Simple Summary
Early development of the intestinal microbiota is considered critical for enteric health in pigs. Dietary strategies that improve gut microbiota development can thus help prevent disturbances associated with weaning. This study evaluated the effects of a soluble oat β-glucan supplement, compared with a placebo, in five litters of piglets during the suckling period. The supplement was introduced at week 1 of age and was provided three times per week until weaning. The effects of the supplement were evaluated in terms of weight development, concentrations of short-chain fatty acids in plasma and intestinal contents, and microbiota composition in rectal swabs and intestinal contents. The results showed that microbiota composition and short-chain fatty acid concentration changed with piglet age, but with no clear effects of the β-glucan supplement. There were no differences in body weight gain between β-glucan-supplemented and control piglets. Several correlations between specific microbes and short-chain fatty acids were identified. Significant litter effects were also found, confirming the importance of genetics and pen environment in suckling piglets.
Abstract
The effects of early supplementation with oat β-glucan during the suckling period on piglet gut microbiota composition, concentrations of short-chain fatty acids, and gut physiological markers were assessed. Fifty piglets from five litters, balanced for sex and birth weight, were divided within litters into two treatment groups: β-glucan and control. Piglets in the β-glucan group received the supplement three times/week from day 7 of age until weaning. Rectal swab samples were collected from 10 piglets per treatment group (balanced across litters) from week 1 to week 4, and plasma samples were collected at 1, 3, and 4 weeks of age. Additional samples of intestinal tissues and jugular and portal vein plasma were collected from 10 animals at weaning (one per treatment group and litter). The concentrations of short-chain fatty acids in plasma and the microbiota composition in rectal swabs were mainly influenced by piglet age, rather than the supplement. There were significant differences in microbiota composition between litters and several correlations between concentrations of short-chain fatty acids in plasma and specific microbial taxa in rectal swabs. Overall, β-glucan supplementation did not have any clear impact on the gut environment in suckling piglets, whereas a clear age-related pattern emerged.
1. Introduction
A range of factors influence the composition of microbiota in the gastrointestinal tract of piglets, including genetics, housing conditions, use of medication and diet [1,2]. The weaning period is known to be a very stressful time in the life of piglets, because weaning is associated with abrupt separation from the sow, major dietary changes, and mixing with unfamiliar piglets. Stress can lead to increased incidence of enteric infections and intestinal inflammation [3,4]. Overgrowth of harmful bacteria is a major risk factor in the development of enteric diseases such as post-weaning diarrhoea, which can lead to financial losses due to decreased growth rate, mortality, and the extra cost of medication. Although the use of antibiotics as growth promotors has been banned in the European Union for almost two decades, antibiotics are still extensively used for therapeutic purposes, e.g., to treat gastrointestinal infections. Antimicrobial use and associated antimicrobial resistance can affect animal and also human health [5]. Thus, it is important to find alternatives to antibiotics to promote enteric health in piglets. One option is diet manipulation, targeting fibre content and quality, to stimulate microbial fermentation, feed intake, and weight gain [6]. Dietary fibre is used as a biological response modifier because it has beneficial effects on the intestinal tract and stimulates the growth of beneficial bacteria. Bacterial fermentation in the small and large intestines produces metabolites such as short-chain fatty acids (SCFA) that have been shown to have positive effects on energy metabolism, intestinal barrier, and immune system in both animals and humans [6,7,8].
Dietary fibre, in particular oat bran, contains large amounts of β-glucans, which are almost fully fermented by bacteria in the large intestine [9]. Fermentation of β-glucans has been shown to increase the concentrations of lactic acid and SCFA, reduce intestinal ammonia production, and stimulate growth of a diverse, beneficial intestinal microbiota in adult pigs [10,11]. β-glucans of microbial origin have also been shown to have immuno-stimulatory and anti-inflammatory properties and may promote survival in experimental animals infected with different microbes [12]. Fermentation of β-glucans is believed to increase the production of butyrate [10] and may be beneficial for intestinal development in young piglets because it provides an energy source for colonocytes and stimulates mucus production [13]. Butyrate has also been shown to lower the number of harmful enterobacteria and reduce inflammation in the gut [14]. Therefore, concentrations of faecal SCFA are considered a marker of gut health status [15]. However, a large fraction of the SCFA produced in the gut is absorbed, so faecal levels of SCFA do not necessarily reflect the amount of SCFA produced in the gut [16]. To obtain more comprehensive data, SCFA excreted in faeces and SCFA absorbed and circulating in blood should both be analysed. In piglets, only a few studies have measured both faecal and circulating levels of SCFA [17,18]. SCFA from plasma appears to be linked to metabolic health and is considered a good biomarker of the effects of prebiotic interventions [19]. Even fewer studies have examined prebiotic fibre supplements and their influence on circulating levels of SCFAs during the suckling period of piglets. Previous studies in piglets have mainly focused on the post-weaning period, with few studies focusing on the early-life establishment of the gut microbiota, its contribution to individual piglet performance, and whether or not it can be modified by nutritional treatments [20,21].
The aim of this study was to evaluate growth performance, gut microbiota composition, SCFA concentration in faeces and blood, and histological development of the gut in suckling piglets provided with an oat β-glucan supplement. The starting hypothesis was that the β-glucan supplementation leads to earlier development of a mature microbiota and higher SCFA production.
2. Materials and Methods
2.1. Ethics Statement
This study was approved by the Ethics Committee for the Uppsala region, with reference number DNR C 1054/16. The study was conducted in accordance with the ARRIVE guidelines [22].
2.2. Animals
The experiment involved 50 Hampshire x Yorkshire pathogen-free piglets from five litters [23]. Ten piglets from each litter were allocated to two equal-sized treatment groups that were balanced for sex and birth weight. In litters with more than 10 piglets, the remaining piglets were allowed to stay with the litter but were not included in the study. These selection criteria were set before the experiment.
2.3. Housing and Management
The experiment was carried out at the Swedish Livestock Research Centre at Lövsta, Uppsala. Pregnant sows were moved to the farrowing unit one week before expected farrowing and stayed with their piglets until weaning at 35 days of age. The farrowing pens (3.35 × 2.0 m) consisted of a heated concrete floor as the lying and feeding area (2.1 m × 2. 0 m), a slatted dung area (1.25 m × 2.0 m), and a heated corner that was only accessible to the piglets. The sows were given 15–20 kg of chopped straw two days prior to the expected farrowing date. An additional small amount of straw (0.5–1 kg/day) was given daily as enrichment after the pens were manually cleaned. Piglets were weighed, marked with an individual number within one day after birth (ear-tattoo), and ear-tagged with their individual number at five days of age. A 1-mL intramuscular injection of an iron supplement (Uniferon, 200 mg/mL) was given at five days and again at two weeks of age. All piglets had ad libitum access to creep feed (Gottfrid, Lantmännen, Sweden, Supplementary Table S1) and water during the suckling period. At weaning, the sow was removed, and the piglets remained in their pen until nine weeks of age, when they were handled according to regular routines at the Research Centre.
2.4. Treatments
Five suckling piglets per litter received an oral supplement of oat β-glucan (40 mg/kg body weight) (BG group) and five piglets per litter received an oral supplement of water (CON group). Disposable syringes were prefilled with a paste form of β-glucan dissolved in water, which was supplemented directly into the mouth of each pig in the BG group. The piglets were assigned to the treatment groups prior to supplementation, which started at seven days of age and was continued three times per week until weaning at five weeks of age. The pigs in the CON group were handled in the same way but received water by syringe, instead of the BG supplement. The dietary supplement used in the experiment was SweOat bran BG28 (Swedish oat fibre, Gothenburg, Sweden), which contained 28% soluble β-glucan with molecular weight 2000 kDa. The total dietary fibre content of BG28 was 52 g/100 g.
2.5. Experimental Procedures
An overview of the experimental set-up is shown in Figure 1. Weight gain was recorded for all piglets on a weekly basis from birth until nine weeks of age, with an extra weight measurement on the day of weaning. Four piglets per litter, two from each treatment, balanced for sex and weight between treatments, were selected for collection of faecal microbiota samples using rectal swabs (E-Swabs, Copan Diagnostics) at 1, 2, 3, and 4 weeks of age. The swabs were immediately placed on ice after sampling and within 2 h were transferred to a freezer and stored at −80 °C until analysis. Blood samples from the jugular vein of the same piglets were collected at 1, 3, and 4 weeks of age, using a 21G vacutainer needle and 4 mL VenoSafe Vacutainer EDTA-tubes. After centrifugation for 20 min at 4 °C and 1500× g, plasma was collected and stored at −80 °C until analysis. The oral supplementation and sampling were performed consecutively. Due to obvious differences in the characteristics of the supplements, the individual collecting the samples was not blind to the treatment. However, the individual performing the laboratory analyses was blind to the treatments, reducing any potential bias in outcome for the two treatment groups. Piglets that needed treatment with antimicrobial drugs were excluded from the study.
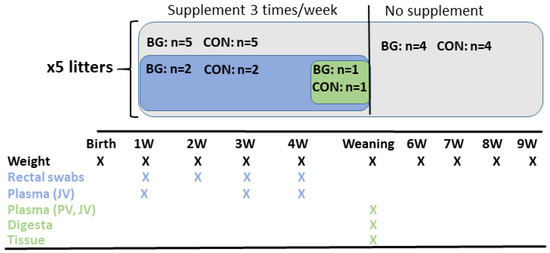
Figure 1.
Overview of the experimental set-up. BG = β-glucan, CON = control, JV = jugular vein, PV = portal vein.
2.6. Post-Mortem Sampling Procedures
On the day before weaning, one piglet per treatment group and litter was euthanized for collection of blood and intestinal samples as described below, while the remaining piglets were weaned and handled according to existing farm routines. The five BG and five CON piglets used for post-mortem sampling were selected so that sex and body weight were balanced between the two diet treatments. The selected piglets were anaesthetized with a combination of medetomidine (Domitor® vet, 1 mg/mL; Orion Pharma Animal Health, Espoo, Finland) at a dose of 0.05 mg/kg BW, and tiletamine and zolazepam (Zoletil® 50 mg + 50 mg/mL; Virbac, Carros, France) at a dose of 2.5 + 2.5 mg/kg BW injected intramuscularly (i.m.). Buprenorphine (Vetergesic® vet, 0.3 mg/mL; Orion Pharma Animal Health, Espoo, Finland) was given at a dose of 0.01 mg/kg BW i.m. for additional analgesia and a 22G cannula (BDTM Venflon; BD, Franklin Lakes, NJ, USA) was placed in the auricular vein. Throughout the sampling procedure, anaesthesia was maintained with intravenous (i.v.) bolus doses of the pharmaceuticals listed above. Blood samples were collected from the jugular vein with a 20G vacutainer needle into EDTA vacutainer tubes. For blood collection from the portal vein, the abdomen was opened with an incision in linea alba and along the last rib. The portal vein was reached by blunt dissection, and blood was collected with a 20G needle and a 10 cc syringe and immediately transferred to an EDTA vacutainer tube. At the end of the procedure, the pigs were euthanized with pentobarbital sodium (Allfatal vet, 100 mg/mL; Omnidea, Stockholm, Sweden) i.v. while still under general anaesthesia. The blood samples were kept on ice and centrifuged and stored as described above. After euthanasia, the gastrointestinal tract was removed. A vertical incision was made immediately on the distal part of the ileum (approximately 15 cm from the caecum) and the central part of the colon, for the collection of intestinal samples. Intestinal contents collected from the colon were snap-frozen in liquid nitrogen and stored at −80 °C until analysis. In addition, one segment of tissue each from the distal ileum and central colon were taken and fixed in 10% formalin. All samples were individually re-coded and labelled before analysis, to avoid any bias in data analysis.
2.7. Histology of Intestinal Tissue
Ileal and colon tissue were stored in formalin with 10% neutral phosphate buffer (Merck KGaA, Darmstadt, Germany) for 48 h. After fixation, three samples per tissue and piglet were trimmed, dehydrated, cleaned, and embedded in paraffin. The paraffin-embedded tissue blocks were then cut into 4 mm thick sections, which were placed on a slide and stained with haematoxylin and eosin. A light microscope (Nikon ECLIPSE 80i, BergmanLabora AB, Danderyd, Sweden), equipped with image analysis software (NIS-Elements D 5.20.02, Nikon Instruments, Melville, NY, USA) was used for examination of gut thickness, mucosal thickness, villus height, crypt depth, and thickness of muscularis externa.
2.8. DNA Extraction, PCR Conditions and Sequence Analysis
DNA was extracted from rectal swabs and intestinal contents using QIAamp DNA Stool Minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, but with the addition of an extra mechanical lysis step using 0.1 mm zirconium/silica beads (Biospec Products, Bartlesville, OK, USA) and 2 × 1 min at 6000 rpm with a Precellys evolution (Bertin Instruments, Montigny-le-Bretonneaux, France). The isolated DNA was stored at −20 °C until analysis. PCR amplicons were generated from 16S rRNA genes using the primers 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC). PCR amplification used DreamTaq PCR chemistry with the following PCR conditions: initial denaturation at 94 °C for 3 min, followed by 25 cycles with 94 °C for 40 s, 58 °C for 40 s, and 72 °C for 60 s, and finally elongation at 72 °C for 7 min. Positive PCR reactions were confirmed with gel electrophoresis (1% agarose) using a Thermo Fisher Scientific Gene Ruler 1 kB DNA ladder as a size marker. The PCR product was purified using Agencourt AMPure purification (Beckmann Coulter, Indianapolis, IN, USA). A second PCR step was then applied to attach sample-specific barcodes and adaptor sequences. Cycling conditions were as follows: initial denaturation at 94 °C for 3 min, 8 cycles of 94 °C for 40 s, 58 °C for 40 s, and 72 °C for 60 s. PCR amplification ended with a final elongation step at 72 °C for 7 min. After a second cleaning step, all samples were quantified using a Qubit Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Samples were pooled in equimolar amounts and sent for sequencing at SciLifeLab in Stockholm, Sweden, using Illumina MiSeq.
The sequencing data generated were processed according to the procedure described by Müller et al. [24]. The Quantitative Insights into Microbial Ecology (QIIME) version 1.8.0 pipeline was used for the quality filtering of data and to generate operational taxonomic units (OTUs) using the open reference OTU picking strategy at a threshold of 97%, with U-CLUST against a Greengenes core set (gg_13_8) [25]. Representative sequences were aligned against the Greengenes core set using PyNAST software (chimeric sequences were removed by ChimeraSlayer [26]). Taxonomy was assigned to each OTU using the Ribosomal Database Project (RDP) classifier with a minimum confidence threshold of 80% [27]. The final OTU table was filtered based on two criteria: an OTU had to be observed in three samples to be retained and an OTU had to contain 87 reads (0.001% of total reads). The sequence dataset was normalised so that each sample contained 14,900 sequences prior to analysis of microbiota. The raw sequencing data have been deposited at the National Center for Biotechnology Information database (NCBI), under accession number PRJNA940420.
2.9. Determination of SCFAs in Plasma and Digesta
Concentrations of SCFAs and caproic acid in EDTA plasma and digesta were analysed by liquid chromatography-mass spectrometry (LC-MS) according to a method described previously [28], with some modifications (for details, see Supplementary File S1).
2.10. Statistical Analysis
Microbiota composition was analysed using both univariate and multivariate approaches. All multivariate statistical analyses were performed using the statistical software PAST, version 4.04 [29]. A multivariate approach involving principal coordinate analysis (PCoA) based on Bray–Curtis distance was performed on OTU data. Clustering patterns identified in the PCoA were validated by analysis of similarity (ANOSIM) based on 9999 permutations.
Relative abundance of the five most abundant phyla and the 10 most abundant genera, alpha diversity index values, plasma concentrations of SCFA and caproic acid, and body weight were analysed in r software (version 4.0.2.). Model assumptions of normality and homoscedasticity of the data were visually checked by QQ-plots (LMERConvenienceFunctions package) [30]. Relative abundance, alpha diversity, SCFA, caproic acid, and weight data were analysed by mixed models (packages lme4 [31] and lmerTest [32]), with treatment, sex, age, and their interactions as fixed effects and with piglet ID nested within sow ID as a random effect. If the data did not meet the normality assumptions, data transformation was applied. Data on acetate and butyrate concentrations and on phylum Proteobacteria remained untransformed, but data on valeric acid, Fusobacteria, S24_7:g, and [Prevotella] were square-root transformed, and all other variables were log-transformed. If the variable still did not meet normality assumption, any outliers with residuals larger than 2.5 units were excluded from the analysis (Supplementary Table S2). After data transformation and exclusion of the outliers, all variables met the normality assumption.
Stepwise backward selection was used for model selection. Post-hoc Tukey HSD tests were performed using the emmeans package (R Foundation for Statistical Computing, Vienna, Austria) [33]. Correlations between the top 10 genera and plasma concentrations of SCFA and caproic acid were analysed with linear correlations (Pearson) on samples from animals for which data on both microbiota and SCFA were available. Effects of treatment on samples collected from the euthanized animals (tissues, jugular, and portal vein plasma) were analysed with t-tests. To correct for multiple analyses in the correlation analysis, the Benjamini–Hochberg procedure was used with a false discovery rate (FDR) of 5% [34].
3. Results
3.1. Animal Growth and Performance
Mean birth weight of the piglets was 1.85 ± 0.04 kg (BG) and 1.86 ± 0.04 kg (CON). The animals grew well in both treatment groups, and there were no differences in growth (Figure 2) or in average daily weight gain (Supplementary Figure S1) between BG and CON piglets. The piglets were healthy during the experiment, none died, and there were no cases of diarrhoea that needed treatment, although loose stools were prevalent within both groups during the experimental period.
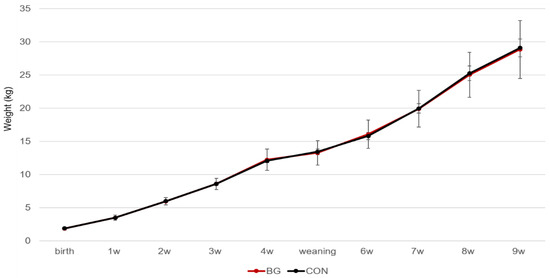
Figure 2.
Weight gain (mean ± SE) of piglets in the β-glucan supplemented group (BG) and control group (CON) from birth to nine weeks of age.
3.2. Development of the Microbiota
Analysis of microbial diversity revealed a significant increase with age in both Shannon index and Chao-1 index, but with no differences between BG and CON piglets (Table 1). Shannon index was influenced by a sex by age interaction (F(3,52.5) = 9.3, p < 0.001), with female piglets showing higher microbiota diversity than males at 4 weeks of age (p < 0.001). Chao-1 index was also influenced by a sex by age interaction (F (3,54) = 3.8, p < 0.05), with females having higher species richness than males at 4 weeks of age (p < 0.05).

Table 1.
Microbial diversity (mean ± SE) determined based on Illumina sequencing data in samples collected from piglets receiving a β-glucan supplement (BG) and control (CON) piglets.
At phylum level, the microbiota was primarily dominated by Firmicutes, Bacteroidetes, and Proteobacteria (Figure 3A). At genus level, Escherichia, Bacteroides, Prevotella, and Lactobacillus were most dominant, comprising more than 50% of relative abundance in swab samples (Figure 3B).
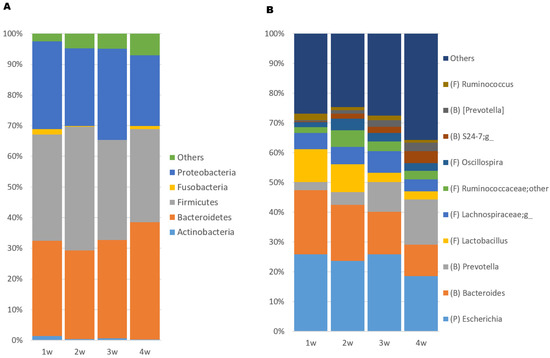
Figure 3.
Stacked bar plot showing relative abundance of (A) the five most abundant bacterial phyla and (B) the 10 most abundant genera in rectal swab samples collected from piglets over the whole suckling period. Others = remaining accumulated abundance of bacterial taxa not among the top 10 in abundance in the samples, B = Bacteroidetes, F = Firmicutes, P = Proteobacteria.
To further assess microbial community composition, a multivariate statistical approach was applied. The ordination with PCoA revealed a clustering pattern that was linked to piglet age but did not indicate any difference in clustering pattern between BG and CON pigs (Figure 4A). This was confirmed in two-way ANOSIM, where age was a significant factor (p = 0.0001, R = 0.24) but not treatment (p = 0.32, R = 0.013). The PCoA and ANOSIM results were also used to evaluate the effect of litter on microbiota composition and revealed an evident effect of litter (Figure 4B), with a significant difference between both age and litter in the two-way ANOSIM (age: p = 0.0001, R = 0.43; litter: p = 0.0001, R = 0.45).
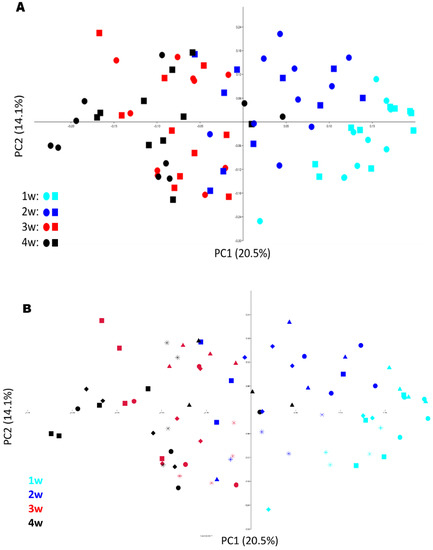
Figure 4.
(A,B) Principal coordinates analysis (PCoA) plots with BrayCurtis distances showing microbiota development in 20 piglets (swab samples) from 1–4 weeks of age (10 receiving a β-glucan (BG) supplement and 10 from the control group (CON)). Symbols in plot (A) represent different treatments (circles = BG, squares = CON) and symbols (squares, diamonds, circles, triangles and stars) in plot (B) different litters.
A univariate statistical approach was also applied to evaluate the effect of treatment and age on the five most abundant phyla and the 10 most abundant genera (Table 2). At phylum level, there was a tendency for a treatment x age interaction for Actinobacteria (F(3,53.4) = 2.5, p = 0.07), with BG piglets tending to have higher relative abundance than CON piglets at week 1 (p = 0.07) (Table 2). Relative abundance of Actinobacteria decreased with age (F(3,53.4) = 8.24, p < 0.001) (Table 2). Males (0.9 ± 0.2%) had a higher relative abundance of Actinobacteria than females (0.4 ± 0.1%), F(1,13.3) = 5.9, p < 0.05) (Table 2). A tendency for a treatment x age interaction was found for Bacteroidetes (F(3.68) =2.4, p = 0.07), with higher relative abundance at week 1 in pigs from the BG group (p = 0.007) (Table 2). Relative abundance of Firmicutes was also influenced by age (F(3,72.0) = 5.1, p < 0.01, with a significant decrease in abundance at week 4 compared with week 2 (p < 0.05), and week 3 compared with week 4 (p < 0.01) (Table 2). Moreover, Fusobacteria was influenced by age (F(3,55,9) = 34.4, p < 0.0001, with abundance decreasing significantly from week 1 to week 2 (p < 0.001), week 3 (p < 0.001) and week 4 (p < 0.001) (Table 2). There was a tendency for a sex effect on Proteobacteria (F(1,14.3) = 4.2, p = 0.06), where male piglets had a higher abundance of Proteobacteria than females (Table 2).

Table 2.
Relative abundance (mean percentage ± SE) on phylum and genus level of microbial taxa in swab samples collected from piglets receiving a β-glucan supplement (BG) and control (CON) piglets during the suckling period.
At genus level, relative abundance of Escherichia was influenced by a treatment x age interaction (F(3,63) = 3.5, p < 0.05), with higher relative abundance in the BG group at 4 weeks of age (p = 0.003) (Table 2). An age x sex interaction was also found (F(3,63) = 4.7, p < 0.01), where female piglets had higher relative abundance of Escherichia than male piglets at week 4 (p < 0.001). Relative abundance of Bacteroides was influenced by a treatment x age interaction (F(3,62) = 3.6, p < 0.05), with higher abundance in the BG group in week 1 (p < 0.05), but higher abundance in the CON group in week 2 (p < 0.05). In addition, an age × sex interaction was found (F(3,62) = 4.0, p < 0.05), with higher relative abundance in male piglets compared with females (p < 0.01) (Table 2). Relative abundance of Prevotella was influenced by a treatment x sex x age interaction (F(3,46.5) = 6.6, p < 0.001), where in week 1 (p < 0.01), week 2 (p < 0.05), and week 3 (p < 0.05), female BG piglets had a higher relative abundance of Prevotella than female CON piglets (Table 2). Relative abundance of Lactobacillus was influenced by age (F(3,56) = 18.6, p < 0.001) and was higher in weeks 1 and 2 compared with weeks 3 and 4 (p < 0.001) (Table 2). The relative abundance of Ruminococcus was influenced by age (F(3,72.2) = 8.9, p < 0.001), with abundance increasing from week 1 to week 2 (p = 0.001) and decreasing from week 2 to week 4 (p < 0.001) (Table 2). The relative abundance of Oscillospira was influenced by age (F(3,71.9) = 6.8, p < 0.001), with lower abundance in week 1 compared with week 2 (p = <0.001), week 3 (p < 0.05), and week 4 (p < 0.05) (Table 2). The abundance of S24_7:g was influenced by an age x sex interaction (F(3,52.9) = 3.3, p < 0.05), where female piglets had higher abundance at week 4 (p < 0.05) than male piglets (Table 2). Relative abundance of [Prevotella] was influenced by age (F(3,56.7) = 8.5, p < 0.001), with a higher abundance at week 3 (p < 0.05) and week 4 (p < 0.001) compared with week 1, and a higher abundance in week 4 (p < 0.05) compared with week 2 (Table 2). Ruminococcus was influenced by age (F(3,72.2) = 8.9, p < 0.001), with abundance decreasing from week 1 to week 2 (p < 0.001) and week 3 (p < 0.05), and from week 2 to week 4 (p < 0.05) (Table 2).
The microbiota in colon digesta from euthanized piglets from the BG and CON groups were also compared, but PCoA did not reveal any separate clustering of these samples according to treatment or any significant effects of the supplement (Supplementary Figure S2).
3.3. SCFA and Caproic Acid Concentrations in Plasma and Colon Digesta
Analysis of SCFA and caproic acid levels in plasma samples collected from the jugular vein at 1, 3, and 4 weeks of age revealed that the concentration of SCFA was mainly affected by age. The only observed effect of treatment was on acetic acid concentration, which was lower in the BG group than in the CON group (F(1,18) = 6.1, p < 0.05) (Table 3). Acetic acid concentration was also influenced by age (F(2,38) = 39.8, p < 0.001), with an increase from week 1 to week 3 (p < 0.001) and week 4 (p < 0.001). The concentration of propionic acid was influenced by age (F(2,38) = 3.3, p < 0.05), with the highest concentration at week 3 (p < 0.05) (Table 3). Plasma concentration of butyric acid was influenced by an age x sex interaction (F(2,34.6) = 4.5, p < 0.05), with higher concentrations in females than in males in week 4 (p < 0.05). Formic acid concentration was influenced by age (F(2,38) = 25.7, p < 0.001), decreasing from week 1 to week 3 (p < 0.01) and week 4 (p < 0.001), and from week 3 to week 4 (p < 0.01) (Table 3). Iso-butyric acid concentration was influenced by age (F(2,38) = 6.3, p < 0.05), and was higher in week 3 (p < 0.05) and week 4 (p < 0.05) than in week 1 (Table 3). Valeric acid concentration tended to be higher in the BG than in the CON group (F(1,18) = 4.0, p = 0.06) and was influenced by age (F(2,38) = 9.3, p < 0.001), with an increase from week 1 to week 3 (p < 0.05) and week 4 (p < 0.001). Succinic acid concentration was influenced by age (F(2,38) = 5.4, p < 0.05) and decreased from week 1 to week 4 (p < 0.05) (Table 3). Caproic acid concentration tended to be higher in BG piglets than in CON piglets (F(1,12) = 3.5, p = 0.08) (Table 3).

Table 3.
Concentrations (µM) of different short-chain fatty acids (SCFA) and caproic acid in plasma collected from piglets receiving a β-glucan supplement (BG) and control (CON) piglets during the suckling period. Mean ± SE.
Jugular vein and portal vein plasma and colon digesta taken from 10 euthanized animals were evaluated for the possible influence of the BG supplement on SCFA concentrations. SCFA concentration varied between individual pigs and was considerably higher in plasma samples from the portal vein than in samples from the jugular vein. However, no significant effects linked to the treatment were found for colon digesta or plasma samples (Supplementary Tables S3 and S4). The molar proportions of SCFA differed between plasma and colon digesta, with higher similarity in portal vein and colon digesta than in jugular vein and colon digesta (Figure 5). However, correlation analysis of individual SCFA in the different sample types did not reveal any significant correlations.
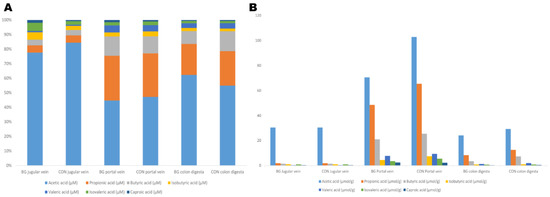
Figure 5.
(A) Relative concentrations (% of total) and (B) mean absolute concentrations (μmol/g) of SCFA and caproic acid in jugular vein and portal vein plasma and colon digesta from 10 euthanized piglets (five piglets in a group receiving a β-glucan supplement and five control (CON) piglets.
3.4. Gut Histological Measurements
Morphological parameters measured in tissue samples are shown in Table 4. No significant differences were detected between BG and CON for any of the measured parameters.

Table 4.
Villus height (µm), crypt depth (µm), and thickness of mucosa, total gut (µm), and muscularis externa of the ileum and colon in piglets receiving a β-glucan supplement (BG) and in control (CON) piglets during the suckling period. Mean ± SE.
3.5. Correlations
Relative abundance data from the 10 most abundant genera were analysed for correlations with SCFA in plasma (Supplementary Figure S2). Among the genera classified to the phylum Bacteroidetes, Bacteroides was negatively correlated with acetic acid (r = −0.34, p = 0.008) and propionic acid (r = −0.35, p = 0.004), while Prevotella was positively correlated with acetic acid (r = 0.42, p = 0.0008), but negatively correlated with formic acid (r = −0.31, p = 0.016). The taxon S24_7:g was negatively correlated with formic acid (r =−0.48, p = 0.0001), but positively correlated with acetic acid (r = 0.55, p = 0.000005), propionic acid (r = 0.34, p = 0.007), butyric acid (r = 0.49, p = 0.00005), iso-butyric acid (r = 0.34, p = 0.009), and valeric acid (r = 0.33, p = 0.01). The taxon [Prevotella] was positively correlated with acetic acid (r = 0.51, p = 0.00003), propionic acid (r = 0.37, p = 0.003), and butyric acid (r = 0.39, p = 0.002). Among the dominating genera within the phylum Firmicutes, Lactobacillus was positively correlated with succinic acid (r = 0.36, p = 0.005) but negatively correlated with acetic acid (r = −0.46, p = 0.0002). Lachnospiraceae;g was positively correlated with valeric acid (r = 0.31, p = 0.016), Ruminococcaceae;g_other was positively correlated with propionic acid (r = 0.36, p = 0.004) and iso-butyric acid (r = 0.31, p = 0.015), and Oscillospira was positively correlated with iso-butyric acid (r = 0.33, p = 0.011). Moreover, Ruminococcus showed a positive correlation with formic acid (r = 0.42, p = 0.0008) and a negative correlation with acetic acid (r = −0.39, p = 0.002). Escherichia, belonging to Proteobacteria, was positively correlated with caproic acid (r = 0.33, p = 0.009). There were also several correlations between individual bacterial taxa (Supplementary Figure S3).
4. Discussion
This study investigated the effect of early dietary supplementation with β-glucan on the development of the gut microbiota, concentrations of SCFA in plasma and digesta, and the morphological structure of the gut in piglets. The results showed mainly age-related effects, with maturation of microbiota composition and higher concentrations of certain SCFA in plasma at older age. Several correlations were found between certain microbial taxa and concentrations of SCFA and caproic acid in plasma samples.
Early in the piglets’ life, Escherichia, Lactobacillus, and Bacteroides were dominating taxa, but with older age both Lactobacillus and Bacteroides showed a reduction in relative abundance. The relative abundance of Lactobacillus decreased from 9% initially to 2% by the end of the suckling period. Some previous studies have found a similar trend, with a significant decrease in the abundance of Lactobacillus from newly weaned piglets compared to piglets at the end of the suckling period [35,36]. In contrast, Frese et al. [37] found an increase in the proportion of Lactobacillus directly after weaning. In the present study, we observed a decrease in the relative abundance of Bacteroides with older age, which is in agreement with findings in other studies [37,38].
A gradual increase in the relative abundance of Prevotella was observed during the suckling period, which is in agreement with findings in earlier studies on gut microbiome sampled at different ages [38,39,40,41,42]. Prevotella is known to harbour specific enzymes that can utilise plant polysaccharides [43]. Thus, it is commonly present in low abundance in nursing animals and usually increases in abundance when pigs are introduced to solid food [38]. Interestingly, in the present study, Prevotella showed a gradual increase over the whole suckling period, possibly due to increasing intake of creep feed with age during the suckling period [44]. Consumption of creep feed has been shown to contribute to microbiota development [20,21]. Creep feed consumption was not recorded in the present study, so no conclusions can be drawn on whether it contributed to the observed increase in the abundance of Prevotella.
Several differences in microbial composition correlated with piglet sex were observed. Relative abundance of the phylum Proteobacteria was higher in male piglets, while at genus level Escherichia showed higher abundance in female piglets and Bacteroides showed higher abundance in male piglets. However, previous studies have found conflicting results, e.g., Zhou et al. [45] found a higher abundance of Proteobacteria in female pigs, and He et al. [46] found a higher abundance of Escherichia in males and Bacteroides in females. The reason for the discrepancies between studies is unknown, but one possible explanation is age differences, as the pigs in those studies were older than the piglets in our study.
It is uncertain to what extent the immature gut microbiota in young piglets can utilise dietary fibre [47]. Microbial metabolism of dietary fibre leads to various end-products [48], and the production of SCFA is considered to be a key factor influencing overall gut health. A substantial part of the energy contribution from the gut in weaned pigs is related to SCFA, with intestinal production of SCFA depending on the size of the gastrointestinal tract and the population and composition of bacteria in the gut [49]. In the present study, changes in SCFA concentrations in plasma were linked more strongly to age than to treatment. The only observed effect linked to the treatment was for acetic acid. However, the acetic acid concentration in plasma was higher in CON piglets at the start of the study, so this difference likely reflected individual differences rather than being an effect of the supplementation.
Formic and succinic acid concentrations in plasma decreased with age, whereas the concentrations of acetic, propionic, butyric, iso-butyric, and valeric acid all increased with age. As the abundance of Bacteroides and Lactobacillus decreased with age, while the levels of acetic and propionic concentration increased, it was not surprising that these variables were negatively correlated. In contrast, Prevotella increased with age and was positively correlated with concentrations of acetic, butyric, and valeric acid. Previous studies have found that Lactobacillus spp., Bacteroides spp., Bifidobacterium spp., Streptococcus spp., Prevotella spp., and Ruminococcus spp. are the most important producers of acetate in the gastrointestinal tract [50,51]. Propionate is mainly produced by Bacteroides spp., Megasphaera elsdenii, Roseburia inulinivorans, Dialister spp., Ruminococcus obeum, Veillonella spp., Coprococcus catus, and Phascolarctobacterium succinatutens [52]. In the present study, Prevotella aligned with prior knowledge of the specific gut bacteria producers of acetate, but Lactobacillus spp. and Bacteroides spp. were negatively correlated with the concentrations of acetic and propionic acid. This could be due to the fact that SCFA were measured in plasma and microbiota taxa in rectal swabs. It has been reported in several previous studies that the concentrations and proportions of circulating SCFA and SCFA in faeces samples are not well correlated [53,54,55]. There was a clear difference in the concentrations of SCFA and caproic acid between portal and jugular vein plasma, with higher concentrations in the portal vein samples (even higher than found in the colon digesta). The observed difference in molar proportions between the portal and jugular vein samples, with reduced proportions of both propionic and butyric acid, suggests that they were metabolised in the liver.
Previous studies on weaned pigs have found that inclusion of oat or barley β-glucan or oat products in the diet is associated with an increased proportion of specific microbial taxa in the colon and ileum, i.e., Lactobacillus spp., Bifidobacterium spp., and Prevotella [56,57,58]. Other studies have found no changes in the gut microbial community, plasma SCFA, or immune function on adding oat β-glucan to the diet of pigs [59,60]. In the present study, there were no significant differences in microbiota, SCFA concentrations, or gut morphological structure that could be linked to the BG treatment alone. The β-glucan supplement was introduced from seven days of age, with three doses per week until weaning. It is possible that more frequent doses or a higher dose than 40 mg/kg body weight could have resulted in an effect of the treatment on the gut microbiota, and hence increased SCFA production. Previous studies on weaned pigs receiving β-glucan from cereals and microbial origin have used doses ranging from 50 to 200 mg/kg body weight [61]. A recent study on weaned pigs receiving a dietary β-glucan supplement and challenged with Escherichia coli used a high dose of β-glucan (500 mg/kg BW) and observed an increased abundance of Lactobacillus in the supplemented group compared with the control group [62].
Apart from most previous studies being performed post-weaning, on older pigs with a more developed microbiota that can utilise β-glucan more effectively, other possible reasons for the differences in response between studies could be related to fermentation rate in the digestive tract, which depends on the chemical composition and physiochemical properties of different types of β-glucans [63,64]. β-glucans can differ widely in both concentration and solubility, depending on the source [65]. For instance, β-glucan from fungi and yeasts is known to have immuno-modulating effects [66,67]. Yeast-derived β-glucan has high bioactivity [68], and effects on the immune system have been demonstrated in vitro [69,70] and in a recent in vivo study [71]. Although barley has a higher β-glucan content than oats [72], β-glucan from oats has been shown to be better for gut health than barley β-glucan when fed to rats at the same dosage [73].
The lack of differences between the treatment groups in the present study could potentially also be related to microbial cross-contamination by allocoprophagy [74], as we provided the CON and BG supplement to individuals within the same litters. However, this study design had many advantages, e.g., siblings from the BG and CON groups had a shared mother, genetic predisposition, and early-life litter exposure. We observed clear litter effects on gut microbial community clusters in our study. The univariate statistical analyses were litter-adjusted, so these effects were accounted for in the statistical model.
Previous studies on weaned pigs fed β-glucan derived from cereal and microbial sources have reported increased levels of butyrate and butyrate-producing bacteria [56,75]. Butyrate can act as an inhibitor of pathogenic microorganisms by reinforcing the mucus layer, enhancing immunological development, and acting as a nutrient source for the colon epithelium [76,77]. In the present study, the concentration of butyric acid was numerically, but not statistically significantly, higher in plasma samples from the BG group, but not in digesta samples, indicating that BG supplementation contributed to higher concentrations of butyrate and butyrate-producing bacteria. Families such as Lachnospiraceae and Ruminococcaceae, which contain several genera known to produce butyrate, were found in the samples, but did not differ in relative abundance between the treatments.
It has been shown that transition to solid feed can cause short-term villus and crypt disorders in piglets [78]. Moreover, it is suggested that 14–21 days of life is a crucial period for maturation of the mucosal structure and mucosal development [79]. However, no measurable changes in intestinal mucosa were seen in BG piglets in our study, and we found no indications of local inflammation in villi and crypts in any of the piglets. The effects of dietary fibre on gut morphology in piglets cannot be assessed based on the literature, as some studies report changes in morphology in piglets fed solid feed before weaning [80] and others report no changes [81].
Comparison of the results from this and previous studies is challenging, due to wide variation in the data obtained, differences in supplement dose and timing, and lack of studies on β-glucan in young piglets during the suckling period.
5. Conclusions
Early supplementation of piglets with oat β-glucan during the suckling period had no obvious or possible long-lasting effects, with piglet age and litter rather than dietary β-glucan supplementation having the most effect on gut microbiota development and on SCFA concentrations. This suggests that cereal β-glucan has a limited capacity to contribute to a beneficial gut microbiota development during the suckling period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13081349/s1, Supplementary Figure S1: Average daily weight gain of piglets included in the study; Supplementary Figure S2: PCoA plot of colon digesta from euthanized piglets; Supplementary Figure S3: Correlation between the top 10 genera and SCFA concentrations; Supplementary Table S1: Dietary composition of creep feed for suckling piglets; Supplementary Table S2: Excluded outliers; Supplementary Table S3: SCFA and caproic acid concentrations in colon digesta from euthanized piglets; Supplementary Table S4: SCFA and caproic acid concentrations in plasma from euthanized piglets; Supplementary File S1: Analysis of plasma SCFA.
Author Contributions
Conceptualization, J.E.L. and J.D.; methodology, J.E.L., R.L. and J.D.; software, L.S.; formal analysis, J.D., E.V. and L.A.; data interpretation, J.E.L., L.K. and T.L.; investigation, J.D., E.M., Y.H. and L.A.; resources, J.D.; data curation, L.S. and J.D.; writing—original draft preparation, L.A. and J.D.; writing, review and editing, J.E.L., T.L., E.V., L.K., L.S., R.L., Y.H. and E.M.; supervision, J.D.; project administration, J.D. and L.A.; funding acquisition, J.E.L., J.D. and L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish research council FORMAS (contracts: DNR 942-2015-630 and DNR 2017-01128). The funder did not have any influence on the experimental design, data analysis, interpretation of the results, or the decision to publish.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Uppsala region, Sweden (DNR C 1054/16.).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequencing data have been deposited at the National Center for Biotechnology Information database (NCBI), under accession number PRJNA940420. Other data from this research study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aluthge, N.D.; Van Sambeek, D.M.; Carney-Hinkle, E.E.; Li, Y.S.; Fernando, S.C.; Burkey, T.E. The pig microbiota and the potential for harnessing the power of the microbiome to improve growth and health1. J. Anim. Sci. 2019, 97, 3741–3757. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kye, Y.C.; Kim, G.; Kim, H.W.; Gu, M.J.; Umboh, J.; Maaruf, K.; Kim, S.W.; Yun, C.H. Stress, Nutrition, and Intestinal Immune Responses in Pigs—A Review. Asian-Australas J. Anim. Sci. 2016, 29, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef]
- Van Hees, H.M.J.; Davids, M.; Maes, D.; Millet, S.; Possemiers, S.; den Hartog, L.A.; van Kempen, T.; Janssens, G.P.J. Dietary fibre enrichment of supplemental feed modulates the development of the intestinal tract in suckling piglets. J. Anim. Sci. Biotechnol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Tian, G.; Wu, X.; Chen, D.; Yu, B.; He, J. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Gosalbes, M.J.; Yuste, S.; Valls, R.M.; Pedret, A.; Llauradó, E.; Jimenez-Hernandez, N.; Artacho, A.; Pla-Pagà, L.; Companys, J.; et al. Gut metagenomic and short chain fatty acids signature in hypertension: A cross-sectional study. Sci. Rep. 2020, 10, 6436. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Jørgensen, H.; Theil, P.K. Changes in short-chain fatty acid plasma profile incurred by dietary fiber composition. J. Anim. Sci. 2016, 94, 476–479. [Google Scholar] [CrossRef]
- Verbeek, E.; Keeling, L.; Landberg, R.; Lindberg, J.E.; Dicksved, J. The gut microbiota and microbial metabolites are associated with tail biting in pigs. Sci. Rep. 2021, 11, 20547. [Google Scholar] [CrossRef]
- Muller, M.; Hernandez, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef]
- Choudhury, R.; Middelkoop, A.; Boekhorst, J.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Early life feeding accelerates gut microbiome maturation and suppresses acute post-weaning stress in piglets. Environ. Microbiol. 2021, 23, 7201–7213. [Google Scholar] [CrossRef]
- Choudhury, R.; Middelkoop, A.; De Souza, J.G.; Van Veen, L.A.; Gerrits, W.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci. Rep. 2021, 11, 1–16. [Google Scholar]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Wallgren, P.; Vallgårda, J. Seroproduktion—Presentation, definition och kravlista. Sven. Vet. Tidn. 1993, 45, 733–735. [Google Scholar]
- Müller, B.; Sun, L.; Westerholm, M.; Schnürer, A. Bacterial community composition and fhs profiles of low- and high-ammonia biogas digesters reveal novel syntrophic acetate-oxidising bacteria. Biotechnol. Biofuels 2016, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classsifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tremblay Antoine, R.J. LMER Convenience Functions: Model Selection and Post-Hoc Analysis for (G)LMER Models, R Package Version 3.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V. Estimated Marginal Means, aka Least-Squares Means, R Package Version 1.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple hypothesis testing. J. Roy. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Qi, R.; Qiu, X.; Du, L.; Wang, J.; Wang, Q.; Huang, J.; Liu, Z. Changes of Gut Microbiota and Its Correlation With Short Chain Fatty Acids and Bioamine in Piglets at the Early Growth Stage. Front. Vet. Sci. 2020, 7, 617259. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, Introduction of Solid Feed and Weaning Are More Important Determinants of Gut Bacterial Succession in Piglets than Breed and Nursing Mother as Revealed by a Reciprocal Cross-Fostering Model: Gut Bacterial Succession in Piglets. Environ. Microbiol. 2016, 18, 1566. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.-J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Ke, S.; Fang, S.; He, M.; Huang, X.; Yang, H.; Yang, B.; Chen, C.; Huang, L. Age-based dynamic changes of phylogenetic composition and interaction networks of health pig gut microbiome feeding in a uniformed condition. BMC Vet. Res. 2019, 15, 172. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.-R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.-J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increases the abundance of Bacteroides–Prevotella–Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Fraser, D.; Pajor, E.A.; Feddes, J.J.R. The relationship between creep feeding behavior of piglets and adaptation to weaning: Effect of diet quality. Can. J. Anim. Sci. 1994, 74, 1–6. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, W.; Shang, W.; Du, H.; Li, G.; Yao, W. How host gender affects the bacterial community in pig feces and its correlation to skatole production. Ann. Microbiol. 2015, 65, 2379–2386. [Google Scholar] [CrossRef]
- He, M.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; Ke, S.; Yang, H.; Chen, C.; Huang, L. Host Gender and Androgen Levels Regulate Gut Bacterial Taxa in Pigs Leading to Sex-Biased Serum Metabolite Profiles. Front. Microbiol. 2019, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yin, J.; Tan, B.; Chen, J.; Zhang, H.; Li, Z.; Ma, X. Physiological function and application of dietary fiber in pig nutrition: A review. Anim. Nutr. 2021, 7, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Hume, I.D. Flow dynamics of digesta and colonic fermentation. In Physiological and Clinical Aspects of Short-Chain Fatty Acids; Cummings, J.H., Rombeau, J.L., Sakata, T., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 119–132. [Google Scholar]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Func. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- van Beers-Schreurs, H.M.; Nabuurs, M.J.; Vellenga, L.; Kalsbeek-van der Valk, H.J.; Wensing, T.; Breukink, H.J. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J. Nutr. 1998, 128, 947–953. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal colonic transit is linked to gut microbiota diversity and microbial fermentation in humans with slow colonic transit. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, 361–369. [Google Scholar] [CrossRef]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, Absorption, and Blood Flow Dynamics of Short-Chain Fatty Acids Produced by Fermentation in Piglet Hindgut during the Suckling-Weaning Period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Zijlstra, R.T.; Mosenthin, R.; Gänzle, M.G. Dietary calcium phosphate content and oat β-glucan influence gastrointestinal microbiota, butyrate-producing bacteria and butyrate fermentation in weaned pigs. FEMS Microbiol. Ecol. 2011, 75, 402–413. [Google Scholar] [CrossRef]
- He, B.; Bai, Y.; Jiang, L.; Wang, W.; Li, T.; Liu, P.; Tao, S.; Zhao, J.; Han, D.; Wang, J. Effects of Oat Bran on Nutrient Digestibility, Intestinal Microbiota, and Inflammatory Responses in the Hindgut of Growing Pigs. Int. J. Mol. Sci. 2018, 19, 2407. [Google Scholar] [CrossRef]
- Pieper, R.; Jha, R.; Rossnagel, B.; Van Kessel, A.G.; Souffrant, W.B.; Leterme, P. Effect of barley and oat cultivars with different carbohydrate compositions on the intestinal bacterial communities in weaned piglets. FEMS Microbiol. Ecol. 2008, 66, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Hiss, S.; Sauerwein, H. Influence of dietary ß-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J. Anim. Physiol. Anim. Nutr. 2003, 87, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.P.; Singh, A.K.; Jha, R. Fermentation characteristics of resistant starch, arabinoxylan, and β-glucan and their effects on the gut microbial ecology of pigs: A review. Anim. Nutr. 2019, 5, 217–226. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Y.; Yu, B.; Zheng, P.; Yu, J.; Huang, Z.; Mao, X.; Luo, J.; Yan, H.; He, J. Effect of β-Glucan Supplementation on Growth Performance and Intestinal Epithelium Functions in Weaned Pigs Challenged by Enterotoxigenic Escherichia coli. Antibiotics 2022, 11, 519. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Physiological Effects of Different Types of Beta-Glucan. Biomed. Pap. 2007, 151, 225–231. [Google Scholar]
- Dong, J.L.; Yu, X.; Dong, L.E.; Shen, R.L. In vitro fermentation of oat [beta]-glucan and hydrolysates by fecal microbiota and selected probiotic strains. J. Sci. Food Agric. 2017, 97, 4198. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, J.; Schloermann, W.; Trautvetter, U.; Glei, M. The effects of β-glucans on intestinal health. Ernahr. Umsch. 2020, 67, 52–59. [Google Scholar]
- Geervliet, M.; Lute, L.C.; Jansen, C.A.; Rutten, V.P.; Savelkoul, H.F.; Tijhaar, E. Differential immunomodulation of porcine bone marrow derived dendritic cells by E. coli Nissle 1917 and β-glucans. PLoS ONE 2020, 15, e0233773. [Google Scholar] [CrossRef] [PubMed]
- Sonck, E.; Devriendt, B.; Goddeeris, B.; Cox, E. Varying effects of different β-glucans on the maturation of porcine monocyte-derived dendritic cells. Clin. Vacc. Immunol. 2011, 18, 1441–1446. [Google Scholar] [CrossRef]
- de Vries, H.; Geervliet, M.; Jansen, C.A.; Rutten, V.; van Hees, H.; Groothuis, N.; Wells, J.M.; Savelkoul, H.F.J.; Tijhaar, E.; Smidt, H. Impact of Yeast-Derived β-Glucans on the Porcine Gut Microbiota and Immune System in Early Life. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704. [Google Scholar] [CrossRef]
- Shen, R.L.; Dang, X.Y.; Dong, J.L.; Hu, X.Z. Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. J. Agric. Food Chem. 2012, 60, 11301–11308. [Google Scholar] [CrossRef]
- Boes, J.; Johansen, M.V.; Eriksen, L.; Bogh, H.O.; Nansen, P.; Stephenson, L.S. False-positive Trichuris suis egg counts in pigs in relation to coprophagia. Parasite 1998, 5, 91–93. [Google Scholar] [CrossRef]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of Different Molecular Weights Enhance Growth Performance of LPS-challenged Piglets via Improved Gut Barrier Function and Microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Lalles, J.P.; Boudry, G.; Favier, C.; Le Floc’H, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Inoue, R.; Tsukahara, T.; Nakatani, M.; Okutani, M.; Nishibayashi, R.; Ogawa, S.; Harayama, T.; Nagino, T.; Hatanaka, H.; Fukuta, K.; et al. Weaning Markedly Affects Transcriptome Profiles and Peyer’s Patch Development in Piglet Ileum. Front. Immunol. 2015, 6, 630. [Google Scholar] [CrossRef]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef]
- Bruininx, E.M.; Binnendijk, G.P.; van der Peet-Schwering, C.M.; Schrama, J.W.; den Hartog, L.A.; Everts, H.; Beynen, A.C. Effect of creep feed consumption on individual feed intake characteristics and performance of group-housed weanling pigs. J. Anim. Sci. 2002, 80, 1413–1418. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).