.; Bradford, B.J.; Penner, G.B.; Tafaj, M.; Südekum, K.-H.; Zebeli, Q. Invited Review: Practical Feeding Management Recommendations to Mitigate the Risk of Subacute Ruminal Acidosis in Dairy Cattle. J. Dairy Sci. 2018, 101, 872–888. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Malašauskienė, D.; Televičius, M. Can Rumination Time and Some Blood Biochemical Parameters Be Used as Biomarkers for the Diagnosis of Subclinical Acidosis and Subclinical Ketosis? Vet. Anim. Sci. 2019, 8, 100077. [Google Scholar] [CrossRef]
- Paudyal, S. Rumination, Activity, Milk Yield and Milk Components Analysis for Disease Detection During the Transition Period of Dairy Cows. Master’s Thesis, West Texas A&M University Canyon, Canyon, TX, USA, 2016. [Google Scholar]
- Kaufman, E.I.; LeBlanc, S.J.; McBride, B.W.; Duffield, T.F.; DeVries, T.J. Association of Rumination Time with Subclinical Ketosis in Transition Dairy Cows. J. Dairy Sci. 2016, 99, 5604–5618. [Google Scholar] [CrossRef] [PubMed]
- Liboreiro, D.N.; Machado, K.S.; Silva, P.R.B.; Maturana, M.M.; Nishimura, T.K.; Brandão, A.P.; Endres, M.I.; Chebel, R.C. Characterization of Peripartum Rumination and Activity of Cows Diagnosed with Metabolic and Uterine Diseases. J. Dairy Sci. 2015, 98, 6812–6827. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Džermeikaitė, K.; Krištolaitytė, J.; Ribelytė, I.; Bespalovaitė, A.; Bulvičiūtė, D.; Rutkauskas, A. Alterations in Rumination, Eating, Drinking and Locomotion Behavior in Dairy Cows Affected by Subclinical Ketosis and Subclinical Acidosis. Animals 2024, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Tozer, P.R. Using Activity and Milk Yield as Predictors of Fresh Cow Disorders. J. Dairy Sci. 2004, 87, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Žilaitis, V.; Kucinskas, A.; Juozaitienė, V.; Leonauskaite, K. Changes in Cow Activity, Milk Yield, and Milk Conductivity before Clinical Diagnosis of Ketosis, and Acidosis. Vet. Ir Zootech. 2015, 70, 3–9. [Google Scholar]
| Parameters | Units | Dairy Cows |
|---|---|---|
| Dry matter | % | 48.8 |
| Net energy lactation | Mcal/kg | 1.6 |
| Crude protein | % | 15.8 |
| Nonfiber carbohydrates | % | 38.7 |
| Neutral detergent fiber | % | 28.2 |
| Acid detergent fiber | % | 19.8 |
| Descriptives | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | p | ||
| Lower Bound | Upper Bound | |||||||||
| pH | HC | 36 | 6.22 | 0.33 | 6.94 × 10−8 M of H+ ion | 6.11 | 6.33 | 5 | 7 | |
| SCA | 23 | 5.28 | 0.11 | 2.42 × 10−7 M of H+ ion. | 5.23 | 5.33 | 5 | 6 | ||
| Total | 59 | 5.85 | 0.53 | 1.56 × 10−7 M of H+ ion. | 5.71 | 5.99 | 5 | 7 | <0.01 | |
| DIM | HC | 36 | 17.85 | 0.84 | 0.32 | 11.52 | 14.23 | 5.00 | 30.00 | |
| SCA | 23 | 19.32 | 0.76 | 0.22 | 13.23 | 15.23 | 5.00 | 30.00 | ||
| Total | 59 | 154.95 | 17.96 | 2.31 | 150.30 | 159.59 | 126.00 | 227.00 | 0.19 | |
| MY (kg/d) | HC | 36 | 32.52 | 1.35 | 0.22 | 8.54 | 9.46 | 7 | 13 | |
| SCA | 23 | 29.43 | 1.60 | 0.32 | 12.66 | 14.01 | 7 | 16 | ||
| Total | 59 | 30.97 | 2.58 | 0.33 | 10.07 | 11.40 | 7 | 16 | <0.01 | |
| Fat (%) | HC | 36 | 4.32 | 0.81 | 0.13 | 4.05 | 4.60 | 3 | 6 | |
| SCA | 23 | 3.67 | 0.25 | 0.05 | 3.57 | 3.78 | 3 | 4 | ||
| Total | 59 | 4.06 | 0.72 | 0.09 | 3.88 | 4.25 | 3 | 6 | <0.01 | |
| Protein (%) | HC | 36 | 3.81 | 0.12 | 0.02 | 3.77 | 3.85 | 4 | 4 | |
| SCA | 23 | 3.63 | 0.05 | 0.01 | 3.60 | 3.65 | 4 | 4 | ||
| Total | 59 | 3.74 | 0.13 | 0.01 | 3.70 | 3.77 | 4 | 4 | <0.01 | |
| Temperature (°C) | HC | 36 | 38.79 | 1.52 | 0.25 | 38.27 | 39.30 | 33 | 40 | |
| SCA | 23 | 38.88 | 1.20 | 0.25 | 38.36 | 39.40 | 35 | 40 | ||
| Total | 59 | 38.82 | 1.40 | 0.18 | 38.46 | 39.19 | 33 | 40 | 0.80 | |
| Cow activity | HC | 36 | 2.92 | 0.97 | 0.16 | 2.59 | 3.25 | 1 | 5 | |
| SCA | 23 | 4.59 | 1.01 | 0.21 | 4.15 | 5.03 | 3 | 6 | ||
| Total | 59 | 3.57 | 1.28 | 0.16 | 3.24 | 3.91 | 1 | 6 | <0.01 | |
| Rumination time (min/d.) | HC | 36 | 520.51 | 47.35 | 8.00 | 504.25 | 536.78 | 393 | 618 | |
| SCA | 23 | 488.31 | 32.51 | 6.93 | 473.89 | 502.73 | 433 | 554 | ||
| Total | 59 | 508.09 | 44.81 | 5.93 | 496.20 | 519.98 | 393 | 618 | 0.01 | |
| Water_intake | HC | 36 | 1.05 | 4.47 | 0.74 | −0.45 | 2.56 | 0.00 | 22.00 | |
| SCA | 23 | 0.95 | 3.29 | 0.68 | −0.46 | 2.38 | 0.00 | 14.00 | ||
| Total | 59 | 1.01 | 4.02 | 0.52 | −0.031 | 2.06 | 0.00 | 22.00 | 0.97 | |
| F/P | HC | 36 | 1.13 | 0.21 | 0.03 | 1.06 | 1.20 | 0.71 | 1.72 | |
| SCA | 23 | 1.01 | 0.06 | 0.01 | 0.98 | 1.04 | 0.88 | 1.13 | ||
| Total | 59 | 1.08 | 0.18 | 0.02 | 1.03 | 1.13 | 0.71 | 1.72 | 0.01 | |
| . | pH | DIM | MY | Fat | Protein | Temperature | Activity | RT | Water_Intake | |
|---|---|---|---|---|---|---|---|---|---|---|
| F/P | Pearson Correlation | 0.344 ** | 0.222 | −0.474 ** | 0.981 ** | 0.167 | −0.180 | −0.328 * | 0.110 | 0.332 ** |
| Sig. (two-tailed) | 0.007 | 0.086 | <0.001 | <0.001 | 0.197 | 0.170 | 0.010 | 0.409 | 0.010 | |
| N | 60 | 61 | 61 | 61 | 61 | 60 | 60 | 58 | 60 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antanaitis, R.; Džermeikaitė, K.; Krištolaitytė, J.; Stankevičius, R.; Daunoras, G.; Televičius, M.; Malašauskienė, D.; Cook, J.; Viora, L. Changes in Parameters Registered by Innovative Technologies in Cows with Subclinical Acidosis. Animals 2024, 14, 1883. https://doi.org/10.3390/ani14131883
Antanaitis R, Džermeikaitė K, Krištolaitytė J, Stankevičius R, Daunoras G, Televičius M, Malašauskienė D, Cook J, Viora L. Changes in Parameters Registered by Innovative Technologies in Cows with Subclinical Acidosis. Animals. 2024; 14(13):1883. https://doi.org/10.3390/ani14131883
Chicago/Turabian StyleAntanaitis, Ramūnas, Karina Džermeikaitė, Justina Krištolaitytė, Rolandas Stankevičius, Gintaras Daunoras, Mindaugas Televičius, Dovilė Malašauskienė, John Cook, and Lorenzo Viora. 2024. "Changes in Parameters Registered by Innovative Technologies in Cows with Subclinical Acidosis" Animals 14, no. 13: 1883. https://doi.org/10.3390/ani14131883
APA StyleAntanaitis, R., Džermeikaitė, K., Krištolaitytė, J., Stankevičius, R., Daunoras, G., Televičius, M., Malašauskienė, D., Cook, J., & Viora, L. (2024). Changes in Parameters Registered by Innovative Technologies in Cows with Subclinical Acidosis. Animals, 14(13), 1883. https://doi.org/10.3390/ani14131883











