Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Rearing System, Feeding Trial, and Fish Sampling
2.3. Biochemical and Cytogenetic Evaluations
2.3.1. Antioxidant System Status and Peroxidative Damage
2.3.2. DNA and Chromosomal Integrity
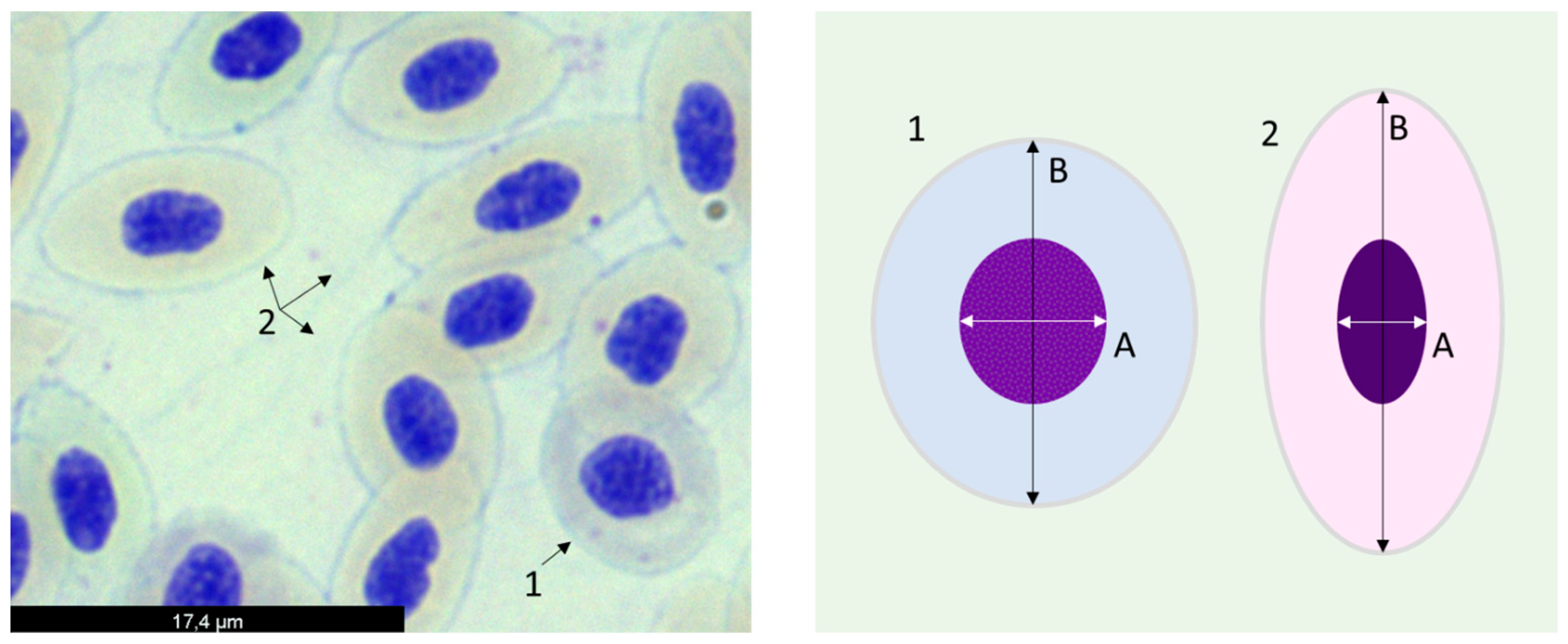
2.3.3. Erythrocyte Population Dynamics
2.4. Statistical Analyses
3. Results
3.1. Growth Performance
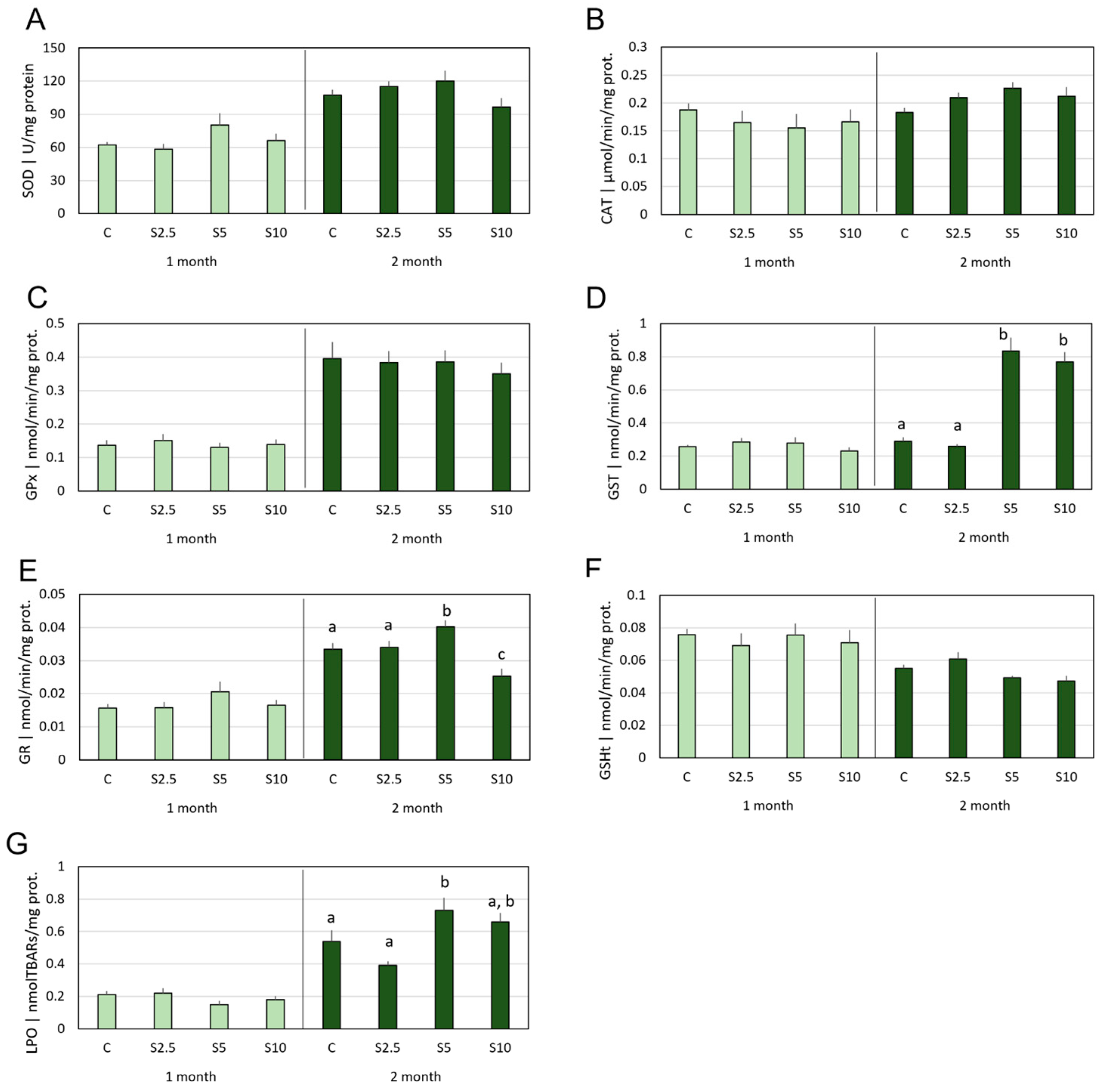
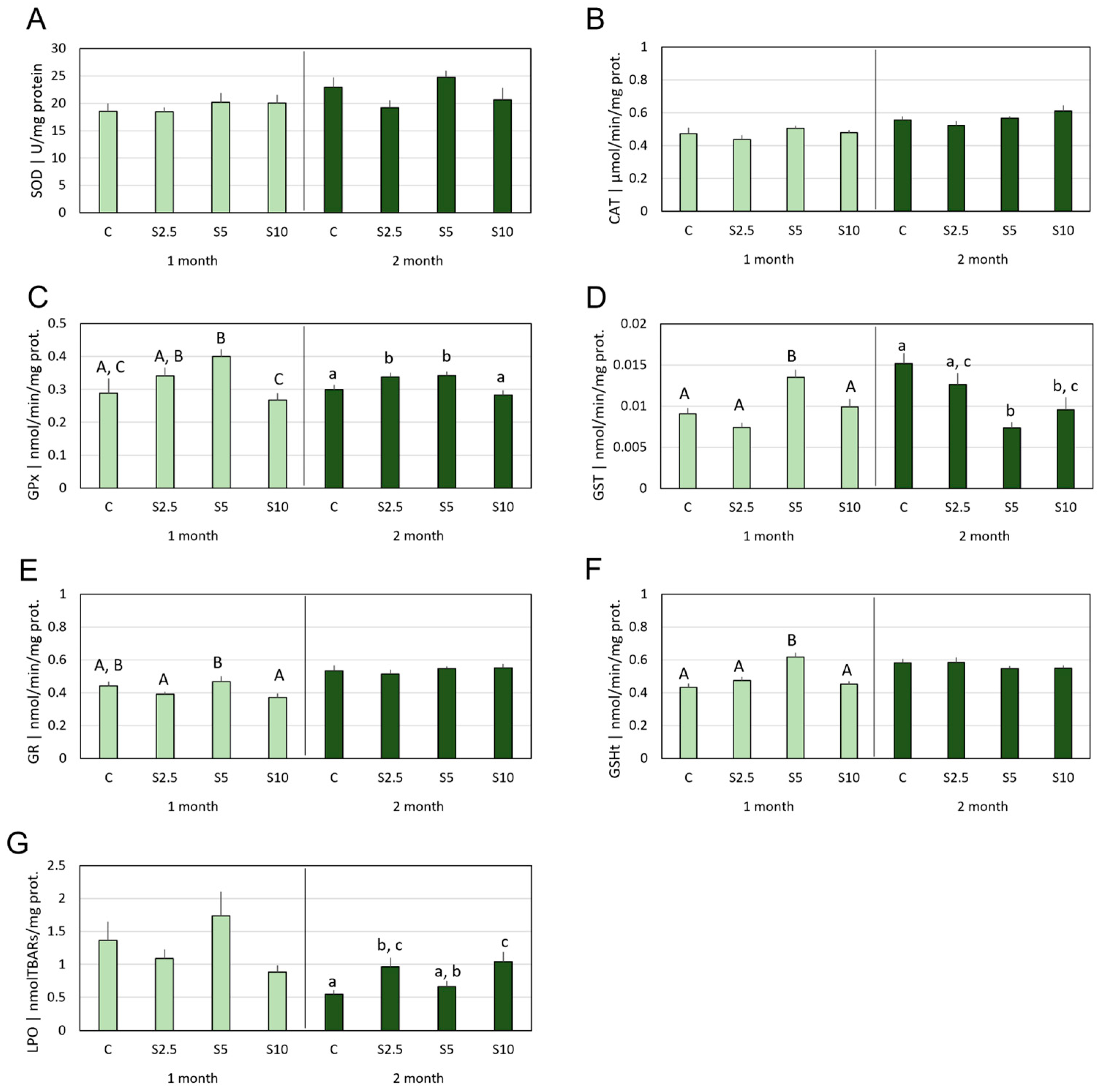
3.2. Antioxidant Defenses vs. Oxidative Damage
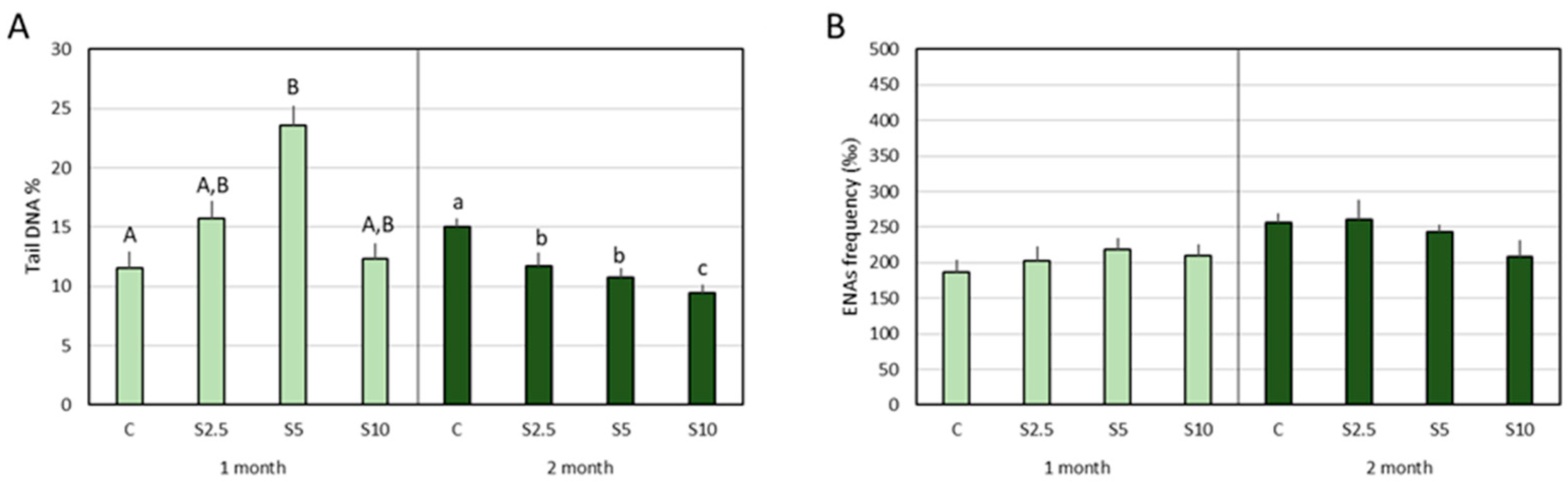
3.3. DNA and Chromosomal Integrity
3.4. Erythrocyte Population Dynamics (EMI Assay)
4. Discussion
4.1. Growth Performance of European Seabass following S. ramosissima Dietary Supplementation
4.2. Incorporation of S. ramosissima in European Seabass Feed and Modulation of the Antioxidant System and Oxidative Status
4.3. Incorporation of S. ramosissima in European Seabass Feed and DNA/Chromosomal Integrity
5. Conclusions
- (i)
- The inclusion of whole-plant biomass up to 10%, for 2 months, replacing wheat meal, showed no impairments on growth performance, pointing out that the use of S. ramosissima routinely as a feed ingredient can be considered, though its scalability and economic viability need to be evaluated.
- (ii)
- S. ramosissima was shown to affect the oxidative status of D. labrax, namely modulating the GSH-related defense subsystem in a tissue-specific manner and with higher acuity in the second month of feeding. Though an antioxidant improvement was apparent, it followed multiple and complex mechanisms, which also included a mild pro-oxidant that, expectantly, triggered beneficial adaptations of the endogenous antioxidant systems.
- (iii)
- A clear protection of DNA integrity (potential to reduce DNA damage or to increase the resistance of DNA to routine challenges) was detected in the second month for all the inclusion levels, though the most prominent benefit was achieved for the highest level (10%). Again, complex genoprotective pathways were disclosed, involving an intermediary step (expressed in the first month) where a genotoxic trigger (regarded as a pro-genoprotective mechanism) was identified.
- (iv)
- The use of Salicornia-enriched formulations as complementary feeds with functional properties showed potential, but the choice of dose and supplementation time tended to be critical.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Fry, J.P.; Love, D.C.; MacDonald, G.K.; West, P.C.; Engstrom, P.M.; Nachman, K.E.; Lawrence, R.S. Environmental Health Impacts of Feeding Crops to Farmed Fish. Environ. Int. 2016, 91, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal Halophytes: Potent Source of Health Promoting Biomolecules with Medical, Nutraceutical and Food Applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef] [PubMed]
- Hulkko, L.S.S.; Chaturvedi, T.; Thomsen, M.H. Extraction and Quantification of Chlorophylls, Carotenoids, Phenolic Compounds, and Vitamins from Halophyte Biomasses. Appl. Sci. 2022, 12, 840. [Google Scholar] [CrossRef]
- Belal, I.E.H.; Al-Dosari, M. Replacement of Fish Meal with Salicornia Meal in Feeds for Nile Tilapia Oreochromis niloticus. J. World Aquac. Soc. 1999, 30, 285–289. [Google Scholar] [CrossRef]
- Ríos-Durán, M.G.; Valencia, I.R.; Ross, L.G.; Martínez-Palacios, C.A. Nutritional Evaluation of Autoclaved Salicornia bigelovii Torr. Seed Meal Supplemented with Varying Levels of Cholesterol on Growth, Nutrient Utilization and Survival of the Nile Tilapia (Oreochromis niloticus). Aquac. Int. 2013, 21, 1355–1371. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The Development of Halophyte-Based Agriculture: Past and Present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia Ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia Europaea l. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef] [PubMed]
- Custódio, M.; Lillebø, A.I.; Calado, R.; Villasante, S. Halophytes as Novel Marine Products—A Consumers’ Perspective in Portugal and Policy Implications. Mar. Policy 2021, 133, 104731. [Google Scholar] [CrossRef]
- Herman, E.M.; Schmidt, M.A. The Potential for Engineering Enhanced Functional-Feed Soybeans for Sustainable Aquaculture Feed. Front. Plant Sci. 2016, 7, 440. [Google Scholar] [CrossRef]
- Soto, J.O.; Paniagua-Michel, J.D.J.; Lopez, L.; Ochoa, L. Functional feeds in aquaculture. In Springer Handbook of Marine Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Guerreiro, I.; Magalhães, R.; Coutinho, F.; Couto, A.; Sousa, S.; Delerue-Matos, C.; Domingues, V.F.; Oliva-Teles, A.; Peres, H. Evaluation of the Seaweeds Chondrus Crispus and Ulva Lactuca as Functional Ingredients in Gilthead Seabream (Sparus aurata). J. Appl. Phycol. 2019, 31, 2115–2124. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant Enzyme Activities and Lipid Peroxidation in the Freshwater Cladoceran Daphnia Magna Exposed to Redox Cycling Compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 175–186. [Google Scholar] [CrossRef]
- Kovacic, P. Free Radicals in Biology and Medicine. J. Pharm. Sci. 1986, 75, 105–106. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally Induced Oxidative Stress in Aquatic Animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, P.; Larroquet, L.; Durand, T.; Oger, C.; Galano, J.M.; Rocher, A.; Vigor, C.; Antony Jesu Prabhu, P.; Véron, V.; Briens, M.; et al. Oxidative Stress and Antioxidant Response in Rainbow Trout Fry Exposed to Acute Hypoxia Is Affected by Selenium Nutrition of Parents and during First Exogenous Feeding. Free Radic. Biol. Med. 2020, 155, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Terova, G.; Makol, A.; Serradell, A.; Valdenegro-Vega, V.; Izquierdo, M.; Acosta, F.; Montero, D. Dietary Phytogenics and Galactomannan Oligosaccharides in Low Fish Meal and Fish Oil-Based Diets for European Sea Bass (Dicentrarchus labrax) Juveniles: Effects on Gill Structure and Health and Implications on Oxidative Stress Status. Front. Immunol. 2021, 12, 663106. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Yazlak, H.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Sahin, N. The Effect of Lycopene on Antioxidant Status in Rainbow Trout (Oncorhynchus mykiss) Reared under High Stocking Density. Aquaculture 2014, 418–419, 132–138. [Google Scholar] [CrossRef]
- Hisar, O.; Yanik, T.; Kocaman, E.M.; Arslan, M.; Slukvin, A.; Goncharova, R. Effects of Diludine Supplementation on Growth Performance, Liver Antioxidant Enzyme Activities and Muscular Trace Elements of Rainbow Trout (Oncorhynchus mykiss) Juveniles at a Low Water Temperature. Aquac. Nutr. 2012, 18, 211–219. [Google Scholar] [CrossRef]
- Marques, A.; Marçal, R.; Pereira, V.; Pereira, P.; Mieiro, C.; Guilherme, S.; Marques, C.; Santos, M.A.; Pereira, R.; Abreu, H.; et al. Macroalgae-Enriched Diet Protects Gilthead Seabream (Sparus aurata) against Erythrocyte Population Instability and Chromosomal Damage Induced by Aqua-Medicines. J. Appl. Phycol. 2020, 32, 1477–1493. [Google Scholar] [CrossRef]
- Cheng, C.H.; Liang, H.Y.; Luo, S.W.; Wang, A.L.; Ye, C.X. The Protective Effects of Vitamin C on Apoptosis, DNA Damage and Proteome of Pufferfish (Takifugu obscurus) under Low Temperature Stress. J. Therm. Biol. 2018, 71. [Google Scholar] [CrossRef]
- Directorate-General for Maritime Affairs and Fisheries. Case Study in the EU Seabass Maritime Affairs and Fisheries Price Structure in the Supply Chain for Seabass focus on Greece, Croatia and Spain. 2021. Available online: https://op.europa.eu/en/publication-detail/-/publication/4577254b-8251-11eb-9ac9-01aa75ed71a1/language-en (accessed on 21 February 2023).
- Messina, M.; Piccolo, G.; Tulli, F.; Messina, C.M.; Cardinaletti, G.; Tibaldi, E. Lipid Composition and Metabolism of European Sea Bass (Dicentrarchus labrax L.) Fed Diets Containing Wheat Gluten and Legume Meals as Substitutes for Fish Meal. Aquaculture 2013, 376–379, 6–14. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Covès, D.; Dutto, G.; Blanc, D. Almost Total Replacement of Fish Meal by Plant Protein Sources in the Diet of a Marine Teleost, the European Seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Gonçalves, P. Partial Replacement of Fishmeal by Brewers Yeast (Saccaromyces cerevisae) in Diets for Sea Bass (Dicentrarchus labrax) Juveniles. Aquaculture 2001, 202, 269–278. [Google Scholar] [CrossRef]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The Role of Currently Used Medicinal Plants in Aquaculture and Their Action Mechanisms: A Review. Rev. Aquac. 2022, 14, 816–847. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Importance of Prebiotics in Aquaculture as Immunostimulants. Effects on Immune System of Sparus aurata and Dicentrarchus labrax. Fish. Shellfish. Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Sousa, V.; Oliveira, B.; Canadas-Sousa, A.; Abreu, H.; Dias, J.; Kiron, V.; Valente, L.M.P. An In-Depth Characterisation of European Seabass Intestinal Segments for Assessing the Impact of an Algae-Based Functional Diet on Intestinal Health. Sci. Rep. 2023, 13, 11686. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, B.; Di Marco, P.; Zarantoniello, M.; Daniso, E.; Cerri, R.; Finoia, M.G.; Capoccioni, F.; Tibaldi, E.; Olivotto, I.; Cardinaletti, G. Effects of Supplementing a Plant Protein-Rich Diet with Insect, Crayfish or Microalgae Meals on Gilthead Sea Bream (Sparus aurata) and European Seabass (Dicentrarchus labrax) Growth, Physiological Status and Gut Health. Aquaculture 2023, 575, 739811. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Hand Book of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Differential Distribution of Glutathione and Glutathione-Related Enzymes in Rabbit Kidney. Possible Implications in Analgesic Nephropathy. Biochem. Pharmacol. 1984, 33, 1801–1807. [Google Scholar] [CrossRef]
- Athar, M.; Iqbal, M. Ferric Nitrilotriacetate Promotes N-Diethylnitrosamine-Induced Renal Tumorigenesis in the Rat: Implications for the Involvement of Oxidative Stress. Carcinogenesis 1998, 19, 1133–1139. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Cribb, A.E.; Leeder, J.; Spielberg, S.P. Use of a Microplate Reader in an Assay of Glutathione Reductase Using 5,5′-Dithiobis(2-Nitrobenzoic Acid). Anal. Biochem. 1989, 183, 195–196. [Google Scholar] [CrossRef]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter Plate Assay for the Measurement of Glutathione and Glutathione Disulfide in Large Numbers of Biological Samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Draper, H.H. Comparative Studies on Different Methods of Malonaldehyde Determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.W.; Torres, M.; Tribess, T.; Pedrosa, R.; Soares, C. Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz. J. Med Biol. Res. 2001, 34, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.W.; Tribess, T.; Gáspari, C.; Claudio, F.; Torres, M.; Magalhães, A. Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, S.; Gaivao, I.; Santos, M.A.; Pacheco, M. European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup(R)—A glyphosate-based herbicide. Mutagenesis 2010, 25, 523–530. [Google Scholar] [CrossRef]
- Shaposhnikov, S.; Azqueta, A.; Henriksson, S.; Meier, S.; Gaivão, I.; Huskisson, N.H.; Smart, A.; Brunborg, G.; Nilsson, M.; Collins, A.R. Twelve-gel slide format optimised for comet assay and fluorescent in situ hybridisation. Toxicol. Lett. 2010, 195, 31–34. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.-V. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar] [CrossRef]
- Kumaravel, T.S.; Vilhar, B.; Faux, S.P.; Jha, A.N. Comet Assay measurements: A perspective. Cell Biol. Toxicol. 2009, 25, 53–64. [Google Scholar] [CrossRef]
- Møller, P. The comet assay: Ready for 30 more years. Mutagenesis 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milić, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Pereira, C.C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for describing comet assay procedures and results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Santos, M.A. Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol. Environ. Saf. 2002, 53, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Maceda-Veiga, A.; Monroy, M.; Viscor, G.; De Sostoa, A. Changes in non-specific biomarkers in the Mediterranean barbel (Barbus meridionalis) exposed to sewage effluents in a Mediterranean stream (Catalonia, NE Spain). Aquat. Toxicol. 2010, 100, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.; Mieiro, C.; Coelho, J.; Guilherme, S.; Marques, A.; Santos, M.; Duarte, A.; Pereira, E.; Pacheco, M. Addressing the impact of mercury estuarine contamination in the European eel (Anguilla anguilla L., 1758)—An early diagnosis in glass eel stage based on erythrocytic nuclear morphology. Mar. Pollut. Bull. 2018, 127, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zar, J. Multiple comparisons. In Biostatistical Analysis; Prentice Hall International Inc.: Upper Saddle River, NJ, USA, 2010; pp. 226–248. [Google Scholar]
- Zarantoniello, M.; Randazzo, B.; Secci, G.; Notarstefano, V.; Giorgini, E.; Lock, E.; Parisi, G.; Olivotto, I. Application of laboratory methods for understanding fish responses to black soldier fly (Hermetia illucens) based diets. J. Insects Food Feed. 2022, 8, 1173–1195. [Google Scholar] [CrossRef]
- Pereira, V.; Marques, A.; Gaivão, I.; Rego, A.; Abreu, H.; Pereira, R.; Santos, M.A.; Guilherme, S.; Pacheco, M. Marine macroalgae as a dietary source of genoprotection in gilthead seabream (Sparus aurata) against endogenous and exogenous challenges. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 12–24. [Google Scholar] [CrossRef]
- Marques, A.; Ferreira, J.; Abreu, H.; Pereira, R.; Pinto, D.; Silva, A.; Gaivão, I.; Pacheco, M. Comparative genoprotection ability of wild-harvested vs. aqua-cultured Ulva rigida coupled with phytochemical profiling. Eur. J. Phycol. 2021, 56, 105–118. [Google Scholar] [CrossRef]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients? A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Troell, M.; Banks, L.K.; Belton, B.; Beveridge, M.C.M.; Klinger, D.H.; Pelletier, N.; Phillips, M.J.; Tran, N. Interventions for improving the productivity and environmental performance of global aquaculture for future food security. One Earth 2021, 4, 1220–1232. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M.F.; Eltholth, M.M.; Auchterlonie, N.A.; Little, D.C.; Harmsen, R.; Newton, R.W.; Davies, S.J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Salas-Leitón, E.; Pereira, L.F.; Queiroz, A.; Magalhães, F.; Pereira, R.; Abreu, H.; Reis, P.A.; Gonçalves, J.F.M.; Ozório, R.O.d.A. Role of dietary seaweed supplementation on growth performance, digestive capacity and immune and stress responsiveness in European seabass (Dicentrarchus labrax). Aquac. Rep. 2016, 3, 189–197. [Google Scholar] [CrossRef]
- Torrecillas, S.; Mompel, D.; Caballero, M.; Montero, D.; Merrifield, D.; Rodiles, A.; Robaina, L.; Zamorano, M.; Karalazos, V.; Kaushik, S.; et al. Effect of fishmeal and fish oil replacement by vegetable meals and oils on gut health of European sea bass (Dicentrarchus labrax). Aquaculture 2017, 468, 386–398. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Feneuil, A.; Wagner, A.; Respondek, F. Use of vital wheat gluten in aquaculture feeds. Aquat. Biosyst. 2013, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; Koubaier, H.B.H.; Casabianca, H.; Abe, N.; El Omri, A.; Chaabouni, M.M.; Bouzouita, N. Phytochemical investigation of Tunisian Salicornia herbacea L., antioxidant, antimicrobial and cytochrome P450 (CYPs) inhibitory activities of its methanol extract. Food Control. 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2012, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Li, Z.-H.; Silovska, S.; Turek, J. Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in rainbow trout. Aquaculture 2011, 310, 369–375. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J.; Andriichuk, A. Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol. Biochem. 2014, 40, 1289–1300. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Dolbeth, M.; Marques, T.A.; Duarte, A.C.; Pereira, M.E.; Pacheco, M. Mercury accumulation and tissue-specific antioxidant efficiency in the wild European sea bass (Dicentrarchus labrax) with emphasis on seasonality. Environ. Sci. Pollut. Res. 2014, 21, 10638–10651. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; Jiang, W.-D.; Jiang, J.; Zhao, J.; Zhang, Y.-A.; Zhou, X.-Q.; Feng, L. A Comparative Study on Antioxidant System in Fish Hepatopancreas and Intestine Affected by Choline Deficiency: Different Change Patterns of Varied Antioxidant Enzyme Genes and Nrf2 Signaling Factors. PLoS ONE 2017, 12, e0169888. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free. Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Cheng, A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006, 29, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.-C.; Duh, P.-D.; Tsai, H.-L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C exhibits pro-oxidant properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Ahmad, I.; Pacheco, M.; Santos, M.A. Anguilla anguilla L. oxidative stress biomarkers: An in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere 2006, 65, 952–962. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gatlin, D.M. Dietary Vitamin C and Vitamin E Interact to Influence Growth and Tissue Composition of Juvenile Hybrid Striped Bass (Morone chrysops ♀ × M. saxatilis ♂) but Have Limited Effects on Immune Responses. J. Nutr. 2002, 132, 748–755. [Google Scholar] [CrossRef]
- Reiser, S.; Wuertz, S.; Schroeder, J.; Kloas, W.; Hanel, R. Risks of seawater ozonation in recirculation aquaculture—Effects of oxidative stress on animal welfare of juvenile turbot (Psetta maxima, L.). Aquat. Toxicol. 2011, 105, 508–517. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Mieiro, C.L.; Ahmad, I.; Pereira, M.E.; Duarte, A.C.; Pacheco, M. Antioxidant system breakdown in brain of feral golden grey mullet (Liza aurata) as an effect of mercury exposure. Ecotoxicology 2010, 19, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ho, C.K.; Siu-Wai, C.; Siu, P.M.; Benzie, I.F. Genoprotection and genotoxicity of green tea (Camellia sinensis): Are they two sides of the same redox coin? Redox Rep. 2013, 18, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Vega, J.A.; Morales-González, J.A.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Sosa-Delgado, S.M.; Sumaya-Martínez, M.T.; Morales-González, Á.; Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 1: Fruits and Polysaccharides. Nutrients 2017, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillán, E.; Fragoso-Antonio, S.; Valadez-Vega, C.; Solano-Solano, G.; Pérez, C.Z.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Gutiérrez-Salinas, J.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Investigation on the Protective Effects of Cranberry Against the DNA Damage Induced by Benzo[a]pyrene. Molecules 2012, 17, 4435–4451. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.B.C.R.; dos Santos, R.S.; Calil, S.d.S.; Niero, R.; Lopes, J.d.S.; Perazzo, F.F.; Rosa, P.C.P.; Andrade, S.F.; Cechinel-Filho, V.; Maistro, E.L. Genotoxic assessment of Rubus imperialis (Rosaceae) extract in vivo and its potential chemoprevention against cyclophosphamide-induced DNA damage. J. Ethnopharmacol. 2014, 153, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Collins, A. Polyphenols and DNA Damage: A Mixed Blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Leandro, L.F.; Munari, C.C.; Sato, V.L.F.L.; Alves, J.M.; de Oliveira, P.F.; Mastrocola, D.F.P.; Martins, S.D.P.L.; da Silva Moraes, T.; de Oliveira, A.I.; Tozatti, M.G. Assessment of the genotoxicity and antigenotoxicity of (+)-usnic acid in V79 cells and Swiss mice by the micronucleus and comet assays. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 753, 101–106. [Google Scholar] [CrossRef]
- Marques, A.; Ferreira, J.; Cerqueda-Pacheco, A.; Pereira, V.; Abreu, H.; Pereira, R.; Pires, M.J.; Seixas, F.; Oliveira, P.; Gaivão, I.; et al. Genoprotection and metabolic benefits of marine macroalgae—Insights into the concept of functional foods through direct and indirect consumption. Food Biosci. 2022, 47. [Google Scholar] [CrossRef]
- El-Bibany, A.H.; Bodnar, A.G.; Reinardy, H.C. Comparative DNA Damage and Repair in Echinoderm Coelomocytes Exposed to Genotoxicants. PLoS ONE 2014, 9, e107815. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, L.; Bounous, D.I.; Elmore, S.A. Chapter 50—Hematopoietic system. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]


| Ingredients (%) | Diets | |||
|---|---|---|---|---|
| C | S2.5 | S5 | S10 | |
| Fish meal LT70 1 | 35.0 | 35.0 | 35.0 | 35.0 |
| Kill meal 2 | 5.0 | 5.0 | 5.0 | 5.0 |
| Soy protein concentrate 3 | 13.0 | 13.0 | 13.0 | 13.0 |
| Wheat gluten 4 | 10.0 | 10.1 | 10.1 | 10.3 |
| Corn gluten meal 5 | 8.0 | 8.0 | 8.0 | 8.0 |
| Wheat meal 6 | 16.3 | 13.7 | 11.2 | 6.0 |
| Vitamin and mineral premix 7 | 1.0 | 1.0 | 1.0 | 1.0 |
| Monocalcium phosphate 8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Fish oil 9 | 5.2 | 5.2 | 5.2 | 5.2 |
| Rapeseed oil 10 | 5.7 | 5.7 | 5.7 | 5.7 |
| Salicomia | - | 2.5 | 5.0 | 10.0 |
| % of Dry Matter | Diets | |||
|---|---|---|---|---|
| C | S2.5 | S5 | S10 | |
| Dry matter | 94.1 | 96.5 | 95.5 | 94.5 |
| Crude protein | 52.4 | 51.4 | 53.7 | 52.1 |
| Crude lipids | 16.8 | 16.8 | 17.3 | 17.3 |
| Ash | 9.0 | 9.8 | 10.5 | 11.3 |
| Energy (KJ g−1 DM) | 23.4 | 23.1 | 23.3 | 22.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marçal, R.; Sousa, P.; Marques, A.; Pereira, V.; Guilherme, S.; Barreto, A.; Costas, B.; Rocha, R.J.M.; Pacheco, M. Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax). Animals 2024, 14, 93. https://doi.org/10.3390/ani14010093
Marçal R, Sousa P, Marques A, Pereira V, Guilherme S, Barreto A, Costas B, Rocha RJM, Pacheco M. Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax). Animals. 2024; 14(1):93. https://doi.org/10.3390/ani14010093
Chicago/Turabian StyleMarçal, Raquel, Pedro Sousa, Ana Marques, Vitória Pereira, Sofia Guilherme, André Barreto, Benjamin Costas, Rui J. M. Rocha, and Mário Pacheco. 2024. "Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax)" Animals 14, no. 1: 93. https://doi.org/10.3390/ani14010093
APA StyleMarçal, R., Sousa, P., Marques, A., Pereira, V., Guilherme, S., Barreto, A., Costas, B., Rocha, R. J. M., & Pacheco, M. (2024). Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax). Animals, 14(1), 93. https://doi.org/10.3390/ani14010093








