The Postictal Phase in Canine Idiopathic Epilepsy: Semiology, Management, and Impact on the Quality of Life from the Owners’ Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hülsmeyer, V.I.; Fischer, A.; Mandigers, P.J.; DeRisio, L.; Berendt, M.; Rusbridge, C.; Bhatti, S.F.; Pakozdy, A.; Patterson, E.E.; Platt, S.; et al. International Veterinary Epilepsy Task Force’s current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet. Res. 2015, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Kearsley-Fleet, L.; O’Neill, D.G.; Volk, H.A.; Church, D.B.; Brodbelt, D.C. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet. Rec. 2013, 172, 338. [Google Scholar] [CrossRef] [PubMed]
- Erlen, A.; Potschka, H.; Volk, H.A.; Sauter-Louis, C.; O’Neill, D.G. Seizure occurrence in dogs under primary veterinary care in the UK: Prevalence and risk factors. J. Vet. Intern. Med. 2018, 32, 1665–1676. [Google Scholar] [CrossRef]
- Casal, M.L.; Munuve, R.M.; Janis, M.A.; Werner, P.; Henthorn, P.S. Epilepsy in Irish Wolfhounds. J. Vet. Intern. Med. 2006, 20, 131–135. [Google Scholar] [CrossRef]
- Zimmermann, R.; Hülsmeyer, V.; Sauter-Louis, C.; Fischer, A. Status epilepticus and epileptic seizures in dogs. J Vet Intern Med 2009, 23, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Berendt, M.; Farquhar, R.G.; Mandigers, P.J.; Pakozdy, A.; Bhatti, S.F.; De Risio, L.; Fischer, A.; Long, S.; Matiasek, K.; Munana, K.; et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet. Res. 2015, 11, 182. [Google Scholar] [CrossRef]
- Pottkämper, J.C.; Hofmeijer, J.; van Waarde, J.A.; van Putten, M.J. The postictal state—What do we know? Epilepsia 2020, 61, 1045–1061. [Google Scholar] [CrossRef]
- Subota, A.; Khan, S.; Josephson, C.B.; Manji, S.; Lukmanji, S.; Roach, P.; Wiebe, S.; Buchhalter, J.; Federico, P.; Teskey, G.C.; et al. Signs and symptoms of the postictal period in epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2019, 94, 243–251. [Google Scholar] [CrossRef]
- Farrell, J.S.; Gaxiola-Valdez, I.; Wolff, M.D.; David, L.S.; Dika, H.I.; Geeraert, B.L.; Rachel Wang, X.; Singh, S.; Spanswick, S.C.; Dunn, J.F.; et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. eLife 2016, 5, e19352. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Volk, H.A. Epilepsy beyond seizures: A review of the impact of epilepsy and its comorbidities on health-related quality of life in dogs. Vet. Rec. 2015, 177, 306–315. [Google Scholar] [CrossRef]
- Shihab, N.; Bowen, J.; Volk, H.A. Behavioral changes in dogs associated with the development of idiopathic epilepsy. Epilepsy Behav. 2011, 21, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jaggy, A.; Bernardini, M. Idiopathic epilepsy in 125 dogs: A long-term study. Clinical and electroencephalographic findings. J. Small Anim. Pract. 1998, 39, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Schachter, S.C. The Postictal State: A Neglected Entity in the Management of Epilepsy. Epilepsy Behav. 2000, 1, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.F.M.; De Risio, L.; Muñana, K.; Penderis, J.; Stein, V.M.; Tipold, A.; Berendt, M.; Farquhar, R.G.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Vet. Res. 2015, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Podell, M.; Volk, H.A.; Berendt, M.; Loscher, W.; Munana, K.; Patterson, E.E.; Platt, S.R. 2015 ACVIM Small Animal Consensus Statement on Seizure Management in Dogs. J. Vet. Intern. Med. 2016, 30, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Wessmann, A.; Volk, H.A.; Parkin, T.; Ortega, M.; Anderson, T.J. Evaluation of Quality of Life in Dogs with Idiopathic Epilepsy. J. Vet. Intern Med. 2014, 28, 510–514. [Google Scholar] [CrossRef]
- D’Silva, J.; Turner, J. Animals, Ethics and Trade: The Challenge of Animal Sentience; Routledge: London, UK, 2012. [Google Scholar]
- Jones, R.C. Science, sentience, and animal welfare. Biol. Philos. 2013, 28, 1–30. [Google Scholar] [CrossRef]
- Proctor, H. Animal sentience: Where are we and where are we heading? Animals 2012, 2, 628–639. [Google Scholar] [CrossRef]
- Ducoté, J.M. Common Neurologic Problems: Impact on Patient Welfare, Caregiver Burden and Veterinarian Wellbeing. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Packer, R.; Volk, H.; Fowkes, R. Physiological reactivity to spontaneously occurring seizure activity in dogs with epilepsy and their carers. Physiol. Behav. 2017, 177, 27–33. [Google Scholar] [CrossRef]
- Christiansen, S.B.; Kristensen, A.T.; Sandøe, P.; Lassen, J. Looking After Chronically III Dogs: Impacts on the Caregiver’s Life. Anthrozoös 2013, 26, 519–533. [Google Scholar] [CrossRef]
- Pergande, A.E.; Belshaw, Z.; Volk, H.A.; Packer, R.M. “We have a ticking time bomb”: A qualitative exploration of the impact of canine epilepsy on dog owners living in England. BMC Vet. Res. 2020, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.C.; Volk, H.A.; Packer, R.M.A. Research priorities for idiopathic epilepsy in dogs: Viewpoints of owners, general practice veterinarians, and neurology specialists. J. Vet. Intern. Med. 2021, 35, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Kähn, C.S.; Bhatti, S.F.; Meller, S.; Meyerhoff, N.; Volk, H.A.; Charalambous, M. Out-of-Hospital Rescue Medication in Dogs with Emergency Seizure Disorders: An Owner Perspective. Front. Vet. Sci. 2023, 10, 1278618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Galardi, M.M.; Pok, B.; Patel, K.K.; Zhao, C.W.; Andrews, J.P.; Singla, S.; McCafferty, C.P.; Feng, L.; Musonza, E.T.; et al. Thalamic Stimulation Improves Postictal Cortical Arousal and Behavior. J. Neurosci. 2020, 40, 7343–7354. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Farooq, F.; Shagufta, S.; Khan, A.M.; Masood, Y.; Saeed, H. Postictal Mania Versus Postictal Psychosis. Cureus 2018, 10, e3338. [Google Scholar] [CrossRef]
- Fischer, A.J.K.; Potschka, H.; Rentmeister, K.; Tipold, A.; Volk, H.A.; von Klopmann, T. Die Idiopathische Epilepsie des Hundes; Enke Verlag: Stuttgard, Germany, 2013; p. 120. [Google Scholar]
- Stoffel, M. Funktionelle Neuroanatomie Für Die Tiermedizin; Georg Thieme Verlag: Stuttgard, Germany, 2021; p. 246. [Google Scholar]
- Shahisavandi, M.; Zeraatpisheh, Z.; Rostaminejad, M.; Asadi-Pooya, A.A. Treatment of postictal headache: A systematic review and future directions. Epilepsy Behav 2021, 119, 107971. [Google Scholar] [CrossRef]
- Ngo, V.T.H.; Camacho, H.; Singh, J. You Are The One Who Was Beating Me”: A Case Report of a Patient with Postictal Psychosis Secondary to Bilateral Temporal Lobe Epilepsy. Cureus 2019, 11, e6069. [Google Scholar] [CrossRef]
- Vonck, K.; Raedt, R.; Boon, P. Vagus nerve stimulation and the postictal state. Epilepsy Behav. 2010, 19, 182–185. [Google Scholar] [CrossRef]
- Muñana, K.R.; Vitek, S.M.; Tarver, W.B.; Saito, M.; Skeen, T.M.; Sharp, N.J.; Olby, N.J.; Haglund, M.M. Use of vagal nerve stimulation as a treatment for refractory epilepsy in dogs. J. Am. Vet. Med. Assoc. 2002, 221, 977–983. [Google Scholar] [CrossRef]
- Harcourt-Brown, T.R.; Carter, M. Implantable vagus nerve stimulator settings and short-term adverse effects in epileptic dogs. J. Vet. Intern. Med. 2021, 35, 2350–2358. [Google Scholar] [CrossRef]
- Hirashima, J.; Saito, M.; Igarashi, H.; Takagi, S.; Hasegawa, D. Case Report: 1-Year Follow-Up of Vagus Nerve Stimulation in a Dog With Drug-Resistant Epilepsy. Front. Vet. Sci. 2021, 8, 708407. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Platt, S.; Stewart, G.; Reno, L.; Barber, R.; Boozer, L. Feasibility of Non-Invasive Vagus Nerve Stimulation (gammaCore VET™) for the Treatment of Refractory Seizure Activity in Dogs. Front. Vet. Sci. 2020, 7, 569739. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.; Meller, S.; Kajin, F.; Sermon, J.J.; Toth, R.; Benjaber, M.; Dijk, D.J.; Bogacz, R.; Worrell, G.A.; Valentin, A.; et al. Case Report: Embedding “Digital Chronotherapy” Into Medical Devices-A Canine Validation for Controlling Status Epilepticus Through Multi-Scale Rhythmic Brain Stimulation. Front. Neurosci. 2021, 15, 734265. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, M.; Van Ham, L.; Broeckx, B.J.G.; Roggeman, T.; Carrette, S.; Vonck, K.; Kervel, R.R.; Cornelis, I.; Bhatti, S.F.M. Repetitive transcranial magnetic stimulation in drug-resistant idiopathic epilepsy of dogs: A noninvasive neurostimulation technique. J. Vet. Intern. Med. 2020, 34, 2555–2561. [Google Scholar] [CrossRef]
- Hobbs, S.L.; Blackwell, E.J.; Wetz, K.E.; Packer, R.M.A. Owner reported management of interictal anxiety behaviours in canine epilepsy. Vet. Rec. 2022, 190, e1321. [Google Scholar] [CrossRef]


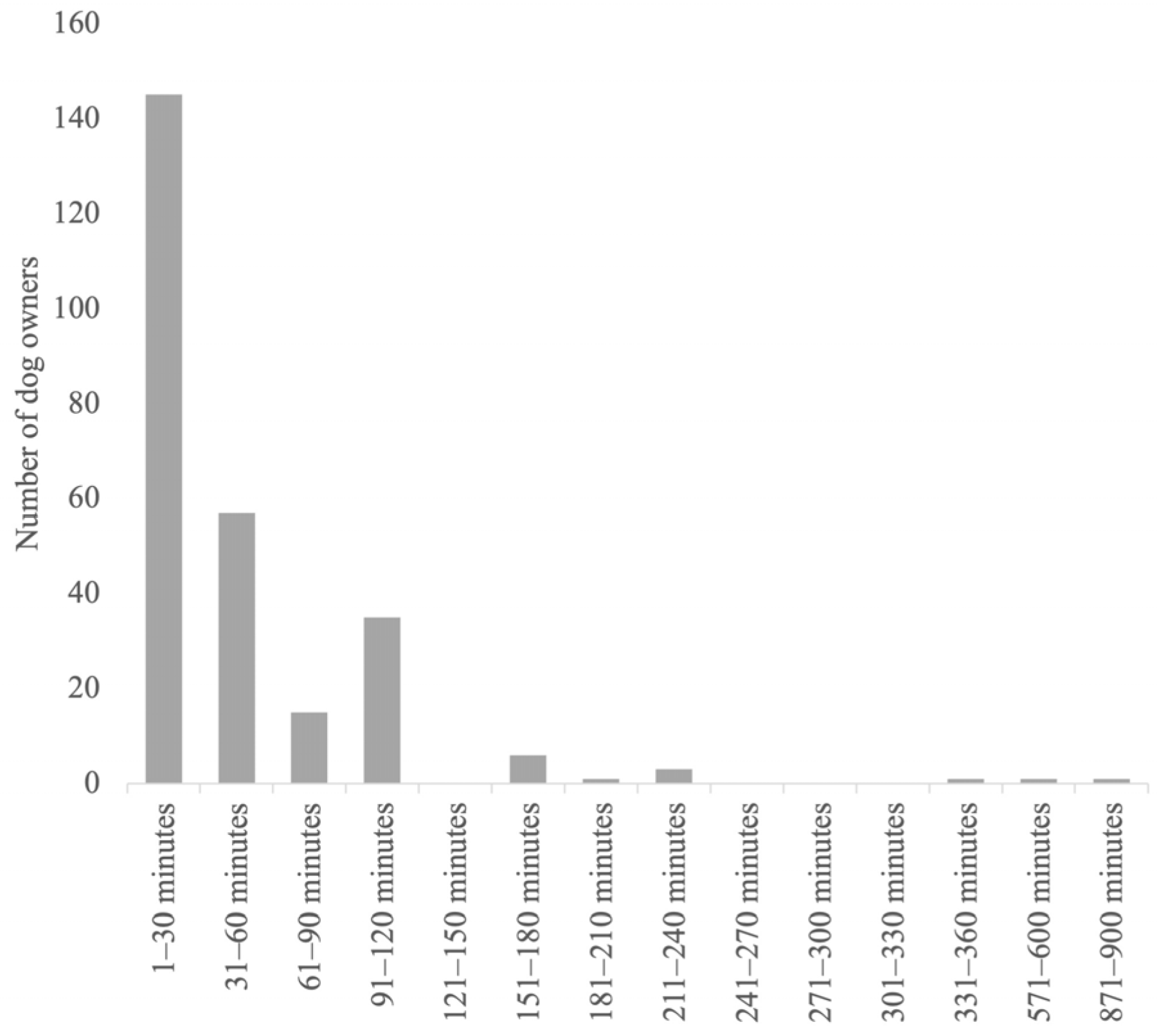
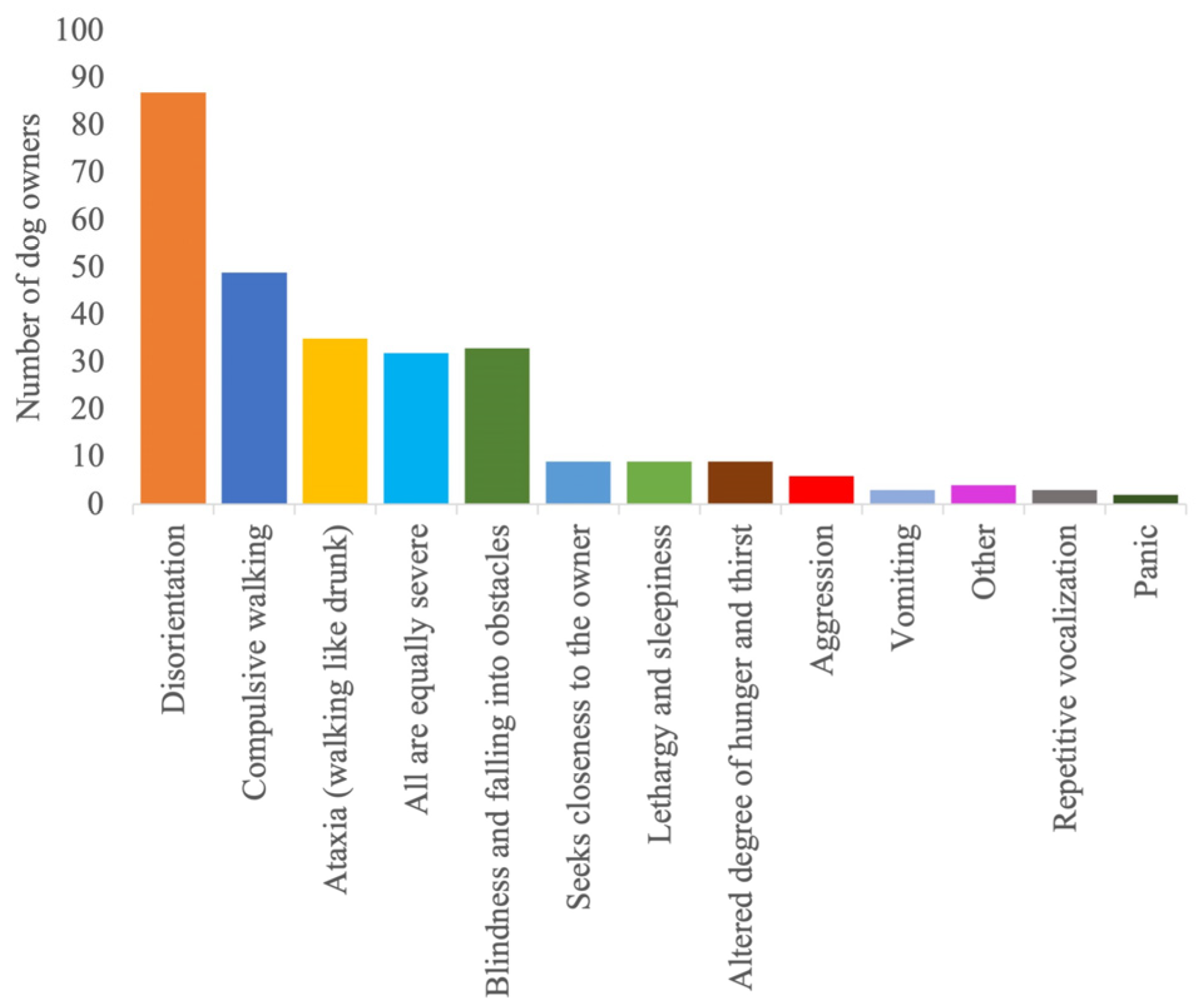
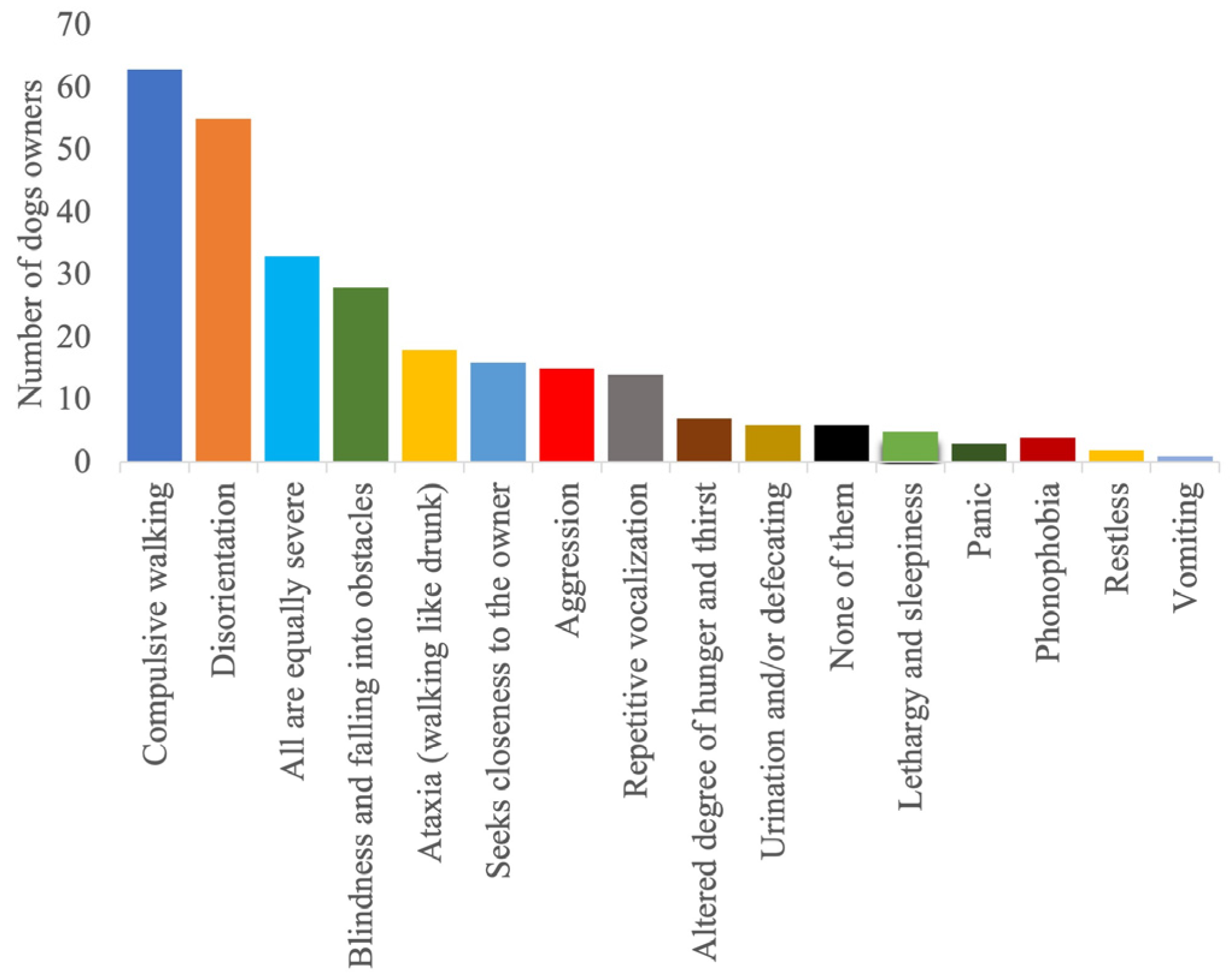
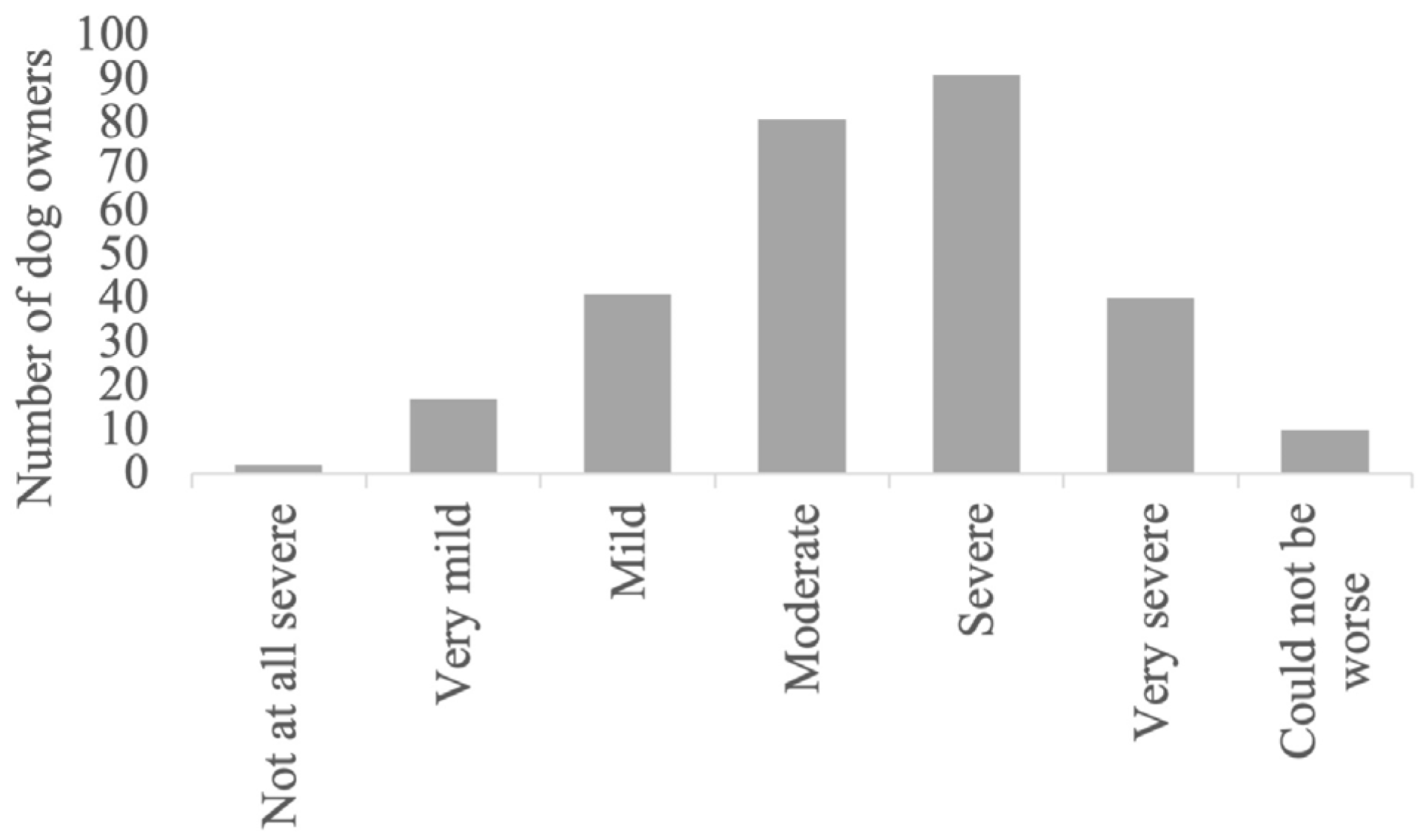
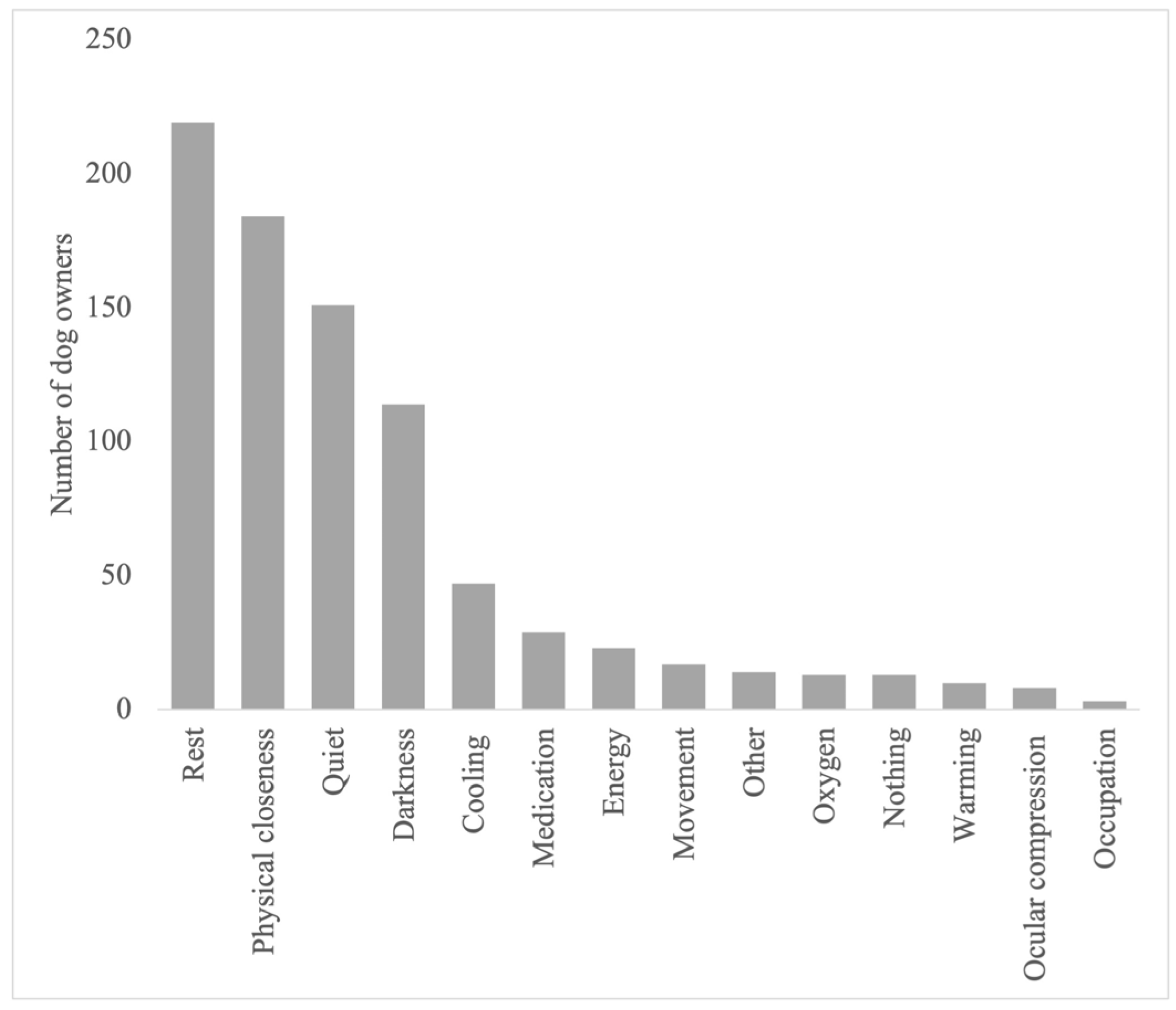
| Country | Germany 65% (190/292); UK 13% (37/292); USA 12% (35/292); Austria 1% (4/292); Belgium 1% (2/292); Other 8% (24/292) |
| Breed | Crossbreed 23% (67/292); Border Collie 8% (24/292); Labrador Retriever 8% (23/292); Australian Shepherd 7% (20/292); Golden Retriever 3% (9/292); Collie 3% (8/292); Belgian Shepherd 2% (5/292); German Shepherd 1% (3/292); English Springer Spaniel 1% (4/292); Other 44% (129/292) |
| Sex | Male-neutered 34% (98/292); Entire-male 27% (80/292); Female-neutered 27% (79/292); Entire-female 12% (35/292) |
| Age | 5.8 years (mean age); 8 years (median); 0–16 (range) |
| Diagnostics | History, signalment, and minimal blood database; urine examination 100% (292/292; Tier I: 100%); Aforementioned examinations plus MRI or CT and CSF 10% (29/292; Tier II: 10%); Aforementioned examinations plus EEG 1.4% (4/292 Tier III: 1.4%) |
| Clinical manifestation of seizures | Generalised 75% (219/292); Focal with secondary generalisation 14% (41/292); Focal 11% (31/292); I do not know 0.5% (1/292) |
| Length of the most common type of seizures | 1–2 min 56% (164/292) 3–5 min 20% (59/292) under 1 min 17% (51/292) more than 5 min 6% (18/292) |
| Approximate monthly number of seizures | 1 = 40% (116/292); Between 1 and 5 = 47% (138/292); Between 6 and 10 = 6% (17/292); Between 11 and 20 = 1% (4/292); >20 = 1% (3/292); I do not know 4% (13/292) |
| Incidence of cluster seizures | Not monthly but more than once per year 31% (91/292); Never 28% (81/292); Once per month 13% (38/292); More than once per month 12% (34/292); Once per year 7% (21/292); I do not know/not applicable 9% (27/292) |
| Incidence of status epilepticus | Never 61% (179/292); Not monthly but more than once per year 11% (32/292); Once per year 8% (23/292); More than once per month 6% (17/292); I do not know/not applicable 11% (31/292); Once per month 3% (10/292) |
| Long-term medication | Phenobarbital 81% (236/292); Levetiracetam 35% (102/292); Potassium bromide 31% (91/292); Imepitoin 14% (42/292); Nothing 9% (25/292); Zonisamide 5% (16/292); Pregabalin 3% (8/292); Gabapentin 2% (5/292); Felbamate 1% (2/292) |
| Rescue medication | Yes 71% (207/292); No 24% (71/292); No answer/not applicable 5% (14/292) |
| Rescue medication product | Diazepam 67% (139/207); Midazolam 15% (32/207); Levetiracetam 8% (16/207); Other 10% (20/207) |
| Rescue medication route | Rectal 64% (133/207); Intranasal 14% (30/207); Oral 14% (29/207); Other 6% (12/207); Buccal 1% (2/207); Sublingual 0.5% (1/207) |
| Positive effects of antiseizure medication on the postictal signs | Yes 52% (148/282); No 48% (134/282) |
| Antiseizure medications with the strongest effect on postictal signs | Phenobarbital 57% (85/148); Potassium bromide 21% (31/148); Levetiracetam 17% (25/148); Imepitoin 7% (10/148); Zonisamide 1% (2/148); Pregabalin 1% (1/148); Gabapentin 1% (1/148), Phenytoin 1% (1/148); Other 3% (4/148) |
| Positive effects of rescue medication on the postictal signs | No 71% (201/282); yes 29% (81/282) |
| Rescue medications with the strongest effect on postictal signs | Rectal diazepam 57% (46/81); Oral levetiracetam 20% (16/81); Intranasal midazolam 17% (14/81); Other 6% (5/81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kähn, C.; Meyerhoff, N.; Meller, S.; Nessler, J.N.; Volk, H.A.; Charalambous, M. The Postictal Phase in Canine Idiopathic Epilepsy: Semiology, Management, and Impact on the Quality of Life from the Owners’ Perspective. Animals 2024, 14, 103. https://doi.org/10.3390/ani14010103
Kähn C, Meyerhoff N, Meller S, Nessler JN, Volk HA, Charalambous M. The Postictal Phase in Canine Idiopathic Epilepsy: Semiology, Management, and Impact on the Quality of Life from the Owners’ Perspective. Animals. 2024; 14(1):103. https://doi.org/10.3390/ani14010103
Chicago/Turabian StyleKähn, Charlotte, Nina Meyerhoff, Sebastian Meller, Jasmin N. Nessler, Holger A. Volk, and Marios Charalambous. 2024. "The Postictal Phase in Canine Idiopathic Epilepsy: Semiology, Management, and Impact on the Quality of Life from the Owners’ Perspective" Animals 14, no. 1: 103. https://doi.org/10.3390/ani14010103
APA StyleKähn, C., Meyerhoff, N., Meller, S., Nessler, J. N., Volk, H. A., & Charalambous, M. (2024). The Postictal Phase in Canine Idiopathic Epilepsy: Semiology, Management, and Impact on the Quality of Life from the Owners’ Perspective. Animals, 14(1), 103. https://doi.org/10.3390/ani14010103





