Phytochemicals in Gastrointestinal Nematode Control: Pharmacokinetic–Pharmacodynamic Evaluation of the Ivermectin plus Carvone Combination
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial 1: Pharmacokinetic and Efficacy of the Coadministration of IVM and R-CNE via Different Routes
2.2. Trial 2: PK/PD of R-CNE Oral Emulsion Administered to Lambs Infected with Susceptible H. contortus
2.3. Trial 3: Target Tissue Distribution and Efficacy of R-CNE/IVM Oral Emulsion against Resistant H. contortus in Lambs
2.4. Chromatographic Analysis
2.5. Data Analysis
3. Results
3.1. Pharmacokinetics and Efficacy of the Coadministration of IVM and R-CNE via Different Routes (Trial 1)
3.2. PK/PD of R-CNE Oral Emulsion Administered to Lambs Infected with Susceptible H. contortus (Trial 2)
3.3. Target Tissue Distribution and Efficacy of R-CNE/IVM Oral Emulsion against Resistant H. contortus in Lambs (Trial 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Biology, epidemiology, diagnosis, and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Deore, A.B.; Dhumane, J.R.; Wagh, H.V.; Sonawane, R.V. The Stages of Drug Discovery and Development Process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar]
- Mengistu, G.; Hoste, H.; Karonen, M.; Salminen, J.P.; Hendriks, W.H.; Pellikaan, W.F. The in vitro anthelmintic properties of browse plant species against Haemonchus contortus is determined by the polyphenol content and composition. Vet. Parasitol. 2017, 237, 110–116. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Costa Junior, L.M.; Lima, A.S.; Silva, C.R.; Ribeiro, M.N.S.; Mesquista, J.W.C.; Rocha, C.Q.; Tangerina, M.M.P.; Vilegas, W. Anthelmintic activity of plant extracts from Brazilian savanna. Vet. Parasitol. 2017, 236, 121–127. [Google Scholar] [CrossRef]
- Silva, C.R.; Lifschitz, A.L.; Macedo, S.R.; Campos, N.R.; Viana-Filho, M.; Alcântara, A.C.; Araújo, J.G.; Alencar, L.M.R.; Costa-Junior, L.M. Combination of synthetic anthelmintics and monoterpenes: Assessment of efficacy, and ultrastructural and biophysical properties of Haemonchus contortus using atomic force microscopy. Vet. Parasitol. 2021, 290, 109345. [Google Scholar] [CrossRef]
- Štrbac, F.; Krnjajić, S.; Maurelli, M.P.; Stojanović, D.; Simin, N.; Orčić, D.; Ratajac, R.; Petrović, K.; Knežević, G.; Cringoli, G.; et al. A Potential Anthelmintic Phytopharmacological Source of Origanum vulgare (L.) Essential Oil against Gastrointestinal Nematodes of Sheep. Animals 2022, 13, 45. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on the safety assessment of carvone, considering all sources of exposure. EFSA J. 2004, 12, 3806. [Google Scholar] [CrossRef]
- Sánchez-Borzone, M.; Delgado-Marín, L.; García, D.A. Inhibitory effects of carvone isomers on the GABAA receptor in primary cultures of rat cortical neurons. Chirality 2014, 26, 368–372. [Google Scholar] [CrossRef]
- Lozon, Y.; Sultan, A.; Lansdell, S.J.; Prytkova, T.; Sadek, B.; Yang, K.H.S.; Howarth, F.C.; Millar, N.S.; Oz, M. Inhibition of human α7 nicotinic acetylcholine receptors by cyclic monoterpene carveol. Eur. J. Pharmacol. 2016, 776, 44–51. [Google Scholar] [CrossRef]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef]
- Aguiar, A.A.R.M.; de Araújo Filho, J.V.; Pinheiro, H.N.; da Silva Campelo, M.; Ribeiro, W.L.C.; Melo, A.C.F.L.; Oliveira da Rocha, L.; Ribeiro, M.E.N.P.; Ricardo, N.M.P.S.; Abreu, F.O.M.; et al. In vitro anthelmintic activity of an R-carvone nanoemulsions towards multiresistant Haemonchus contortus. Parasitology 2022, 149, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Katiki, L.M.; Araujo, R.C.; Ziegelmeyer, L.; Gomes, A.C.P.; Gutmanis, G.; Rodrigues, L.; Bueno, M.S.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S.; et al. TEvaluation of encapsulated anethole and carvone in lambs artificially-and naturally-infected with Haemonchus contortus. Exp. Parasitol. 2019, 197, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.V.; Luque, S.; Cardozo, P.; Lloberas, M.; Sousa, D.M.; Soares, A.M.S.; Costa-Junior, L.M.; Virkel, G.L.; Lifschitz, A.L. Plant-Derived Compounds as a Tool for the Control of Gastrointestinal Nematodes: Modulation of Abamectin Pharmacological Action by Carvone. Front. Vet Sci. 2020, 7, 601750. [Google Scholar] [CrossRef]
- Roberts, F.H.S.; O’sullivan, P.J. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust. J. Agric. Res. 1950, 1, 99–102. [Google Scholar] [CrossRef]
- MAFF (Ministry of Agriculture, Fisheries and Food). Manual of Veterinary Parasitological Laboratory Techniques, 3rd ed.; Her Majesty’s Stationery Office: London, UK, 1986.
- Muchiut, S.M.; Fernández, A.S.; Lloberas, M.; Steffan, P.E.; Luque, S.E.; Cardozo, P.A.; Bernat, G.A.; Riva, E.; Fiel, C.A. Recovery of fenbendazole efficacy on resistant Haemonchus contortus by management of parasite refugia and population replacement. Vet. Parasitol. 2019, 271, 31–37. [Google Scholar] [CrossRef]
- Maté, L.; Ballent, M.; Cantón, C.; Ceballos, L.; Lifschitz, A.; Lanusse, C.; Alvarez, L.; Liron, J.P. Assessment of P-glycoprotein gene expression in adult stage of Haemonchus contortus in vivo exposed to ivermectin. Vet. Parasitol. 2018, 264, 1–7. [Google Scholar] [CrossRef]
- Lifschitz, A.; Virkel, G.; Sallovitz, J.; Sutra, J.; Galtier, P.; Alvinerie, M.; Lanusse, C. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet. Parasitol. 2000, 87, 327–338. [Google Scholar] [CrossRef]
- Lifschitz, A.; Fiel, C.; Steffan, P.; Cantón, C.; Muchiut, S.; Dominguez, P.; Lanusse, C.; Alvarez, L. Failure of ivermec-tin efficacy against Psoroptes ovis infestation in cattle: Integrated pharmacokinetic-pharmacodynamic evaluation of two commercial formulations. Vet Parasitol. 2018, 263, 18–22. [Google Scholar] [CrossRef]
- Lloberas, M.; Alvarez, L.; Entrocasso, C.; Virkel, G.; Lanusse, C.; Lifschitz, A. Measurement of ivermectin concentrations in target worms and host gastrointestinal tissues: Influence of the route of administration on the activity against resistant Haemonchus contortus in lambs. Exp. Parasitol. 2012, 131, 304–309. [Google Scholar] [CrossRef]
- de Montigny, P.; Shim, J.S.K.; Pivnichny, J.V. Liquid chromatographic determination of ivermectin in animal plasma with trifluoroacetic anhydride and N-methylimidazole as the derivatization reagent. J. Pharm. Biomed. Anal. 1990, 8, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Pereira, M.A. Quantification of carvone, cineole, perillaldehyde, perillyl alcohol and sobrerol by isocratic high-performance liquid chromatography. J. Chromatogr. A 1998, 793, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Marcel Dekker: NewYork, NY, USA, 1982; pp. 45–109. [Google Scholar]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- McKenna, P. The detection of anthelmintic resistance by the faecal egg count reduction test: An examination of some of the factors affecting performance and interpretation. N. Z. Vet. J. 1990, 38, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Alvinerie, M.; Dupuy, J.; Kiki-Mvouaka, S.; Sutra, J.F.; Lespine, A. Ketoconazole increases the plasma levels of ivermectin in sheep. Vet. Parasitol. 2008, 157, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.L.; Entrocasso, C.; Alvarez, L.I.; Lloberas, M.; Ballent, M.; Manazza, G.; Virkel, G.; Borda, B.; Lanusse, C.E. Interference with P-glycoprotein improves ivermectin activity against adult resistant nematodes in sheep. Vet. Parasitol. 2010, 172, 291–298. [Google Scholar] [CrossRef]
- Lifschitz, A.; Suarez, V.H.; Sallovitz, J.; Cristel, S.L.; Imperiale, F.; Ahoussou, S.; Schiavi, C.; Lanusse, C. Cattle nematodes resistant to macrocyclic lactones: Comparative effects of P-glycoprotein modulation on the efficacy and disposition kinetics of ivermectin and moxidectin. Exp. Parasitol. 2010, 125, 172–178. [Google Scholar] [CrossRef]
- Pacheco, P.A.; Louvandini, H.; Giglioti, R.; Wedy, B.C.R.; Ribeiro, J.C.; Verissimo, C.J.; Ferreira, J.F.D.S.; Amarante, A.F.T.D.; Katiki, L.M. Phytochemical modulation of P-Glycoprotein and its gene expression in an ivermectin-resistant Haemonchus contortus isolate in vitro. Vet. Parasitol. 2022, 305, 109713. [Google Scholar] [CrossRef]
- Sasaki, K.; Wada, K.; Tanaka, Y.; Yoshimura, T.; Matuoka, K.; Anno, T. Thyme (Thymus vulgaris L.) leaves and its constituents increase the activities of xenobiotic-metabolizing enzymes in mouse liver. J. Med. Food 2005, 8, 184–189. [Google Scholar] [CrossRef]
- Lloberas, M.; Alvarez, L.; Entrocasso, C.; Ballent, M.; Virkel, G.; Luque, S.; Lanusse, C.; Lifschitz, A. Comparative pharmacokinetic and pharmacodynamic response of single and double intraruminal doses of ivermectin and moxidectin in nematode-infected lambs. N. Z. Vet. J. 2015, 63, 227–234. [Google Scholar] [CrossRef]
- Glendinning, S.K.; Buckingham, S.D.; Sattelle, D.B.; Wonnacott, S.; Wolstenholme, A.J. Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans. PLoS ONE 2011, 6, e22390. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Estrada-Mondragon, A.; Nguyen, B.; Lynch, J.W.; Keramidas, A. Effects of glutamate and ivermectin on single glutamate-gated chloride channels of the parasitic nematode H. contortus. PLoS Pathog. 2017, 13, e1006663. [Google Scholar] [CrossRef] [PubMed]
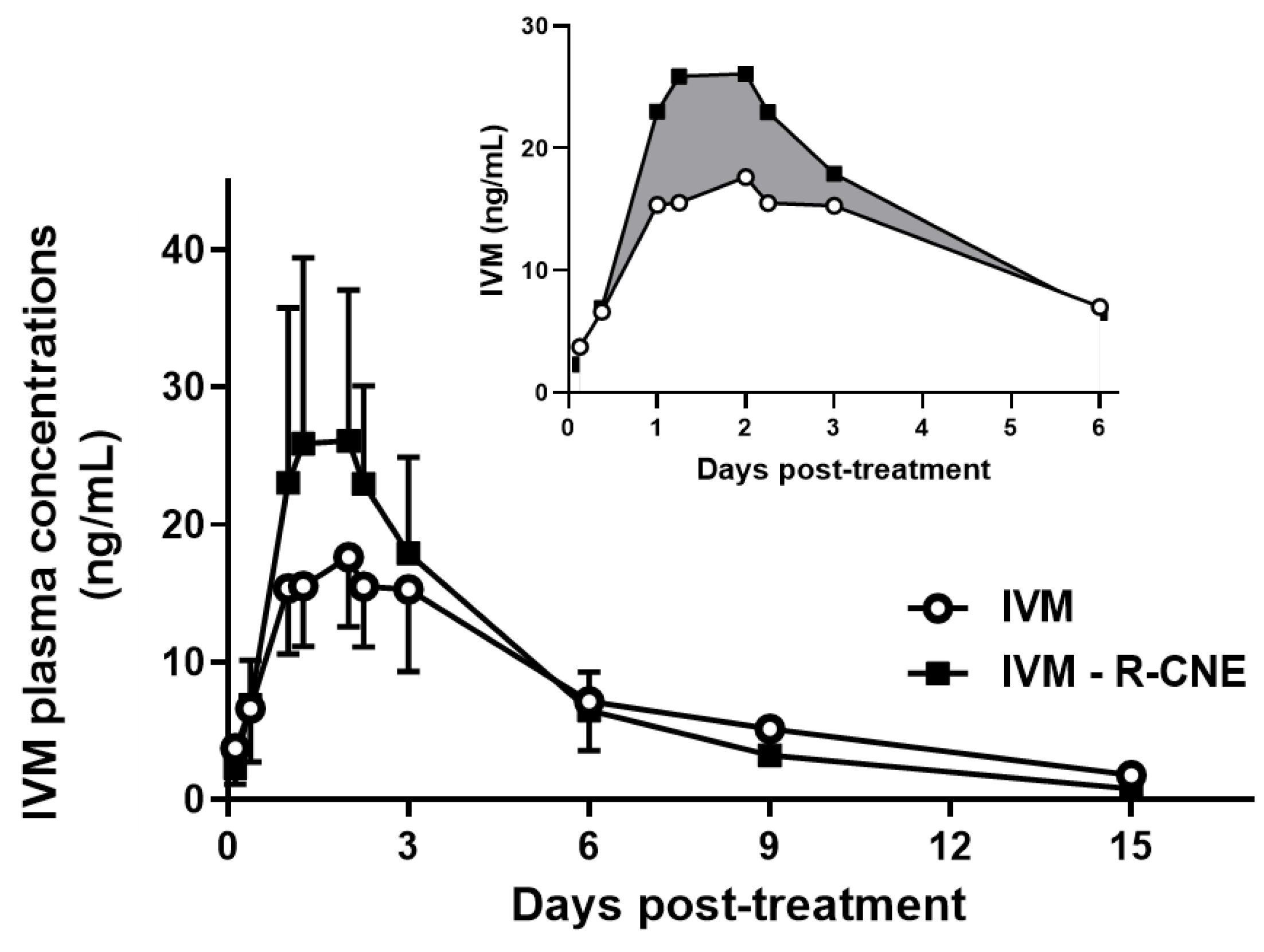

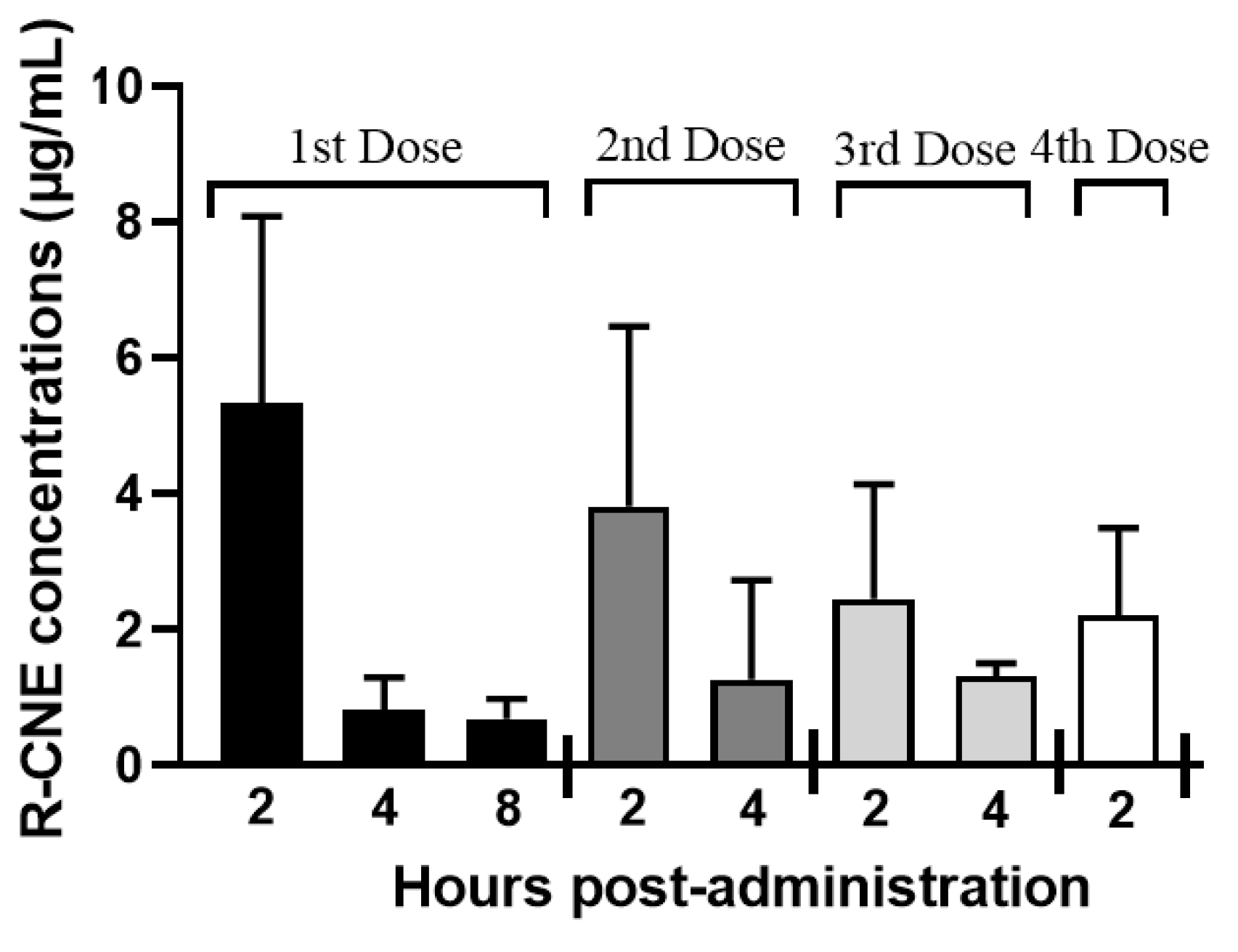
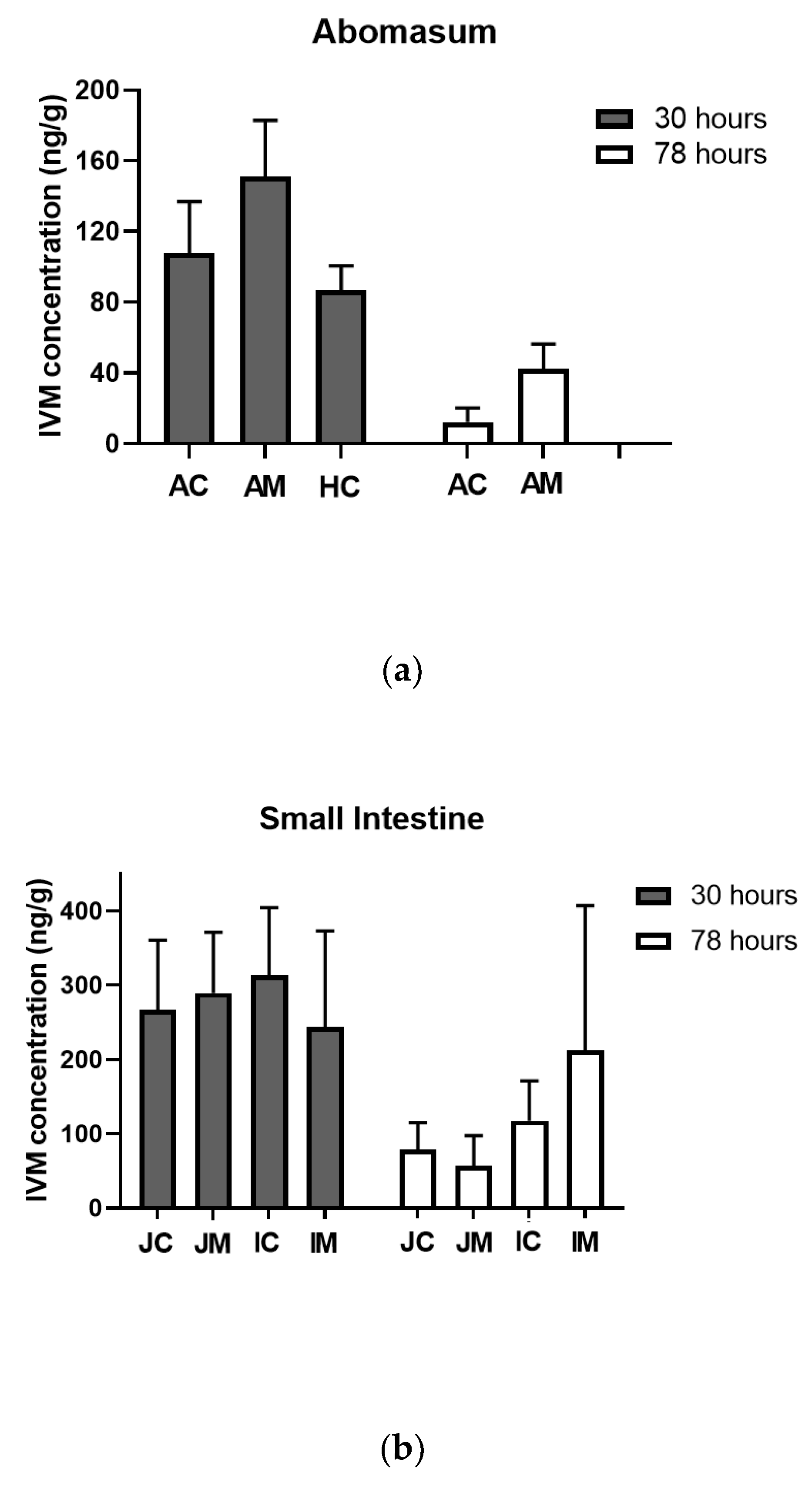
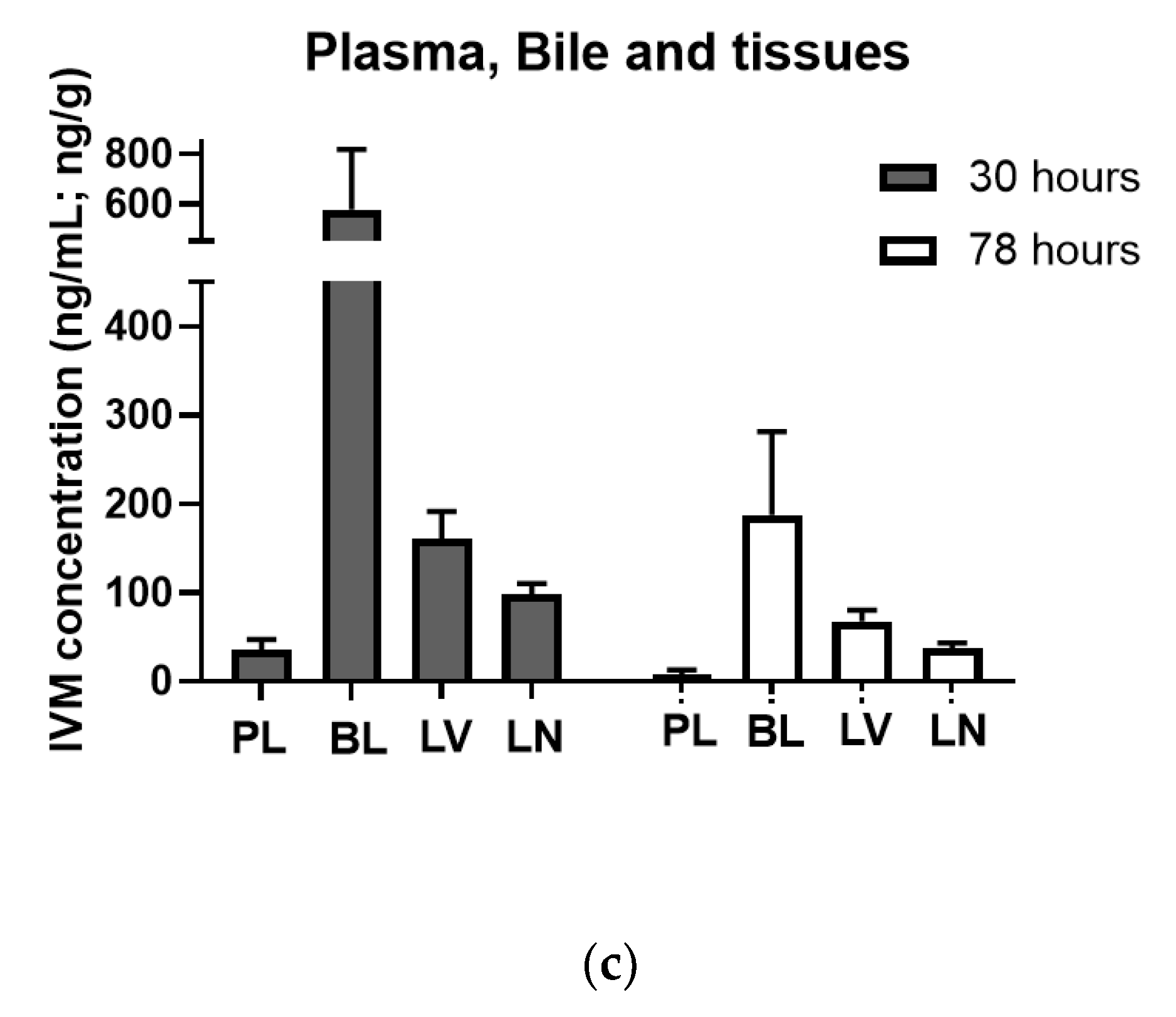

| Kinetic Parameters | IVM | IVM + R-CNE |
|---|---|---|
| Cmax (ng/mL) | 18.4 ± 5.40 a | 35.7 ± 13.9 b |
| T max (days) | 2.00 ± 0.58 a | 1.83 ± 0.56 a |
| AUC0–t (ng d/mL) | 113 ± 34.9 a | 121 ± 36.6 a |
| AUC0–2.25d (ng d/mL) | 28.7 ± 7.93 a | 42.4 ± 12.3 b |
| T ½ ab (days) | 0.38 ± 0.15 a | 0.50 ± 0.08 a |
| T ½ el (days) | 3.29 ± 0.47 a | 2.52 ± 0.41 b |
| MRT (days) | 4.82 ± 1.07 a | 3.80 ± 0.40 a |
| Day | EPG | FECR (%) (LCI-UCL) |
|---|---|---|
| 0 | 13,953 ± 13,932 a | - |
| 7 | 2170 ± 2725 b | 84.5 (40.5–95.9) |
| 14 | 1706 ± 2249 b | 87.8 (51.3–96.9) |
| Day | EPG | FECR (%) (LCI-UCL) |
|---|---|---|
| 0 | 3407 ± 2715 a | - |
| 7 | 1832 ± 2068 b | 46.2 (0–78) |
| 14 | 2192 ± 2417 b | 35.7 (0–73.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miró, M.V.; Costa-Júnior, L.M.; Lloberas, M.; Cardozo, P.; Lanusse, C.; Virkel, G.; Lifschitz, A. Phytochemicals in Gastrointestinal Nematode Control: Pharmacokinetic–Pharmacodynamic Evaluation of the Ivermectin plus Carvone Combination. Animals 2023, 13, 1287. https://doi.org/10.3390/ani13081287
Miró MV, Costa-Júnior LM, Lloberas M, Cardozo P, Lanusse C, Virkel G, Lifschitz A. Phytochemicals in Gastrointestinal Nematode Control: Pharmacokinetic–Pharmacodynamic Evaluation of the Ivermectin plus Carvone Combination. Animals. 2023; 13(8):1287. https://doi.org/10.3390/ani13081287
Chicago/Turabian StyleMiró, María Victoria, Livio Martins Costa-Júnior, Mercedes Lloberas, Patricia Cardozo, Carlos Lanusse, Guillermo Virkel, and Adrián Lifschitz. 2023. "Phytochemicals in Gastrointestinal Nematode Control: Pharmacokinetic–Pharmacodynamic Evaluation of the Ivermectin plus Carvone Combination" Animals 13, no. 8: 1287. https://doi.org/10.3390/ani13081287
APA StyleMiró, M. V., Costa-Júnior, L. M., Lloberas, M., Cardozo, P., Lanusse, C., Virkel, G., & Lifschitz, A. (2023). Phytochemicals in Gastrointestinal Nematode Control: Pharmacokinetic–Pharmacodynamic Evaluation of the Ivermectin plus Carvone Combination. Animals, 13(8), 1287. https://doi.org/10.3390/ani13081287





