Anaplasma Species in Ticks Infesting Mammals of Sardinia, Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection
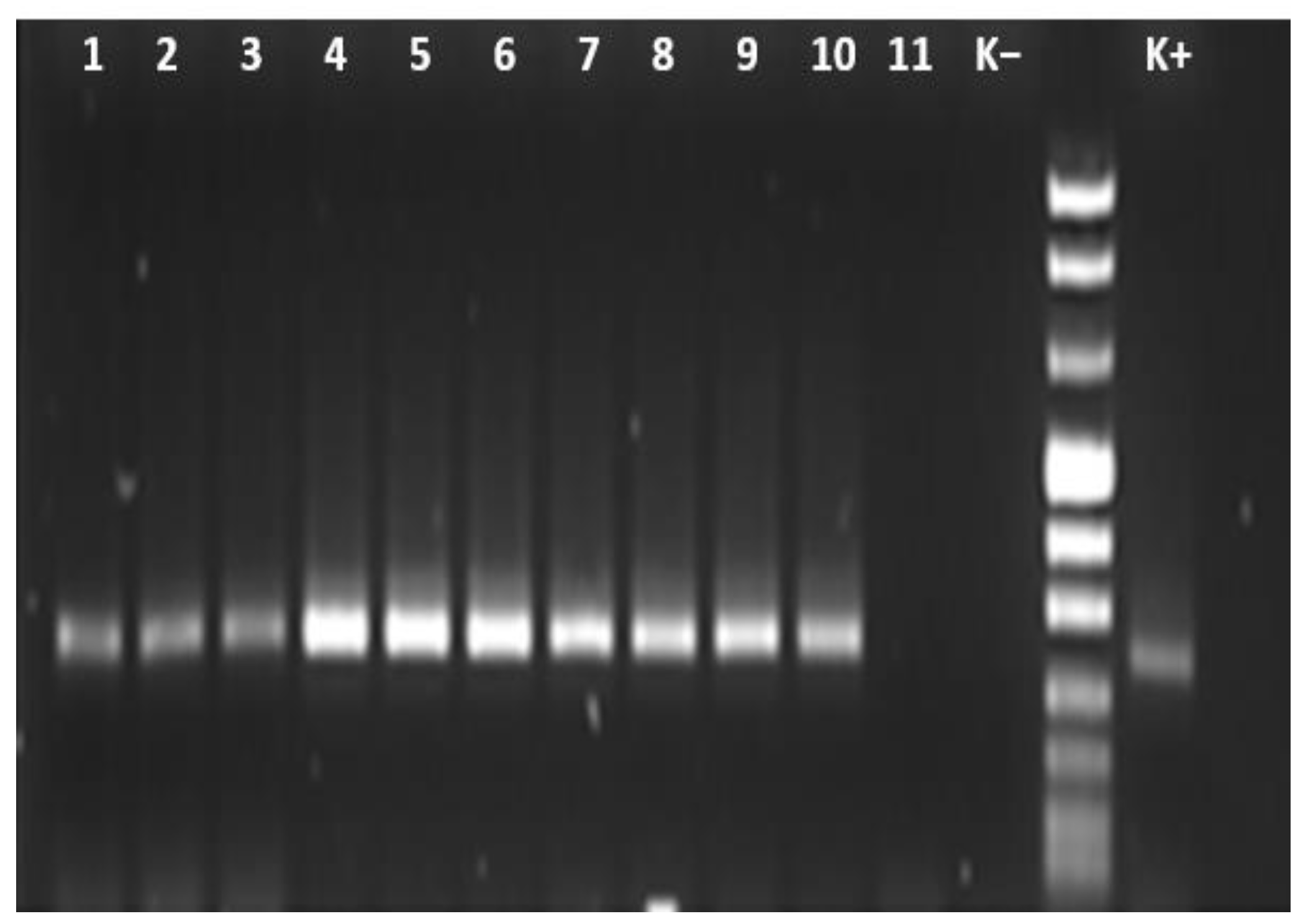
2.2. DNA Extraction and PCR
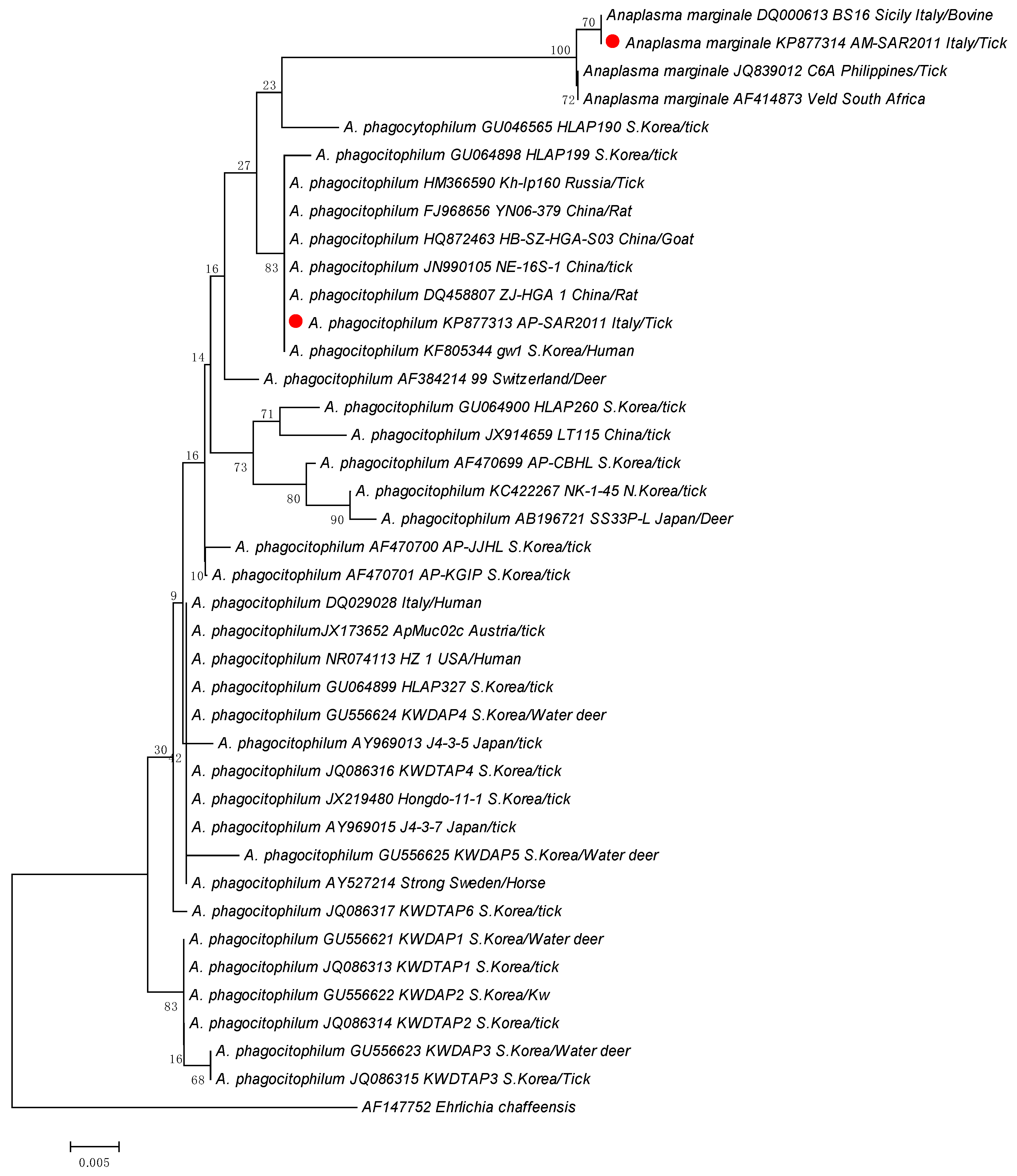
2.3. Purification, Sequencing, and Phylogenetic Analyses
3. Results
3.1. Tick Collection
3.2. Molecular Detection of Anaplasma spp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brites-Neto, J.; Duarte, K.M.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301–315. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Gallois, M.; Fontugne, M.; Allain, E.; Denoual, M.; Moutailler, S.; Devillers, E.; Zientara, S.; Memmi, M.; Chauvin, A.; et al. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasit. Vectors 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.J.; Xu, J.M.; Chen, Y.Z.; Li, J.H.; Holmes, E.C.; Zhang, Y.Z. Epidemiology and diversity of rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef] [PubMed]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Lysholm, S.; Ådén, F.; Aspán, A.; Högberg, A.; Wensman, J.J.; Omazic, A. Presence of Anaplasma spp. and their associated antibodies in the Swedish goat population. Animals 2023, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, Q.; Qin, X.; Lyu, Y.; Teng, Z.; Li, K.; Yu, L.; Jin, X.; Chang, H.; Wang, W.; et al. Anaplasma bovis Infection in Fever and Thrombocytopenia Patients-Anhui Province, China, 2021. China CDC Wkly. 2022, 4, 249–253. [Google Scholar] [CrossRef]
- Arraga-Alvarado, C.M.; Qurollo, B.A.; Parra, O.C.; Berrueta, M.A.; Hegarty, B.C.; Breitschwerdt, E.B. Case report: Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. J. Trop. Med. Hyg. 2014, 91, 1161–1165. [Google Scholar] [CrossRef]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G. Human infection with a novel tick-borne Anaplasma species in China, a surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Zobba, R.; Lecis, R.; Sotgiu, F.; Masala, G.; Foxi, C.; Pisu, D.; Alberti, A. GroEL typing and phylogeny of Anaplasma species in ticks from domestic and wild vertebrates. Ticks Tick. Borne Dis. 2018, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T. Ticks of Europe and North Africa. In A Guide to Species Identification; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; p. 404. ISBN 978-3-319-63759-4. [Google Scholar]
- Kolbert, C. Detection of the agent of human granulocytic ehrlichiosis. In PCR Protocols for Emerging Infectious Diseases; Persing, D.H., Ed.; American Society for Microbiology: Washington, DC, USA, 1996; pp. 106–111. [Google Scholar]
- de la Fuente, J.; Torina, A.; Naranjo, V.; Caracappa, S.; Vicente, J.; Mangold, A.J.; Vicari, D.; Alongi, A.; Scimeca, S.; Kocan, K.M. Genetic diversity of Anaplasma marginale strains from cattle farms in the province of Palermo, Sicily. J. Vet. Med. 2005, 52, 226–229. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Chisu, V.; Foxi, C.; Mannu, R.; Satta, G.; Masala, G. A five-year survey of tick species and identification of tick-borne bacteria in Sardinia, Italy. Ticks Tick Borne Dis. 2018, 9, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Loi, F.; Foxi, C.; Chessa, G.; Masu, G.; Rolesu, S.; Masala, G. Coexistence of tick-Borne pathogens in ticks collected from their hosts in Sardinia: An Update. Acta Parasitol. 2020, 65, 999–1004. [Google Scholar] [PubMed]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick. Borne Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef]
- de la Fuente, J.; Vicente, J.; Hofle, U.; Ruiz-Fons, F.; Fernandez De Mera, I.G.; Van Den Bussche, R.A.; Kocan, K.M.; Gortazar, C. Anaplasma infection in free-ranging Iberian red deer in the region of Castilla-La Mancha, Spain. Vet. Microbiol. 2004, 100, 163–173. [Google Scholar] [CrossRef]
- de la Fuente, J.; Torina, A.; Caracappa, S.; Tumino, G.; Furla, R.; Almazan, C.; Kocan, K.M. Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet. Parasitol. 2005, 133, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Ben Said, M.; Vinueza, R.L.; Leon, R.; Ahmad, N.; Parveen, A.; Khan, A.; Ejaz, A.; Ali, M.; Khan, A.U.; et al. Seasonal investigation of Anaplasma marginale infection in Pakistani cattle reveals hematological and biochemical changes, multiple associated risk factors and msp5 gene conservation. Pathogens 2022, 11, 1261. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Wang, T.; Sun, W.; Yang, X.; Liu, J. Tick-borne pathogens and the vector potential of ticks in China. Parasit. Vectors 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Broce, A.B.; Lysyk, T.J.; Palmer, G.H. Relative efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni (Acari: Ixodidae) compared with mechanical transmission by Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2005, 42, 668–675. [Google Scholar] [CrossRef]
- Hornok, S.; Boldogh, S.A.; Takács, N.; Sándor, A.D.; Tuska-Szalay, B. Zoonotic ecotype-I of Anaplasma phagocytophilum in sympatric wildcat, pine marten and red squirrel-Short communication. Acta Vet. Hung. 2022, 26, 215–219. [Google Scholar] [CrossRef]
- Ismail, N.; McBride, J.W. Tick-borne emerging infections: Ehrlichiosis and Anaplasmosis. Clin. Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef]
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Mi-Croorganisms 2022, 10, 1433. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Kauffmann, M.; Passos, L.M.F.; Pfister, K.; Zweygarth, E. Isolation, propagation and preliminary characterization of Anaplasma phagocytophilum from roe deer (Capreolus capreolus) in the tick cell line IDE8. Ticks Tick. Borne Dis. 2011, 2, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit. Vectors 2014, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, K.; Zhao, S.; Yan, Y.; Wang, H.; Jing, J.; Jian, F.; Wang, R.; Zhang, L.; Ning, C. Detection and phylogenetic characterization of Anaplasma capra: An emerging pathogen in sheep and goats in China. Front. Cell. Infect. Microbiol. 2018, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Lillini, E.; Macrì, G.; Proietti, G.; Scarpulla, M. New findings on anaplasmosis caused by infection with Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2006, 1081, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Maioli, G.; Pistone, D.; Bonilauri, P.; Pajoro, M.; Barbieri, I.; Mulatto, P.; Vicari, N.; Dottori, M. Etiological [corrected] agents of rickettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp. Appl. Acarol. 2012, 57, 199–208. [Google Scholar] [CrossRef]
- Carpi, G.; Bertolotti, L.; Pecchioli, E.; Cagnacci, F.; Rizzoli, A. Anaplasma phagocytophilum groEL gene heterogeneity in Ixodes ricinus larvae feeding on roe deer in Northeastern Italy. Vector Borne Zoonotic Dis. 2009, 9, 179–184. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum-a widespread multi-host pathogen with highly adaptive strategies. Cell Infect. Microbiol. 2013, 3, 1–33. [Google Scholar] [CrossRef]
- Chastagner, A.; Bailly, X.; Leblond, A.; Pradier, S.; Vourc’h, G. Single genotype of Anaplasma phagocytophilum identified from ticks, Camargue, France. Emerg. Infect. Dis. 2013, 19, 825–827. [Google Scholar] [CrossRef]
| N. of Ticks | Sex | Host (n.) | Collection Sites | |
|---|---|---|---|---|
| Ogliastra Province | ||||
| Rh. sanguineus s.l. | 97 | 40 males 57 females | Goat (18) | Talana |
| Rh. bursa | 13 | 6 males 7 females | Goat (1) | Jerzu |
| 1 | 1 male | Goat (1) | Talana | |
| Sassari Province | ||||
| Rh. sanguineus s.l. | 23 | 19 males 4 females | Marten (1) | Bono |
| Rh. bursa | 22 | 1 male 21 females | Cattle (1) | Villanova Monteleone |
| Tick Species | N. of Positive Ticks and Relative Sex | Host | Anaplasma Identification | Strain | GenBank Accession Number |
|---|---|---|---|---|---|
| Rh. sanguineus s.l. | 2♂—2♀ | Goat | A. phagocitophilum | AP-SAR2011 | KP877313 |
| 1♂ | Marten | A. marginale | AM-SAR2011 | KP877314 | |
| Rh. Bursa | 4♀ | Goat | A. phagocitophilum | AP-SAR2011 | KP877313 |
| 1♀ | Cattle | A. marginale | AM-SAR2011 | KP877314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisu, V.; Dei Giudici, S.; Foxi, C.; Chessa, G.; Peralta, F.; Sini, V.; Masala, G. Anaplasma Species in Ticks Infesting Mammals of Sardinia, Italy. Animals 2023, 13, 1332. https://doi.org/10.3390/ani13081332
Chisu V, Dei Giudici S, Foxi C, Chessa G, Peralta F, Sini V, Masala G. Anaplasma Species in Ticks Infesting Mammals of Sardinia, Italy. Animals. 2023; 13(8):1332. https://doi.org/10.3390/ani13081332
Chicago/Turabian StyleChisu, Valentina, Silvia Dei Giudici, Cipriano Foxi, Giovanna Chessa, Francesca Peralta, Valentina Sini, and Giovanna Masala. 2023. "Anaplasma Species in Ticks Infesting Mammals of Sardinia, Italy" Animals 13, no. 8: 1332. https://doi.org/10.3390/ani13081332
APA StyleChisu, V., Dei Giudici, S., Foxi, C., Chessa, G., Peralta, F., Sini, V., & Masala, G. (2023). Anaplasma Species in Ticks Infesting Mammals of Sardinia, Italy. Animals, 13(8), 1332. https://doi.org/10.3390/ani13081332






