Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor
Abstract
Simple Summary
Abstract
1. Introduction
2. Factors Affecting the Vitality of the Newborn
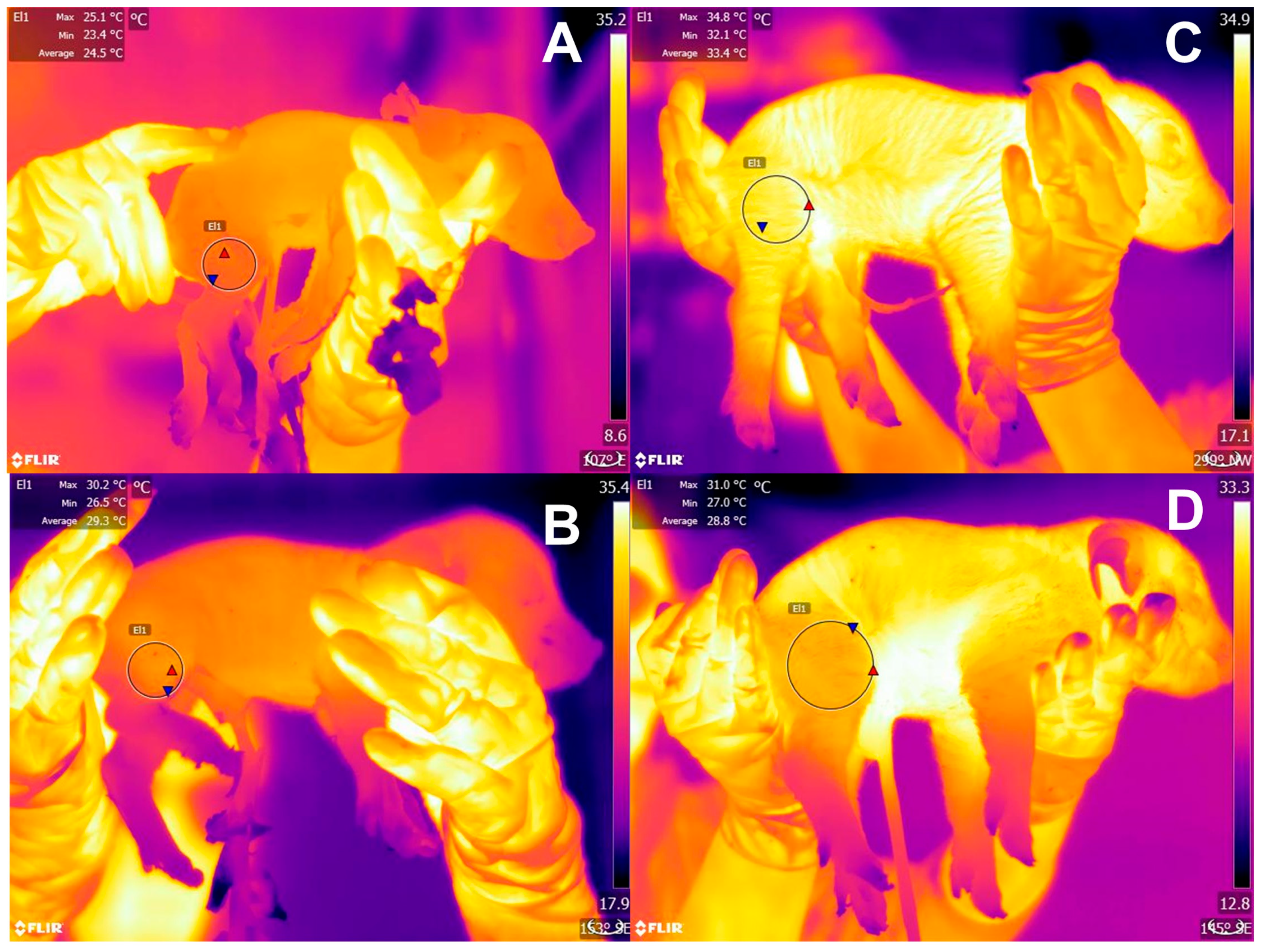
2.1. Hypothermia
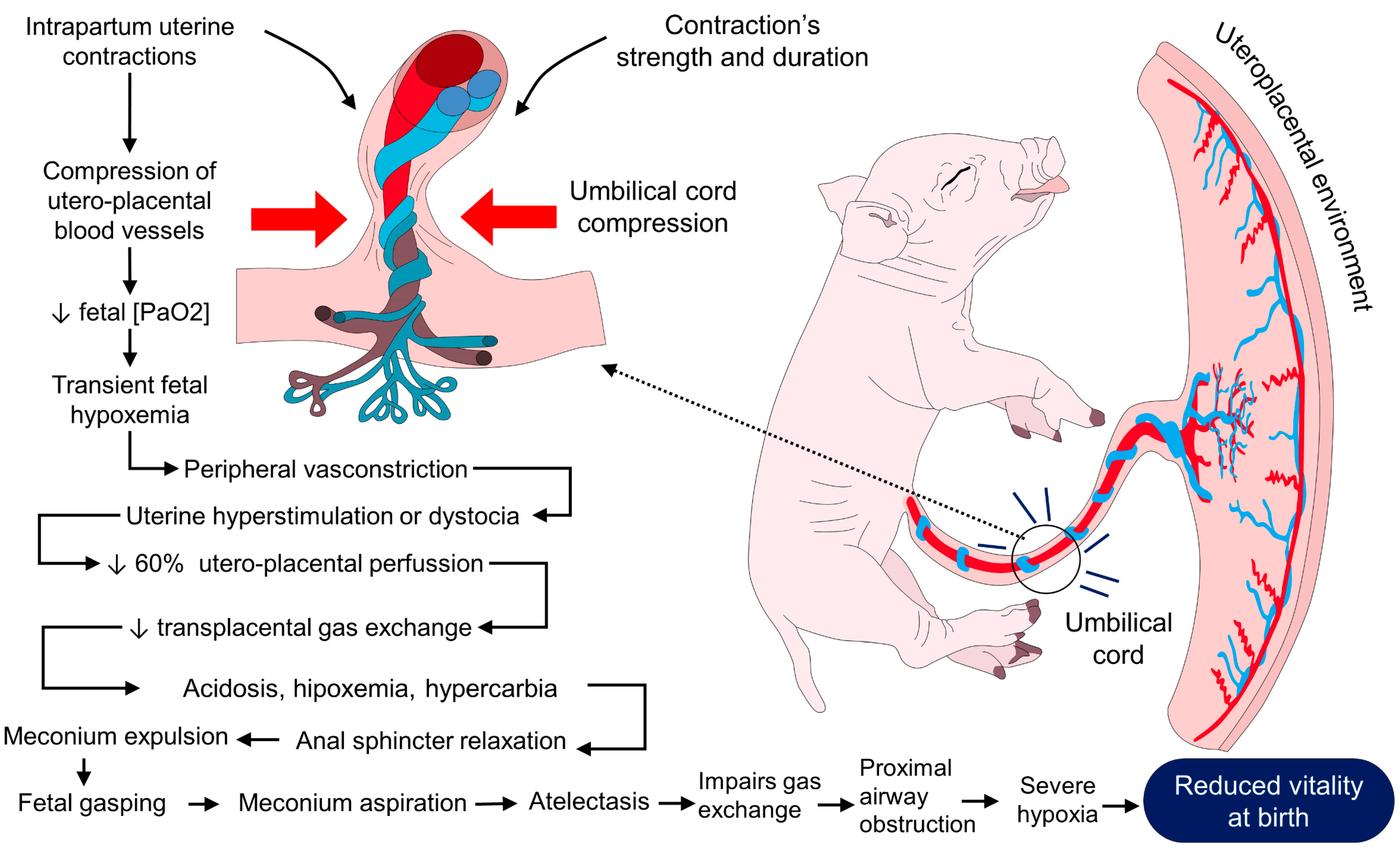
2.2. Hypoxia
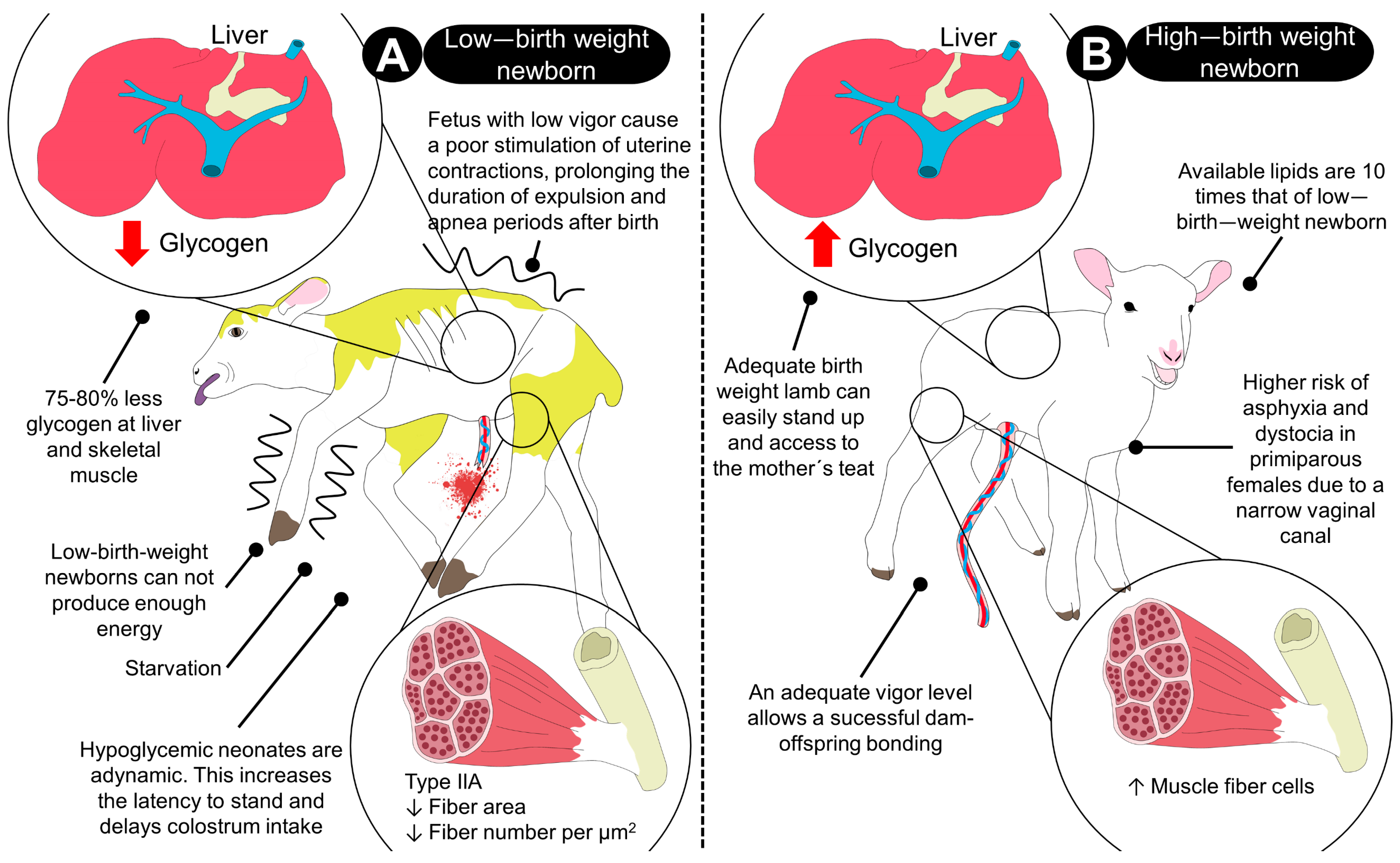
2.3. Birth Weight
2.4. Glycogen Depletion
2.5. Neurodevelopment
2.6. Dystocia
3. Physiological Mechanisms of the Newborn to Achieve Thermostability and Improve Vitality
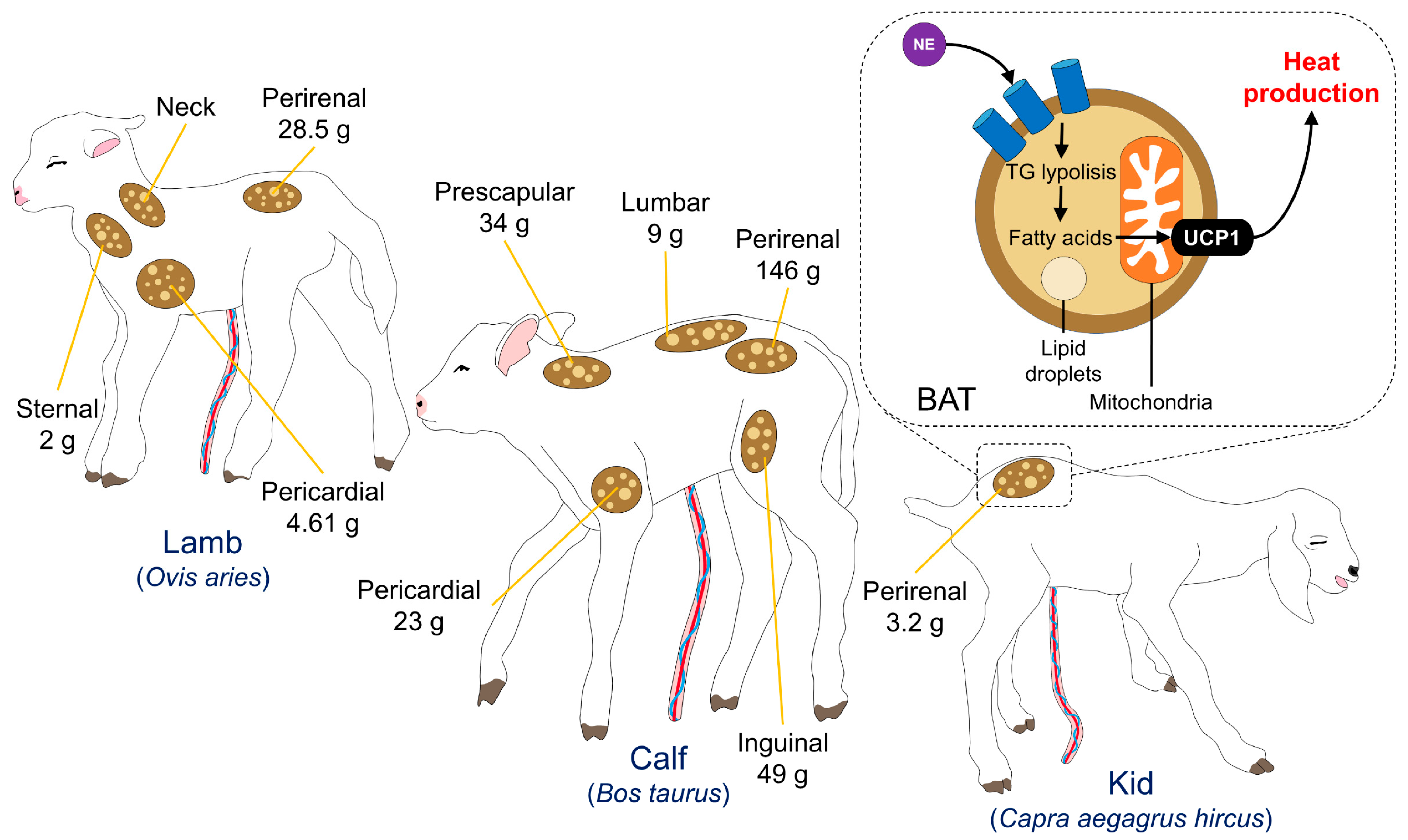
3.1. Brown Adipose Tissue
3.2. Shivering
3.3. Vasomotor Control
3.4. Postural and Behavioral Changes
4. Therapies and Methods Applied in Neonates to Promote Vitality
4.1. Pharmacologic Therapies
4.1.1. Energetic Supplements: Dextrose and Colostrum
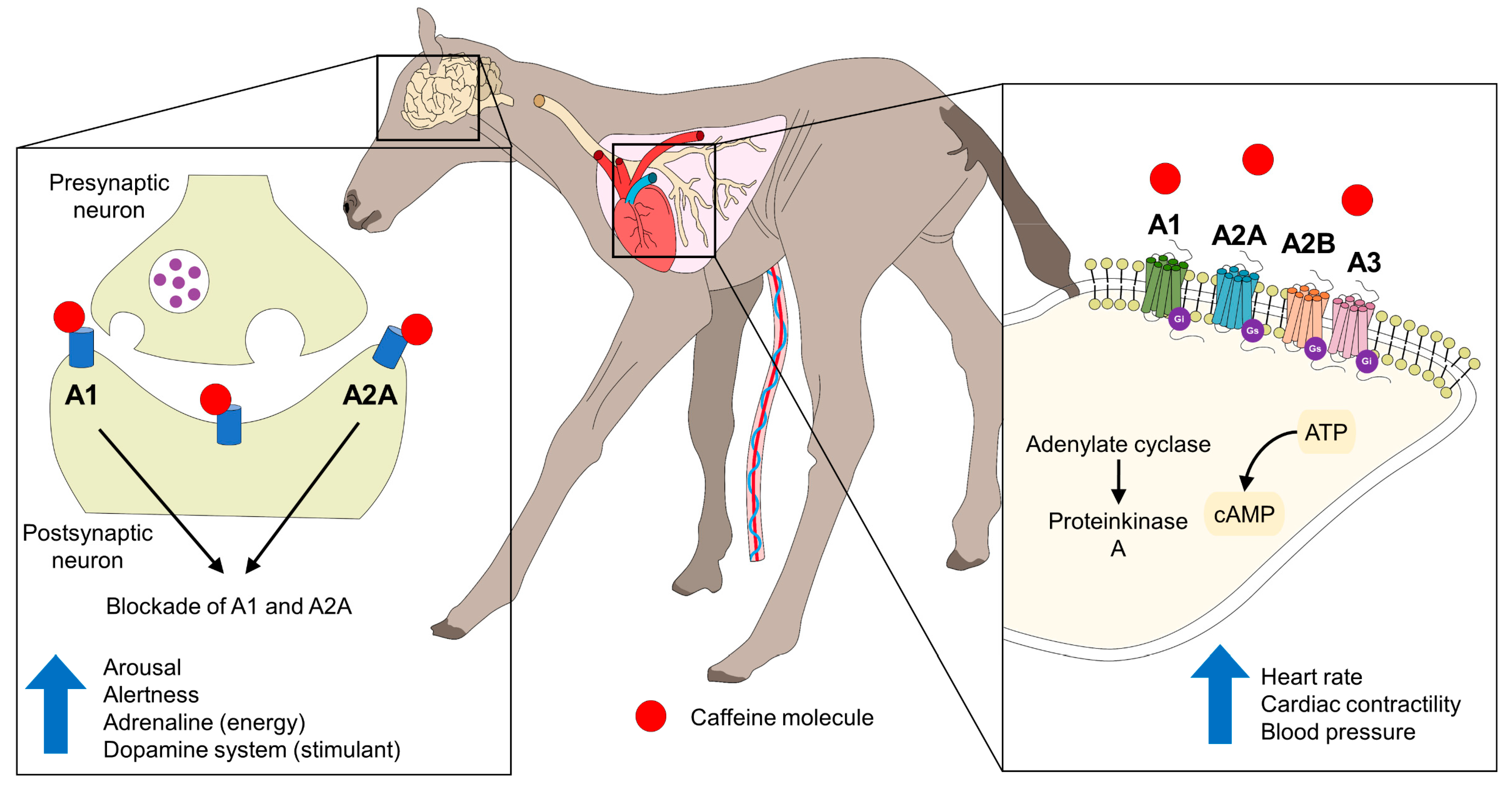
4.1.2. Caffeine
4.1.3. Naloxone
4.1.4. Oxygen Therapy
4.2. Physical Methods
4.2.1. Colostrum: Natural and Artificial Supplementation
4.2.2. Temperature Drops Immediately after Birth—Sources of External Heat and Drying
5. Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Farmer, C. The Suckling and Weaned Piglet; Wageningen Academic Publishers: Amsterdam, The Netherlands, 2020; 312p. [Google Scholar]
- Mota-Rojas, D.; López, A.; Martínez-Burnes, J.; Muns, R.; Villanueva-García, D.; Mora-Medina, P.; González-Lozano, M.; Olmos-Hernández, A.; Ramírez-Necoechea, R. Is vitality assessment important in neonatal animals? CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martinez-Burnes, J.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M.L.; Ramirez-Necoechea, R.; Lopez, A. Effect of oxytocin treatment in sows on umbilical cord morphology, meconium staining, and neonatal mortality of piglets. Am. J. Vet. Res. 2002, 63, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Fierro, R.; Santiago, R.; Gonzalez-Lozano, M.; Martínez-Rodríguez, R.; García-Herrera, R.; Mora-Medina, P.; Flores-Peinado, S.; Sánchez, M. Outcomes of gestation length in relation to farrowing performance in sows and daily weight gain and metabolic profiles in piglets. Anim. Prod. Sci. 2015, 55, 93–100. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; López, A.; Muns, R.; Mainau, E.; Martínez, J. Piglet welfare. In Bienestar Animal. Una Visión Global en Iberoamérica; Mota-Rojas, D., Velarde, A., Huerta, S., Cajiao, M., Eds.; Elsevier: Barcelona, España, 2016; pp. 51–62. [Google Scholar]
- Hillman, N.H.; Kallapur, S.G.; Jobe, A.H. Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 2012, 39, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Spilsbury, M.; Mota-Rojas, D.; Villanueva-García, D.; Martínez-Burnes, J.; Gregorio, O.; Ramírez-Necoechea, R.; Mayagoitia, A.L.; Trujillo, M.E. Perinatal asphyxia pathophysiology in pig and human: A review. Anim. Reprod. Sci. 2005, 90, 1–30. [Google Scholar] [CrossRef]
- Cavaliere, T.A. From fetus to neonate: A sensational journey. Newborn Infant Nurs. Rev. 2016, 16, 43–47. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernández, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Sánchez-Millán, J.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother′s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef]
- Bovbjerg, M.L.; Dissanayake, M.V.; Cheyney, M.; Brown, J.; Snowden, J.M. Utility of the 5-minute apgar score as a research endpoint. Am. J. Epidemiol. 2019, 188, 1695–1704. [Google Scholar] [CrossRef]
- Santiago, R.; Martínez-Burnes, J.; Mayagoitia, A.L.; Ramírez-Necoechea, R.; Mota-Rojas, D. Relationship of vitality and weight with the temperature of newborn piglets born to sows of different parity. Livest. Sci. 2019, 220, 26–31. [Google Scholar] [CrossRef]
- Groppetti, D.; Pecile, A.; Del Carro, A.P.; Copley, K.; Minero, M.; Cremonesi, F. Evaluation of newborn canine viability by means of umbilical vein lactate measurement, apgar score and uterine tocodynamometry. Theriogenology 2010, 74, 1187–1196. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Villanueva-García, D.; Hernández-González, R.; Martínez-Rodríguez, R.; Mora-Medina, P.; Sánchez-Hernández, M.; Trujillo, O.M. Assessment of the vitality of the newborn: An overview. Sci. Res. Essays 2012, 7, 869. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martinez-Burnes, J.; Villanueva-Garcia, D.; Santiago, P.; Trujillo-Ortega, M.; Gregorio, O.; Lopez-Mayagoitia, A. Animal welfare in the newborn piglet: A review. Vet. Med. 2012, 57, 338–349. [Google Scholar] [CrossRef]
- Murray, C.F. Characteristics, Risk Factors and Management Programs for Vitality of Newborn Dairy Calves. Ph.D. Thesis, University of Guelp, Guelph, ON, Canada, 2014. [Google Scholar]
- Flora, T.; Smallman, M.; Kutzler, M. Developing a modified Apgar scoring system for newborn lambs. Theriogenology 2020, 157, 321–326. [Google Scholar] [CrossRef]
- Murray, C.F.; Leslie, K.E. Newborn calf vitality: Risk factors, characteristics, assessment, resulting outcomes and strategies for improvement. Vet. J. 2013, 198, 322–328. [Google Scholar] [CrossRef]
- Barrier, A.C.; Ruelle, E.; Haskell, M.J.; Dwyer, C.M. Effect of a difficult calving on the vigour of the calf, the onset of maternal behaviour, and some behavioural indicators of pain in the dam. Prev. Vet. Med. 2012, 103, 248–256. [Google Scholar] [CrossRef]
- Moon, P.F.; Massat, B.J.; Pascoe, P.J. Neonatal critical care. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 343–367. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.E.; Mora-Medina, P.; Hernández-Avalos, I.; Casas-Alvarado, A.; Olmos-Hernández, A.; Martínez-Burnes, J. Neurobehavioral and neuroprotector effects of caffeine in animal models. J. Anim. Behav. Biometeorol. 2020, 8, 298–307. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Miranda-Cortés, A.; Ibarra-Ríos, D.; Casas-Alvarado, A.; Mora-Medina, P.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Avalos, I. Caffeine: Cardiorespiratory effects and tissue protection in animal models. Exp. Anim. 2021, 70, 431–439. [Google Scholar] [CrossRef]
- Lezama-García, K.; Mota-Rojas, D.; Martínez-Burnes, J.; Villanueva-García, D.; Domínguez-Oliva, A.; Gómez-Prado, J.; Mora-Medina, P.; Casas-Alvarado, A.; Olmos-Hernández, A.; Soto, P.; et al. Strategies for hypothermia compensation in altricial and precocial newborn mammals and their monitoring by infrared thermography. Vet. Sci. 2022, 9, 246. [Google Scholar] [CrossRef]
- Watt, B.; Wright, B. The Importance of Colostrum to Foals. Colostrum and Passive Transfer Assessment. Available online: https://www.equineguelph.ca/pdf/facts/ImportanceofColostrumtoFoalsApr_08.pdf (accessed on 13 March 2023).
- Ramirez, B.C.; Hayes, M.D.; Condotta, I.C.F.S.; Leonard, S.M. Impact of housing environment and management on pre-/post-weaning piglet productivity. J. Anim. Sci. 2022, 100, skac142. [Google Scholar] [CrossRef]
- Knol, E.F.; van der Spek, D.; Zak, L.J. Genetic aspects of piglet survival and related traits: A review. J. Anim. Sci. 2022, 100, skac190. [Google Scholar] [CrossRef]
- Nel, C.L.; Cloete, S.W.P.; Kruger, A.C.M.; Dzama, K. Long term genetic selection for reproductive success affects neonatal lamb vitality across cold stress conditions. J. Therm. Biol. 2021, 98, 102908. [Google Scholar] [CrossRef]
- Canario, L.; Bidanel, J.-P.; Rydhmer, L. Genetic trends in maternal and neonatal behaviors and their association with perinatal survival in French Large White swine. Front. Genet. 2014, 5, 410. [Google Scholar] [CrossRef]
- Kapell, D.N.R.G.; Ashworth, C.J.; Knap, P.W.; Roehe, R. Genetic parameters for piglet survival, litter size and birth weight or its variation within litter in sire and dam lines using Bayesian analysis. Livest. Sci. 2011, 135, 215–224. [Google Scholar] [CrossRef]
- Swanson, J.R.; Sinkin, R.A. Transition from fetus to newborn. Pediatr. Clin. N. Am. 2015, 62, 329–343. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011, 589, 3641–3658. [Google Scholar] [CrossRef]
- Mellor, D.J.; Stafford, K.J. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 2004, 168, 118–133. [Google Scholar] [CrossRef]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef]
- Vande Pol, K.D.; Tolosa, A.F.; Shull, C.M.; Brown, C.B.; Alencar, S.A.S.; Ellis, M. Effect of drying and warming piglets at birth on preweaning mortality. Transl. Anim. Sci. 2021, 5, txab016. [Google Scholar] [CrossRef]
- Lawler, D. Neonatal and pediatric care of the puppy and kitten. Theriogenology 2008, 70, 384–392. [Google Scholar] [CrossRef]
- Schild, S.-L.A.; Foldager, L.; Rangstrup-Christensen, L.; Pedersen, L.J. Characteristics of piglets born by two highly prolific sow hybrids. Front. Vet. Sci. 2020, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.J.; Berg, P.; Jørgensen, G.; Andersen, I.L. Neonatal piglet traits of importance for survival in crates and indoor pens. J. Anim. Sci. 2011, 89, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Edwards, S.A. Review: Improving the performance of neonatal piglets. Animal 2022, 16, 100350. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 2101. [Google Scholar] [CrossRef]
- Ahmed, B.M.S.; Younas, U.; Asar, T.O.; Dikmen, S.; Hansen, P.J.; Dahl, G.E. Cows exposed to heat stress during fetal life exhibit improved thermal tolerance. J. Anim. Sci. 2017, 95, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Tao, S.; Thompson, I.; Dahl, G. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef] [PubMed]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Cobos, L.; Rosetti, M.; Distel, H.; Hudson, R. To stay or not to stay: The contribution of tactile and thermal cues to coming to rest in newborn rabbits. J. Comp. Physiol. A 2003, 189, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Martínez-Burnes, J.; Pérez-Jiménez, J.C.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Mota-Rojas, D. Relation between the dam’s weight on superficial temperature of her puppies at different stages of the post-partum. Vet. Sci. 2022, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Noblet, J.; Le Dividich, J. Energy metabolism of the newborn pig during the first 24 h of life. Neonatology 1981, 40, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Fyda, T.J.; Spencer, C.; Jastroch, M.; Gaudry, M. Disruption of thermogenic UCP1 predated the divergence of pigs and peccaries. J. Exp. Biol. 2020, 223, jeb223974. [Google Scholar] [CrossRef] [PubMed]
- Tucker, B.S.; Craig, J.R.; Morrison, R.S.; Smits, R.J.; Kirkwood, R.N. Piglet viability: A review of identification and pre-weaning management strategies. Animals 2021, 11, 2902. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.-H.; Gonçalves-Titto, C.; Martinez-Burnes, J.; Villanueva-García, D.; Lezama-García, K.; Domínguez-Oliva, A.; Avalos, I.H.; Mora-Medina, P.; Verduzco-Mendoza, A.; et al. Neonatal infrared thermography images in the hypothermic ruminant model: Anatomical-morphological-physiological aspects and mechanisms for thermoregulation. Front. Vet. Sci. 2022, 9, 963205. [Google Scholar] [CrossRef]
- Kredatusova, G.; Hajurka, J.; Szakallova, I.; Valencakova, A.; Vojtek, B. Physiological events during parturition and possibilities for improving puppy survival: A review. Vet. Med. 2011, 56, 589–594. [Google Scholar] [CrossRef]
- Randall, G.C.B. Changes in fetal and maternal blood at the end of pregnancy and during parturition in the pig. Res. Vet. Sci. 1982, 32, 278–282. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Villanueva, D.; Suárez, X.; Hernandez, R.; Santiago, R.; Trujillo, M. Foetal and neonatal energy metabolism in pigs and humans: A review. Vet. Med. 2011, 56, 215–225. [Google Scholar] [CrossRef]
- Diehl, B.; Oster, M.; Vernunft, A.; Wimmers, K.; Bostedt, H. Intrinsic challenges of neonatal adaptation in swine. Arch. Anim. Breed. 2022, 65, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, L.d.M.S.; Parisotto, E.B.; Salomão, E.d.A.; Maldonado, M.J.M.; Silva, I.S. Characterization of oxidative stress in animal model of neonatal hypoxia. Acta Cir. Bras. 2021, 36, e361108. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Burnes, J.; Mota- Rojas, D.; Villanueva- García, D.; Ibarra- Ríos, D.; Lezama- García, K.; Barrios- García, H.; López- Mayagoitia, A. Meconium aspiration syndrome in mammals. CAB Rev. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Xodo, S.; Londero, A.P. Is It Time to Redefine Fetal Decelerations in Cardiotocography? J. Pers. Med. 2022, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.A.; Wassink, G.; Westgate, J.A.; Nijhuis, J.G.; Ugwumadu, A.; Galinsky, R.; Bennet, L.; Gunn, A.J. The peripheral chemoreflex: Indefatigable guardian of fetal physiological adaptation to labour. J. Physiol. 2018, 596, 5611–5623. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Mitchell, M.D.; Kumar, S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020, 222, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Villanueva-García, D.; Mota-Reyes, A.; Orihuela, A.; Hernández-Ávalos, I.; Domínguez-Oliva, A.; Casas-Alvarado, A.; Flores-Padilla, K.; Jacome-Romero, J.; Martínez-Burnes, J. Meconium Aspiration Syndrome in Animal Models: Inflammatory Process, Apoptosis, and Surfactant Inactivation. Animals 2022, 12, 3310. [Google Scholar] [CrossRef]
- Marcet-Rius, M.; Bienboire-Frosini, C.; Lezama-García, K.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Mora-Medina, P.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Gazzano, A. Clinical Experiences and Mechanism of Action with the Use of Oxytocin Injection at Parturition in Domestic Animals: Effect on the Myometrium and Fetuses. Animals 2023, 13, 768. [Google Scholar] [CrossRef]
- Steinhardt, M.; Bünger, U.; Furcht, G. Zum Eisenbedarf des Schweines in den ersten 2 Lebensmonaten. Arch. Exp. Vet. Med. 1984, 29, 104–111. [Google Scholar]
- Szudzik, M.; Starzyński, R.; Jończy, A.; Mazgaj, R.; Lenartowicz, M.; Lipiński, P. Iron supplementation in suckling piglets: An ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals 2018, 11, 128. [Google Scholar] [CrossRef]
- Bünger, B.; Bünger, U.; Lemke, E. Verhaltensbiologische Vitalitätseinschätzung von Ferkeln mit hoch- und mittelgradiger konnataler Eisenmangelanämie. Monatsh. Vet. 1988, 43, 583–587. [Google Scholar]
- Gondret, F.; Lefaucheur, L.; Louveau, I.; Lebret, B.; Pichodo, X.; Le Cozler, Y. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod. Sci. 2005, 93, 137–146. [Google Scholar] [CrossRef]
- Beaulieu, A.D.; Aalhus, J.L.; Williams, N.H.; Patience, J.F. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 2010, 88, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.; Galan, H. Fetal growth restriction. In Gabbe’s Obstretrics: Normal and Problem Pregnancies; Landon, M., Galan, H., Jauniaux, E., Driscoll, D., Berghella, V., Grobman, W., Kilpatrock, S., Cahil, A., Eds.; Elsevier: Philadelphi, PA, USA, 2020; pp. 555–585. [Google Scholar]
- Mota-Rojas, D.; Alonso-Spilsbury, M.L.; Ramírez-Necoechea, R.; Moles y Cervantes, P.; González-Lozano, M. Involved factors in the immune response of neonatal pigs. In Animal Perinatology: Clinical and Experimental Approaches; Mota-Rojas, D., Nava-Ocampo, A.A., Villanueva-García, D., Alonso-Spilbury, M.L., Eds.; BM Editores: Mexico City, Mexico, 2008; pp. 443–454. [Google Scholar]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- England, D.C. Husbandry components in prenatal and perinatal development in swine. J. Anim. Sci. 1974, 38, 1045–1049. [Google Scholar] [CrossRef]
- Vermorel, M.; Dardillat, C.; Vernet, J.; Saido; Dardillat, C.; Demigne, C. Energy metabolism and thermoregulation in the newborn calf. Ann. Rech. Vet. 1983, 14, 382–389. [Google Scholar]
- Mota-Rojas, D.; Rosales, A.M.; Trujillo, M.E.; Gregorio, O.; Ramírez, R.; Alonso-Spilsbury, M. The effects of vetrabutin chlorhydrate and oxytocin on stillbirth rate and asphyxia in swine. Theriogenology 2005, 64, 1889–1897. [Google Scholar] [CrossRef]
- Şirin, E.; Aksoy, Y.; Șen, U.; Ulutaș, Z.; Kuran, M. Effect of lamb birth weight on fiber number and type of semitendinosus muscle. Anadolu Tarım Bilim. Derg. 2011, 26, 63–67. [Google Scholar]
- Greenwood, P.L.; Hunt, A.S.; Hermanson, J.W.; Bell, A.W. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J. Anim. Sci. 2000, 78, 50. [Google Scholar] [CrossRef]
- Gama, L.T.; Dickerson, G.E.; Young, L.D.; Leymaster, K.A. Effects of breed, heterosis, age of dam, litter size, and birth weight on lamb mortality1. J. Anim. Sci. 1991, 69, 2727–2743. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Morgan, C.A. Maintenance of body temperature in the neonatal lamb: Effects of breed, birth weight, and litter size1. J. Anim. Sci. 2006, 84, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. INVITED REVIEW: Piglet mortality: Management solutions. J. Anim. Sci. 2013, 91, 3361–3389. [Google Scholar] [CrossRef] [PubMed]
- Johanson, J.M.; Berger, P.J. Birth weight as a predictor of calving ease and perinatal mortality in holstein cattle. J. Dairy Sci. 2003, 86, 3745–3755. [Google Scholar] [CrossRef] [PubMed]
- Schmidek, A.; da Costa, M.J.R.P.; Mercadante, M.E.Z.; de Toledo, L.M.; Cyrillo, J.N.d.S.G.; Branco, R.H. Genetic and non-genetic effects on calf vigor at birth and preweaning mortality in Nellore calves. Rev. Bras. Zootec. 2013, 42, 421–427. [Google Scholar] [CrossRef]
- Lezama-García, K.; Martínez-Burnes, J.; Marcet-Rius, M.; Gazzano, A.; Olmos-Hernández, A.; Mora-Medina, P.; Domínguez-Oliva, A.; Pereira, A.M.F.; Hernández-Ávalos, I.; Baqueiro-Espinosa, U.; et al. Is the weight of the newborn puppy related to its thermal balance? Animals 2022, 12, 3536. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.A.; Abou-Ismail, U.A.; El-Kholya, S.Z. Differences in post-parturient behaviour, lamb performance and survival rate between purebred Egyptian Rahmani and its crossbred Finnish ewes. Small Rumin. Res. 2010, 89, 57–61. [Google Scholar] [CrossRef]
- Mellor, D.J.; Cockburn, F. A comparison of energy metabolism in the new-born infant, piglet and lamb. Q. J. Exp. Physiol. 1986, 71, 361–379. [Google Scholar] [CrossRef]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Szymeczko, R.; Kapelański, W.; Piotrowska, A.; Dybała, J.; Bogusławska-Tryk, M.; Burlikowska, K.; Hertig, I.; Sassek, M.; Pruszyńska-Oszmałek, E.; Maćkowiak, P. Changes in the content of major proteins and selected hormones in the blood serum of piglets during the early postnatal period. Folia Biol. 2008, 57, 97–103. [Google Scholar] [CrossRef]
- Girard, J. Metabolic adaptations to change of nutrition at birth. Neonatology 1990, 58, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Parada, P.R. Cori Cycle. Available online: https://www.lifeder.com/ciclo-de-cori/ (accessed on 13 March 2023).
- Ophardt, E.C. Cori Cycle. Available online: http://www.elmhurst.edu/~chm/vchembook/615coricycle.html (accessed on 13 March 2023).
- Mellor, D. Preparing for Life After Birth: Introducing the concepts of intrauterine and extrauterine sensory entrainment in mammalian young. Animals 2019, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Lawrence, A.B. A review of the behavioural and physiological adaptations of hill and lowland breeds of sheep that favour lamb survival. Appl. Anim. Behav. Sci. 2005, 92, 235–260. [Google Scholar] [CrossRef]
- Mellor, D.; Lentle, R. Survival implications of the development of behavioural responsiveness and awareness in different groups of mammalian young. N. Z. Vet. J. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martínez-Burnes, J.; Casas-Alvarado, A.; Gómez-Prado, J.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Jacome-Romero, J.; Rodríguez-González, D.; Pereira, A.M. Clinical usefulness of infrared thermography to detect sick animals: Frequent and current. CABI Rev. 2022, 17, 1–17. [Google Scholar] [CrossRef]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation mechanisms and perspectives for validating thermal windows in pigs with hypothermia and hyperthermia: An overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef]
- Larson, M.A.; Stein, B.E. The use of tactile and olfactory cues in neonatal orientation and localization of the nipple. Dev. Psychobiol. 1984, 17, 423–436. [Google Scholar] [CrossRef]
- André, V.; Henry, S.; Lemasson, A.; Hausberger, M.; Durier, V. The human newborn’s umwelt: Unexplored pathways and perspectives. Psychon. Bull. Rev. 2018, 25, 350–369. [Google Scholar] [CrossRef]
- Fitzgerald, M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005, 6, 507–520. [Google Scholar] [CrossRef]
- Serra, J.; Nowak, R. Olfactory preference for own mother and litter in 1-day-old rabbits and its impairment by thermotaxis. Dev. Psychobiol. 2008, 50, 542–553. [Google Scholar] [CrossRef]
- Lopez, A.; Bildfell, R. Pulmonary inflammation associated with aspirated meconium and epithelial cells in calves. Vet. Pathol. 1992, 29, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Haakonsen Lindenskov, P.H.; Castellheim, A.; Saugstad, O.D.; Mollnes, T.E. Meconium aspiration syndrome: Possible pathophysiological mechanisms and future potential therapies. Neonatology 2015, 107, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Mee, J.F. Newborn dairy calf management. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- González-Lozano, M.; Trujillo-Ortega, M.E.; Becerrill- Herrera, M.; Alonso-Spilsbury, M.L.; Ramírez-Necoechea, R.; Hernández- González, R.; Mota-Rojas, D. Effects of oxytocin on critical blood variables from dystocic sows. Vet. México 2009, 40, 231–245. [Google Scholar]
- González-Lozano, M.; Mota-Rojas, D.; Velázquez-Armenta, E.Y.; Nava-Ocampo, A.A.; Hernández-González, R.; Becerril-Herrera, M.; Trujillo-Ortega, M.E.; Alonso-Spilsbury, M. Obstetric and fetal outcomes in dystocic and eutocic sows to an injection of exogenous oxytocin during farrowing. Can. Vet. J. = Rev. Vet. Can. 2009, 50, 1273–1277. [Google Scholar]
- Muro, B.B.D.; Carnevale, R.F.; Andretta, I.; Leal, D.F.; Monteiro, M.S.; Poor, A.P.; Almond, G.W.; Garbossa, C.A.P. Effects of uterotonics on farrowing traits and piglet vitality: A systematic review and meta-analysis. Theriogenology 2021, 161, 151–160. [Google Scholar] [CrossRef]
- Randall, G.C.B. Perinatal adaptation in animals. Anim. Reprod. Sci. 1992, 28, 309–318. [Google Scholar] [CrossRef]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic peroxisome proliferator-activated receptor γ (pparγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, ucp1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef]
- Rowan, T.G. Thermoregulation in neonatal ruminants. BSAP Occas. Publ. 1992, 15, 13–24. [Google Scholar] [CrossRef]
- Smith, S.; Carstens, G. Ontogeny and metabolism of brown adipose tissue in livestock species. In Biology of Growing Animals; Burrin, D., Mersmann, H., Eds.; Elsevier: Philadephia, PA, USA, 2005; pp. 303–322. [Google Scholar]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown adipose tissue: Physiological function and evolutionary significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Robinson, L.; Yazdani, M.; Symonds, M.E.; Budge, H. Brown adipose tissue genes in pericardial adipose tissue of newborn sheep are downregulated by maternal nutrient restriction in late gestation. Pediatr. Res. 2013, 74, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Bryant, M.J.; Clarke, L.; Darby, C.J.; Lomax, M.A. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. J. Physiol. 1992, 455, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and thermoregulation in animals: Structural biology and neurophysiological aspects. Animals 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Haman, F. Shivering in the cold: From mechanisms of fuel selection to survival. J. Appl. Physiol. 2006, 100, 1702–1708. [Google Scholar] [CrossRef]
- Nakamura, K. Afferent pathways for autonomic and shivering thermoeffectors. Handb. Clin. Neurol. 2018, 156, 263–279. [Google Scholar] [CrossRef]
- Kerman, I.A.; Enquist, L.W.; Watson, S.J.; Yates, B.J. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J. Neurosci. 2003, 23, 4657–4666. [Google Scholar] [CrossRef]
- Hohtola, E. Shivering thermogenesis in birds and mammals. In Life in the Cold: Evolution, Mechanisms, Adaptation, and Application; Barnes, M., Carey, H.V., Eds.; ARCUS: Fairbanks, AK, USA, 2004; pp. 241–252. [Google Scholar]
- Carstens, G.E. Cold thermoregulation in the newborn calf. Vet. Clin. N. Am. Food Anim. Pract. 1994, 10, 69–106. [Google Scholar] [CrossRef]
- Alexander, G.; Williams, D. Shivering and non-shivering thermogenesis during summit metabolism in young lambs. J. Physiol. 1968, 198, 251–276. [Google Scholar] [CrossRef]
- Phillips, D.I.W.; Jones, A. Fetal programming of autonomic and HPA function: Do people who were small babies have enhanced stress responses? J. Physiol. 2006, 572, 45–50. [Google Scholar] [CrossRef]
- Krogstad, A.-L.; Elam, M.; Karlsson, T.; Wallin, B.G. Arteriovenous anastomoses and the thermoregulatory shift between cutaneous vasoconstrictor and vasodilator reflexes. J. Auton. Nerv. Syst. 1995, 53, 215–222. [Google Scholar] [CrossRef]
- Cook, N.; Schaefer, A.; Warren, L.; Burwash, L.; Anderson, M.; Baron, V. Adrenocortical and metabolic responses to ACTH injection in horses: An assessment by salivary cortisol and infrared thermography of the eye. Can. J. Anim. Sci. 2001, 81, 621. [Google Scholar]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.P.; Aoki, K.; Kosiba, W.A.; Johnson, J.M. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am. J. Physiol. Circ. Physiol. 2001, 280, H1496–H1504. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.P.; Saad, A.R.; Bennett, L.A.T.; Kosiba, W.A.; Johnson, J.M. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am. J. Physiol. Circ. Physiol. 2004, 287, H1404–H1409. [Google Scholar] [CrossRef]
- Mai, T.C.; Braun, A.; Bach, V.; Pelletier, A.; Seze, R. Low-level radiofrequency exposure induces vasoconstriction in rats. Bioelectromagnetics 2021, 42, 455–463. [Google Scholar] [CrossRef]
- Hrupka, B.J.; Leibbrandt, V.D.; Crenshaw, T.D.; Benevenga, N.J. Effect of sensory stimuli on huddling behavior of pigs. J. Anim. Sci. 2000, 78, 592–596. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Velarde, A.; Maris-Huertas, S.; Cajiao, M.N. Animal Welfare, a Global Vision in Ibero-America, 3rd ed.; Elsevier: Barcelona, Spain, 2016; p. 516. [Google Scholar]
- Regueiro, M.; López-Mazz, C.; Jorge-Smeding, E.; Baldi, F.; Banchero, G. Duration of phase II of labour negatively affects maternal behaviour and lamb viability in wool-type primiparous ewes under extensive rearing. Appl. Anim. Behav. Sci. 2021, 234, 105207. [Google Scholar] [CrossRef]
- García-Torres, E.; Hudson, R.; Castelán, F.; Martínez-Gómez, M.; Bautista, A. Differential metabolism of brown adipose tissue in newborn rabbits in relation to position in the litter huddle. J. Therm. Biol. 2015, 51, 33–41. [Google Scholar] [CrossRef]
- Ferner, K.; Schultz, J.A.; Zeller, U. Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J. Anat. 2017, 231, 798–822. [Google Scholar] [CrossRef]
- Bautista, A.; García-Torres, E.; Martínez-Gómez, M.; Hudson, R. Do newborn domestic rabbits Oryctolagus cuniculus compete for thermally advantageous positions in the litter huddle? Behav. Ecol. Sociobiol. 2008, 62, 331–339. [Google Scholar] [CrossRef]
- McCauley, S.R. Glucose Metabolism in Low Birth Weight Neonatal Pigs. Doctoral Dissertation, Virginia Polytethic Institute and State University, Blacksburg, VA, USA, 2019. [Google Scholar]
- Napolitano, F.; Bragaglio, A.; Braghieri, A.; El-Aziz, A.H.A.; Titto, C.G.; Villanueva-García, D.; Mora-Medina, P.; Pereira, A.M.F.; Hernández-Avalos, I.; José-Pérez, N.; et al. The effect of birth weight and time of day on the thermal response of newborn water buffalo calves. Front. Vet. Sci. 2023, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.A.; Lamberski, N. Approaches to management and care of the neonatal nondomestic ruminant. Vet. Clin. N. Am. Exot. Anim. Pract. 2012, 15, 265–277. [Google Scholar] [CrossRef]
- Eales, F.; Small, J.; Gilmour, J. Resuscitation of hypothermic lambs. Vet. Rec. 1982, 110, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, J.E.; Harding, J.E.; Oliver, M.H.; Gamble, G.; Dickson, J.L.; Chase, G.; Jaquiery, A.L. Oral dextrose gel to improve survival in less vigorous newborn triplet lambs: A randomised controlled trial. N. Z. J. Agric. Res. 2017, 60, 54–69. [Google Scholar] [CrossRef]
- Engelsmann, M.N.; Hansen, C.F.; Nielsen, M.N.; Kristensen, A.R.; Amdi, C. Glucose injections at birth, warmth and placing at a nurse sow improve the growth of IUGR piglets. Animals 2019, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Le Dividich, J.; Noblet, J. Thermoregulation and energy metabolism in the neonatal pig. Ann. Rech. Vet. 1983, 14, 375–381. [Google Scholar] [PubMed]
- Le Dividich, J.; Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 1981, 40, 167–174. [Google Scholar] [CrossRef]
- Silva, F.L.M.; Miqueo, E.; da Silva, M.D.; Torrezan, T.M.; Rocha, N.B.; Salles, M.S.V.; Bittar, C.M.M. Thermoregulatory responses and performance of dairy calves fed different amounts of colostrum. Animals 2021, 11, 703. [Google Scholar] [CrossRef]
- Silva, F.L.M.; Bittar, C.M.M. Thermogenesis and some rearing strategies of dairy calves at low temperature—A review. J. Appl. Anim. Res. 2019, 47, 115–122. [Google Scholar] [CrossRef]
- Pakkanen, R.; Aalto, J. Growth factors and antimicrobial factors of bovine colostrum. Int. Dairy J. 1997, 7, 285–297. [Google Scholar] [CrossRef]
- Muns, R.; Silva, C.; Manteca, X.; Gasa, J. Effect of cross-fostering and oral supplementation with colostrums on performance of newborn piglets. J. Anim. Sci. 2014, 92, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IgG. J. Anim. Sci. 2017, 95, 353. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Ramirez-Mares, M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014, 25, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Kumar; Lipshultz Caffeine and Clinical Outcomes in Premature Neonates. Children 2019, 6, 118. [CrossRef] [PubMed]
- Swinbourne, A.M.; Kind, K.L.; Flinn, T.; Kleemann, D.O.; van Wettere, W.H.E.J. Caffeine: A potential strategy to improve survival of neonatal pigs and sheep. Anim. Reprod. Sci. 2021, 226, 106700. [Google Scholar] [CrossRef]
- Orozco, G.; Mota-Rojas, D.; Trujillo-Ortega, M.E.; Becerril-Herrera, M.; Hernández-González, R.; Villanueva-García, D. Effects of administration of caffeine on metabolic variables in neonatal pigs with peripartum asphyxia. Am. J. Vet. Res. 2010, 71, 1214–1219. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kind, K.; Hebart, M.L.; van Wettere, W.H.E.J. Caffeine supplementation at birth, but not 8 to 12 h post-birth, increased 24 h pre-weaning mortality in piglets. Animal 2020, 14, 1529–1535. [Google Scholar] [CrossRef]
- Robertson, S.M.; Edwards, S.H.; Doran, G.S.; Friend, M.A. Maternal caffeine administration to ewes does not affect perinatal lamb survival. Anim. Reprod. Sci. 2021, 231, 106799. [Google Scholar] [CrossRef]
- Jarratt, L.; James, S.E.; Kirkwood, R.N.; Nowland, T.L. Effects of caffeine and glucose supplementation at birth on piglet pre-weaning growth, thermoregulation, and survival. Animals 2023, 13, 435. [Google Scholar] [CrossRef]
- Murdock, N.J.; Weaver, A.C.; Kelly, J.M.; Kleemann, D.O.; van Wettere, W.H.E.J.; Swinbourne, A.M. Supplementing pregnant Merino ewes with caffeine to improve neonatal lamb thermoregulation and viability. Anim. Reprod. Sci. 2021, 226, 106715. [Google Scholar] [CrossRef] [PubMed]
- Binder-Heschl, C.; Crossley, K.; te Pas, A.; Polglase, G.; Blank, D.; Zahra, V.; Moxham, A.; Rodgers, K.; Hooper, S. Haemodynamic effects of prenatal caffeine on the cardiovascular transition in ventilated preterm lambs. PLoS ONE 2018, 13, e0200572. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, A.; Mazzoni, C.; Serventi, P.; Zanardelli, P.; Bertini, S. Pharmacokinetics of oral caffeine in sows: A pilot study. Large Anim. Rev. 2015, 21, 207–210. [Google Scholar]
- Mainau, E.; Manteca, X. Pain and discomfort caused by parturition in cows and sows. Appl. Anim. Behav. Sci. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Gupta, M. Physiology, Opioid Receptor; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hazinski, T.A.; Grunstein, M.M.; Schlueter, M.A.; Tooley, W.H. Effect of naloxone on ventilation in newborn rabbits. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 50, 713–717. [Google Scholar] [CrossRef]
- Long, W.A.; Lawson, E.E. Developmental aspects of the effect of naloxone on control of breathing in piglets. Respir. Physiol. 1983, 51, 119–129. [Google Scholar] [CrossRef]
- Hsia, C.C.W.; Schmitz, A.; Lambertz, M.; Perry, S.F.; Maina, J.N. Evolution of Air Breathing: Oxygen Homeostasis and the Transitions From Water to Land and Sky. In Comprehensive Physiology; Wiley: New York, NY, USA, 2013; pp. 849–915. [Google Scholar]
- Soraci, A.; Decundo, J.; Dieguez, S.; Martinez, G.; Romanelli, A.; Perez Gaudio, D.; Fernandez Paggi, M.; Amanto, F. Practical oxygen therapy for newborn piglets. N. Z. Vet. J. 2020, 68, 331–339. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Berry, M.J.; Quinn, M.; Holman, S.L.; Bradshaw, E.L.; Jesse, S.M.; Haller, C.; Seed, M.; Morrison, J.L. Haemodynamics and cerebral oxygenation of neonatal piglets in the immediate ex utero period supported by mechanical ventilation or ex utero oxygenator. J. Physiol. 2021, 599, 2751–2761. [Google Scholar] [CrossRef]
- Bleul, U.T.; Bircher, B.M.; Kähn, W.K. Effect of intranasal oxygen administration on blood gas variables and outcome in neonatal calves with respiratory distress syndrome: 20 cases (2004–2006). J. Am. Vet. Med. Assoc. 2008, 233, 289–293. [Google Scholar] [CrossRef]
- Kim, E.; Nguyen, M. Oxygen therapy for neonatal resuscitation in the delivery room. Neoreviews 2019, 20, e500–e512. [Google Scholar] [CrossRef]
- Silva, L.C.G.; Angrimani, D.S.R.; Regazzi, F.M.; Lúcio, C.F.; Veiga, G.A.L.; Fernandes, C.B.; Vannucchi, C.I. Pulmonary changes and redox status after fractionalized dose of prophylactic surfactant treatment in preterm neonatal lambs. J. Appl. Anim. Res. 2020, 48, 220–227. [Google Scholar] [CrossRef]
- Rawat, M.; Chandrasekharan, P.; Gugino, S.F.; Koenigsknecht, C.; Nielsen, L.; Wedgwood, S.; Mathew, B.; Nair, J.; Steinhorn, R.; Lakshminrusimha, S. Optimal oxygen targets in term lambs with meconium aspiration syndrome and pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 63, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Chigerwe, M.; Hagey, J.V.; Aly, S.S. Determination of neonatal serum immunoglobulin G concentrations associated with mortality during the first 4 months of life in dairy heifer calves. J. Dairy Res. 2015, 82, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Conneely, M.; Berry, D.P.; Sayers, R.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 2013, 7, 1824–1832. [Google Scholar] [CrossRef]
- Jaster, E.H. Evaluation of quality, quantity, and timing of colostrum feeding on immunoglobulin G1 absorption in Jersey calves. J. Dairy Sci. 2005, 88, 296–302. [Google Scholar] [CrossRef]
- Argüello, A.; Castro, N.; Zamorano, M.J.; Castroalonso, A.; Capote, J. Passive transfer of immunity in kid goats fed refrigerated and frozen goat colostrum and commercial sheep colostrum. Small Rumin. Res. 2004, 54, 237–241. [Google Scholar] [CrossRef]
- Bragg, R.; Macrae, A.; Lycett, S.; Burrough, E.; Russell, G.; Corbishley, A. Prevalence and risk factors associated with failure of transfer of passive immunity in spring born beef suckler calves in Great Britain. Prev. Vet. Med. 2020, 181, 105059. [Google Scholar] [CrossRef]
- Turini, L.; Conte, G.; Bonelli, F.; Sgorbini, M.; Madrigali, A.; Mele, M. The relationship between colostrum quality, passive transfer of immunity and birth and weaning weight in neonatal calves. Livest. Sci. 2020, 238, 104033. [Google Scholar] [CrossRef]
- Cabrera, R.A.; Lin, X.; Campbell, J.M.; Moeser, A.J.; Odle, J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 2012, 3, 42. [Google Scholar] [CrossRef]
- Maciag, S.S.; Bellaver, F.V.; Bombassaro, G.; Haach, V.; Morés, M.A.Z.; Baron, L.F.; Coldebella, A.; Bastos, A.P. On the influence of the source of porcine colostrum in the development of early immune ontogeny in piglets. Sci. Rep. 2022, 12, 15630. [Google Scholar] [CrossRef]
- Mellado, M.; Pittroff, W.; García, J.E.; Mellado, J. Serum IgG, blood profiles, growth and survival in goat kids supplemented with artificial colostrum on the first day of life. Trop. Anim. Health Prod. 2008, 40, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Berge, A.C.B.; Besser, T.E.; Moore, D.A.; Sischo, W.M. Evaluation of the effects of oral colostrum supplementation during the first fourteen days on the health and performance of preweaned calves. J. Dairy Sci. 2009, 92, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Chiba, A.; Itoh, M.; Nambo, Y.; Yamagishi, N.; Shibano, K.; Cheong, S.H. Colostral and foal serum immunoglobulin G levels and associations with perinatal abnormalities in heavy draft horses in Japan. J. Equine Sci. 2020, 31, 29–34. [Google Scholar] [CrossRef]
- Lester, G.D. Colostrum: Assessment of Quality and Artificial Supplementation. In Equine Reproduction; McKinnon, A.O., Squires, E.L., Vaala, W.E., Varner, D.D., Eds.; Wiley Blackwell: Oxford, UK, 2011; pp. 342–345. [Google Scholar]
- Grigaleviciute, R.; Planciuniene, R.; Prikockyte, I.; Radzeviciute-Valciuke, E.; Baleviciute, A.; Zelvys, A.; Zinkeviciene, A.; Zigmantaite, V.; Kucinskas, A.; Matusevicius, P.; et al. The Influence of feeding with colostrum and colostrum replacer on major blood biomarkers and growth performance in dairy calves. Vet. Sci. 2023, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C.; Junnikkala, S.; Peltoniemi, O. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 2019, 54, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Han, K.; Kim, I.H. Impact of artificial colostrum supplement on the growth performance and blood profile in piglets. Anim. Nutr. Feed Technol. 2022, 22, 665–672. [Google Scholar] [CrossRef]
- Uddin, M.K.; Hasan, S.; Peltoniemi, O.; Oliviero, C. The effect of piglet vitality, birth order, and blood lactate on the piglet growth performances and preweaning survival. Porc. Health Manag. 2022, 8, 52. [Google Scholar] [CrossRef]
- Aleman, M.; Weich, K.; Madigan, J. Survey of Veterinarians Using a Novel Physical Compression Squeeze Procedure in the Management of Neonatal Maladjustment Syndrome in Foals. Animals 2017, 7, 69. [Google Scholar] [CrossRef]
- Piccione, G.; Caola, G.; Refinetti, R. Maturation of the daily body temperature rhythm in sheep and horse. J. Therm. Biol. 2002, 27, 333–336. [Google Scholar] [CrossRef]
- Aleksiev, Y.; Gudev, D.; Dimov, G. Thermal status in three breeds of newborn lambs during the first 24 h of postnatal life. Bulg. J. Agrisultural Sci. 2007, 13, 563–573. [Google Scholar]
- Giannetto, C.; Arfuso, F.; Fazio, F.; Giudice, E.; Panzera, M.; Piccione, G. Rhythmic function of body temperature, breathing and heart rates in newborn goats and sheep during the first hours of life. J. Vet. Behav. 2017, 18, 29–36. [Google Scholar] [CrossRef]
- Labeur, L.; Villiers, G.; Small, A.H.; Hinch, G.N.; Schmoelzl, S. Infrared thermal imaging as a method to evaluate heat loss in newborn lambs. Res. Vet. Sci. 2017, 115, 517–522. [Google Scholar] [CrossRef]
- Vande Pol, K.D.; Tolosa, A.F.; Bautista, R.O.; Willard, N.C.; Gates, R.S.; Shull, C.M.; Brown, C.B.; Alencar, S.A.S.; Lents, C.A.; Ellis, M. Effects of drying and providing supplemental oxygen to piglets at birth on rectal temperature over the first 24 h after birth. Transl. Anim. Sci. 2021, 5, txab095. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.G.; Quinn, C.T.; Nielsen, S.G.; Raidal, S.L. Respiratory support for pharmacologically induced hypoxia in neonatal calves. Vet. Med. Int. 2016, 2016, 2129362. [Google Scholar] [CrossRef] [PubMed]
- Milan, H.F.M.; Campos Maia, A.S.; Gebremedhin, K.G. Prediction of optimum supplemental heat for piglets. Trans. ASABE 2019, 62, 321–342. [Google Scholar] [CrossRef]
- Malmkvist, J.; Damgaard, B.M.; Pedersen, L.J.; Jørgensen, E.; Thodberg, K.; Chaloupková, H.; Bruckmaier, R.M. Effects of thermal environment on hypothalamic-pituitary-adrenal axis hormones, oxytocin, and behavioral activity in periparturient sows. J. Anim. Sci. 2009, 87, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Vasdal, G.; Glærum, M.; Melišová, M.; Bøe, K.E.; Broom, D.M.; Andersen, I.L. Increasing the piglets’ use of the creep area—A battle against biology? Appl. Anim. Behav. Sci. 2010, 125, 96–102. [Google Scholar] [CrossRef]
- Menzies, P.I. Lambing Management and Neonatal Care. In Current Therapy in Large Animal Theriogenology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 680–695. [Google Scholar]
- Davis, J.D.; Xin, H.; MacDonald, R.D. MacDonald infrared thermographic evaluation of commercially available incandescent heat lamps. Appl. Eng. Agric. 2008, 24, 685–693. [Google Scholar] [CrossRef]
- Pedersen, L.J.; Larsen, M.L.V.; Malmkvist, J. The ability of different thermal aids to reduce hypothermia in neonatal piglets1. J. Anim. Sci. 2016, 94, 2151–2159. [Google Scholar] [CrossRef]
- Nowak, R.; Poindron, P. From birth to colostrum: Early steps leading to lamb survival. Reprod. Nutr. Dev. 2006, 46, 431–446. [Google Scholar] [CrossRef]
- Andersen, I.L.; Haukvik, I.A.; Bøe, K.E. Drying and warming immediately after birth may reduce piglet mortality in loose-housed sows. Animal 2009, 3, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Vande Pol, K.D.; Ellis, M.; Xiong, Y.; Gates, R. 7 Effect of piglet birth weight and drying on post-natal changes in rectal temperature. J. Anim. Sci. 2019, 97, 4. [Google Scholar] [CrossRef]
- Vasdal, G.; Østensen, I.; Melišová, M.; Bozděchová, B.; Illmann, G.; Andersen, I.L. Management routines at the time of farrowing—Effects on teat success and postnatal piglet mortality from loose housed sows. Livest. Sci. 2011, 2–3, 225–231. [Google Scholar] [CrossRef]
- Cote, A.; Blanchard, P.W.; Meehan, B. Metabolic and cardiorespiratory effects of doxapram and theophylline in sleeping newborn piglets. J. Appl. Physiol. 1992, 72, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Silvera, F.E.; Blasina, M.F.; Vaamonde, L.; Tellechea, S.; Godoy, C.; Zabala, S.; Mañana, G.; Martell, M.; Olivera, W. Sildenafil prevents the increase of extravascular lung water and pulmonary hypertension after meconium aspiration in newborn piglets. Braz. J. Med. Biol. Res. 2011, 44, 778–785. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienboire-Frosini, C.; Muns, R.; Marcet-Rius, M.; Gazzano, A.; Villanueva-García, D.; Martínez-Burnes, J.; Domínguez-Oliva, A.; Lezama-García, K.; Casas-Alvarado, A.; Mota-Rojas, D. Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor. Animals 2023, 13, 1542. https://doi.org/10.3390/ani13091542
Bienboire-Frosini C, Muns R, Marcet-Rius M, Gazzano A, Villanueva-García D, Martínez-Burnes J, Domínguez-Oliva A, Lezama-García K, Casas-Alvarado A, Mota-Rojas D. Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor. Animals. 2023; 13(9):1542. https://doi.org/10.3390/ani13091542
Chicago/Turabian StyleBienboire-Frosini, Cécile, Ramon Muns, Míriam Marcet-Rius, Angelo Gazzano, Dina Villanueva-García, Julio Martínez-Burnes, Adriana Domínguez-Oliva, Karina Lezama-García, Alejandro Casas-Alvarado, and Daniel Mota-Rojas. 2023. "Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor" Animals 13, no. 9: 1542. https://doi.org/10.3390/ani13091542
APA StyleBienboire-Frosini, C., Muns, R., Marcet-Rius, M., Gazzano, A., Villanueva-García, D., Martínez-Burnes, J., Domínguez-Oliva, A., Lezama-García, K., Casas-Alvarado, A., & Mota-Rojas, D. (2023). Vitality in Newborn Farm Animals: Adverse Factors, Physiological Responses, Pharmacological Therapies, and Physical Methods to Increase Neonate Vigor. Animals, 13(9), 1542. https://doi.org/10.3390/ani13091542







