Simple Summary
The mitochondrial genome (mitogenome) has been extensively employed in the investigation of phylogenetic relationships at different taxonomic levels. The mitochondrial genomes of insects are important for understanding their evolution and relationships. Herein, the entire mitogenome of Cydalima perspectalis was sequenced and characterized. Comparative mitogenomics and phylogenetic relationships were performed within the Pyraloidea. Our comparative studies show that mitochondrial genomes are a useful tool for phylogenetic studies at the level of the subfamilies in the Pyraloidea.
Abstract
To resolve and reconstruct phylogenetic relationships within Pyraloidea based on molecular data, the mitochondrial genome (mitogenome) was widely applied to understand phylogenetic relations at different taxonomic levels. In this research, a complete mitogenome of Cydalima perspectalis was recorded, and the phylogenetic position of C. perspectalis was inferred based on the sequence in combination with other available sequence data. According to the research, the circular mitochondrial genome is 15,180 bp in length. It contains 22 transfer RNA genes (tRNAs), two ribosomal RNA genes (rRNAs), 13 typical protein-coding genes (PCGs), and a non-coding control region. The arrangement of a gene of the C. perspectalis mitogenome is not the same as the putative ancestral arthropod mitogenome. All of the PCGs are initiated by ATN codons, except for the cytochrome c oxidase subunit 1 (cox1) gene, which is undertaken by CGA. Five genes have incomplete stop codons that contain only ‘T’. All tRNA genes display a typical clover–leaf structure of mitochondrial tRNA, except for trnS1 (AGN). The control region contained an ‘ATAGG(A)’-like motif followed by a poly-T stretch. Based on the mitochondrial data, phylogenetic analysis within Pyraloidea was carried out using Bayesian inference (BI) and maximum likelihood (ML) analyses. Phylogenetic analysis showed that C. perspectalis is more closely related to Pygospila tyres within Spilomelinae than those of Crambidae and Pyraloidea.
1. Introduction
Lepidoptera, with more than 157,000 known species and 137 families among 43 superfamilies, is the world’s third most significant order after Diptera and Coleoptera [1]. One of several Lepidoptera superfamilies, Pyraloidea, includes the Pyralidae and Crambidae families. To date, over 15,500 different species of Pyraloidea have been identified around the world [2]. Pyraloidea insects contain a large number of economically significant pests that affect forests, agriculture, stored goods, and ornamental plants, and they have been used as model insects to research biodiversity, community ecology, management, behavioral ecology, genetics, and the evolution of pheromone communication networks [3,4,5,6,7]. The box tree moth C. perspectalis (Walker 1859) (Lepidoptera: Crambidae) is native to East Asia and invasive in Europe; however, it is currently a completely unique species to Middle and South Europe [8]. However, the Pyraloidea classification has not yet reached a satisfactory or stable state. The phylogenetic hypothesis for the higher-level taxa of Pyraloidea was demonstrated through molecular data, which are new and derived from mitochondrial genomes.
Mitochondrial genomes are considered robust phylogenetic relationship markers due to maternal inheritance [9], infrequent recombination [10], a relatively high rate of evolution, and immobile gene components [11]. The insect mitochondrial genomes are often rounded molecules of around 15–16 kb, which include two ribosomal RNA (rrnL and rrnS) genes, 22 tRNA genes, 37 genes, and 13 PCGs. A non-coding element containing initiation sites for replication and transcription is called the A + T rich region (CR) [12,13]. With the great developments in PCR techniques and high-throughput sequencing, many animal group’s complete mitogenomic data including insects are easier to obtain and have been widely applied in the research of phylogenetics, molecular evolution, evolutionary and comparative genomics, and population genetics [14,15,16].
In this study, we presented a complete mitogenome sequence of C. perspectalis and compared its structures with some of the determined Pyraloidea species. Meanwhile, the gene sequence data were incorporated from other available Pyraloidea species listed in GenBank. We also reconstructed phylogenetic trees from PCG sequences to analyze the evolutionary relationships in Pyraloidea insects.
2. Materials and Methods
2.1. Ethics Standards
The Committee of the Yancheng Teachers University and Nanjing University of Chinese Medicine approved the animal protocols, and all experiments were performed under the applicable standards, with access no. YCTU-2020007 and SP-2020003, respectively.
2.2. Sample Collection and DNA Extraction
The moths of C. perspectalis were gathered in Yancheng, Jiangsu Province, China. The specimens were stored in 100% ethanol at −20 °C until DNA extraction. The total genomic DNA was extracted from the legs of moths using the Ezup Column Animal Genomic DNA Purification Kit (SangonBiotech, Shanghai, China) in accordance with the manufacturer’s protocol.
2.3. Mitogenome Sequencing
Universal primer sets for mitogenomic sequences from other Lepidopteran insects were designed to amplify the C. perspectalis mitogenome [17,18,19,20]. PCR was conducted in the following series: 3 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 1–3 min at 50–62 °C, and 10 min at 72 °C. All amplifications were conducted in 50 μL reaction volumes using the Mastercycler gradient and Eppendorf Mastercycler. The PCR products were separated by agarose gel electrophoresis (1% w/v) and then purified using a DNA Gel Extraction Kit (Vazyme, Nanjing, China). The refined PCR products were ligated into T-vector (SangonBiotech, Shanghai, China) and sequenced at least three times.
2.4. Gene Annotation and Sequence Assembly
Sequence annotation was applied by NCBI Internet BLAST function for the searching and packaging of MITOS (http://mitos2.bioinf.uni-leipzig.de/index.py (accessed on 10 January 2023)). Alignments of C. perspectalis PCGs and different Pyraloidea mitogenomes were applied by MAFFT17. The following rules were calculated using composition skewness: GC-skew = [G − C]/[G + C] and AT-skew = [A − T]/[A + T]. Nucleotide composition statistics and codon usage were computed using PhyloSuite [21].
2.5. Phylogenetic Analysis
GenBank provides the Pyraloidea species used for mitogenomic phylogeny to determine the phylogenetic relationships among Pyraloidea insects based on nucleotide alignments (Table 1). Spodoptera litura was used as an outgroup. Using default concatenation and settings, nucleotide sequences were aligned for each of the 13 mitochondrial PCGs. MrBayes v 3.2.2 [22] and IQ-Tree [23] performed phylogenetic analyses using the maximum likelihood (ML) and Bayesian inference (BI), respectively. Each of the PCGs was individually aligned using MAFFT [24]. Gblocks were applied to ensure protected areas and eliminate undependably aligned sequences in the datasets [25]. For ML and BI analyses, GTR + I + G was the suitable model for nucleotide sequences by MrModeltest 2.3 on Akaike’s information criterion (AIC) [26]. Bayesian analysis was conducted under the following circumstances: 10,000,000 generations, four chains, and a burn-in step for the first 5000 generations, 100 sample frequency. We evaluated the reliability of the results through two methods: first, the average standard deviation of split frequencies was lower than 0.01 in the Bayesian method. The value of ESS was over 200. This showed that our data combined cumulatively. The results of the phylogenetic trees are presented in it [27].

Table 1.
Pyraloidea species used in the phylogenetic analyses.
3. Results and Discussion
3.1. Base Composition and Genome Organization
The complete mitogenome sequence of C. perspectalis is a closed circular molecule 15,180 bp in length. The composition of the gene is similar to that of other Pyraloidea insect mitogenomes such as 13 PCGs (cox1-3, nad1-6, nad4L, cob, atp6 and atp8), 22 tRNA genes, two mitochondrial rRNA genes (rrnS and rrnL), and a central non-coding region known as the AT-rich region. The majority strand (F strand) encodes 23 genes. The opposite (R) strand encodes 14 genes (Figure 1, Table 2). Four of the 13 PCGs (nad1, nad4, nad4L and nad5), eight tRNAs (trnQ, trnV, trnY, trnF, trnC, trnP, trnH, and trnL [CUN]), and two rRNAs (rrnS and rrnL) were coded with minority-strands. The remaining 23 genes were encoded by the majority strands.
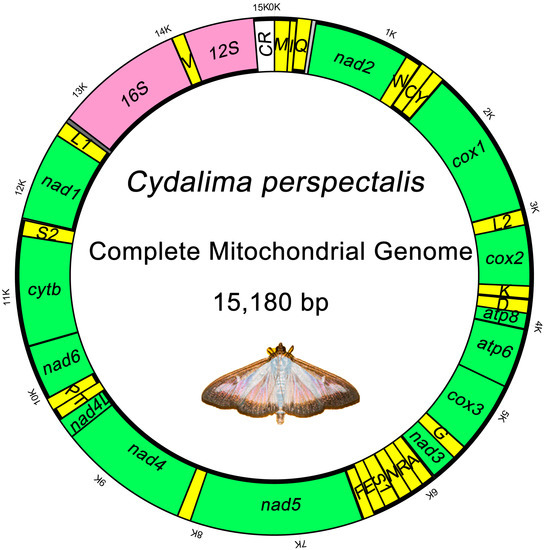
Figure 1.
Circular map of the mitochondrial genome of C. perspectalis.

Table 2.
Summary of the mitogenome of C. perspectalis.
The nucleotide composition of the C. perspetives mitogenome is as follows (Table 3): A = 6058 (39.9%), T = 6231 (41.0%), G = 1162 (7.7%), and C = 1729 (11.4%). The A + T of the C. perspectalis mitogenome’s nucleotide composition was 81.0%. The entire GC-skew and AT-skew of the C. perspectalis mitogenome were −0.014 and −0.196, respectively. The AT skew for the C. perspectalis mitogenome was slightly negative. This suggests that T nucleotides are more abundant than A nucleotides. The GC-skew for the C. perspectalis mitogenome was scarcely negative, with C nucleotides outnumbering G nucleotides. In addition, AT-skew (0.014) and GC-skew (0.185) of the tRNAs indicate that tRNAs include more As and Gs than Ts and Cs. Similarly, AT-skew (0.050) and GC-skew (0.337) of the rRNAs clearly suggest that rRNAs have more As and Gs than Ts and Cs.

Table 3.
Composition and skewness in the C. perspectalis mitogenome.
3.2. Protein-Coding Genes
In total, 13 PCGs of C. perspectalis contain 3723 codons, except for the termination codons. The beginning and ending codons of 13 PCGs in the C. perspectalis mitogenome are presented in Table 2. The CGA codon encoded arginine, with the exception of cox1. All of the PCGs were launched by ATN codons. The CGA codon is incredibly protected across almost all groups of the insect [28,29,30]. In the C. perspectalis mitogenome, eight PCGs (atp6, atp8, cox1, cox3, nad2, nad3, nad6, and cob) had the whole stop codon TAA, but the other five ended with a single T (nad1, nad4, nad4L, cox2, and nad5). The ordinary A + T of the 13 PCGs was 79.6%. Moreover, 13 PCGs had a slightly negative AT skew, although it was a marginally positive GC skew (Table 3). For the C. perspectalis mitogenome, the related synonymous codon usage (RSCU) is valuable, as outlined in Table 4 and Figure 2, where NNT and NNA were higher than 1.0, apart from Leu (CUR), showing a great Ts or As bias in the 3rds. Leu (UUR) (484), Ile (469), and Phe (374) (Figure 3) are the most frequent amino acids found in mitochondrial proteins.

Table 4.
Codon number and RSCU in the C. perspectalis mitochondrial PCGs. (* the termination codons).
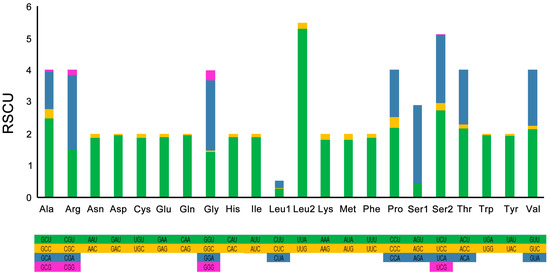
Figure 2.
The relative synonymous codon usage (RSCU) in the mitogenome of C. perspectalis.
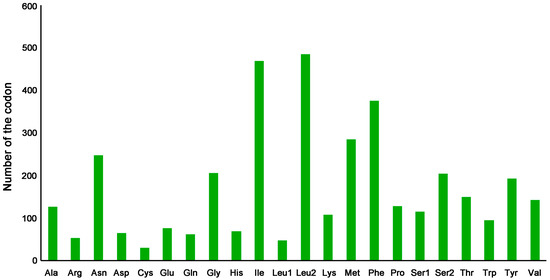
Figure 3.
Composition of the amino acids in the mitogenome of C. perspectalis.
3.3. Control Region
The control region (AT-rich region) plays a crucial role in the introduction of the transcription and replication of the mitogenome [31]. The AT-rich part (288 bp) of the C. perspectalis mitogenome is situated among trnM and rrnS. The entire AT content of the PCGs was 96.2% and it was highest in the mitogenome of C. perspectalis. The entire GC-skew and AT-skew in the AT-rich part of C. perspectalis were 0.26 and 0.01, respectively (Table 3). The GC-skew and AT-skew for the AT-rich part of C. perspectalis were marginally positive, showing that G and A are more abundant than C and T.
Some protected structures were discovered in an AT-rich part of C. perspectalis (Figure 4). The motif ATAGG plus 17 bp poly-T stretch downstream of rrnS was the first protected structure and may demonstrate the source of light strands or minority replication [32,33]. In the A + T rich region, the microsatellite-like repeat (AT)14 elements were detected. In addition, a 10 bp poly-A stretch was discovered just in front of the trnM region. Many tandem repeat elements are usually present in the A + T-rich regions of most insects. No repetitions were discovered in the A + T-rich region of the C. perspectalis mitogenome (Figure 4).

Figure 4.
AT-rich region of the C. perspectalis mitogenome. Coloured nucleotides indicate the ATAGG motif (red), the poly-T stretch (blue), a microsatellite A/T repeat sequence (green), and the poly-A stretch (pink).
3.4. Rearrangement of Gene
The arrangement of genes of Pyraloidea insects is often remarkably conserved. In contrast to the putative ancestral arthropod mitogenome, the order of the C. perspectalis differs from that of traditional insects. The trnM gene’s placement in the C. perspectalis mitogenome is trnM-trnI-trnQ-nad2. This differs from conventional insects, in which trnM is situated between nad2 and trnQ (Figure 5). The ancestral insect placement of the trnM gene clusters has been discovered in ghost moths [34]. The rearrangement of genes in C. perspectalis stands for the opinion that the ancestral arrangement of the trnM gene cluster goes through rearrangement after Hepialoidea departs from the Pyraloidea lineages. Rearrangements of tRNA are believed to be the result of a tandem copy of the mitogenome’s part as a whole. This was followed by non-random or random loss of identical copies [35,36,37,38].
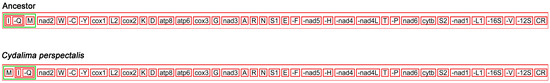
Figure 5.
The mitochondrial gene order of C. perspectalis and ancestral insects. tRNA genes are indicated by singer letter IUPAC-IUB abbreviation with S1 = AGN, S2 = UCN, L1 = CUN, and L2 = UUR. Protein and rRNA genes are labelled with three letter code.
3.5. Phylogenetic Analyses
Based on nucleotide alignments (NT dataset), phylogenetic trees were constructed using two methods (ML and BI) and the MAFFT alignment technique. As an outgroup, S. litura was used. The monophyly of every superfamily is usually strongly suggested by Bayesian inference (BI), and the maximum likelihood method based on the nucleotide sequence of 13 mitochondrial PCGs. The BI and ML trees had identical tree topologies; monophyly of the families and subfamilies was powerfully recommended, as shown by the morphological characteristics and phylogeny of the completed mitogenome [39]. In the research, the trees’ comparative analyses show high node support values, together with 13 PCG datasets (Figure 6). The phylogenetic analysis shows that C. perspectalis is more closely related to Pygospila tyres than other species, indicating that C. perspectalis belongs to the Spilomelinae, Crambidae, and Pyraloidea. As shown in Figure 6, the monophyly of each superfamily is generally well-supported, typically with posterior probabilities greater than 0.9 and bootstrap support (BS) greater than 75. It is obvious that three families belong to the Pyraloidea: Thyrididae, Pyralidae, and Crambidae. Regier et al. presented molecular phylogenetic research on Pyraloidea using five nuclear genes. The findings led to a new classification of Crambidae into ‘non-PS Clade’ and ‘PS Clade’. The two sister lineages correspond suitably to the ‘PS clade’ (Pyraustinae and Spilomelinae) and the ‘non-PS clade’ (Glaphyriinae, Acentropinae, Crambinae, Schoenobiinae, and Scopariinae) [40]. Our phylogenetic analysis outcome demonstrates that the same topological structures were derived from some traditional classifications and molecular data. Four of the subfamilies, Galleriinae + (Phycitinae + (Pyralinae + Epipaschiinae)) have been widely supported based on a variety of combinations of mitogenomic data or multiple gene markers in Pyralidae [40,41,42,43], and these phylogenetic relationships were also obtained based on 14 nuclear gene data. Meanwhile, the limited availability of a mitogenome precluded the Chrysauginae from being sampled in this case [44]. Orybina was regarded as a member of Pyralina based on the morphological method. However, a molecular phylogenetic analysis of Orybina revealed that the phylogenetic position was away from the Pyralina and close to Galleriinae, which is consistent with a previous study with significant value support [45]. Within the Crambidae, the ‘PS clade’ Pyraustinae and Spilomelinae formed sister lineages, while the ‘non-PS clade’ was divided into two sister lineages: one group included Glaphyriinae and Odontiinae, while the other group included the remaining four subfamilies (Schoenobiinae, Crambinae, Scopariinae, and Nymphulinae). The family-level topology of the phylogenetic analyses can be described as follows: (Glaphyriinae + Odontiinae) (Schoenobiinae + (Crambinae + (Scopariinae + Nymphulinae))) and the results were strongly supported (BS ≥ 95, PP = 1.00) and consistent with the previous research results [1,46]. Nevertheless, since we identified a separate sample in the research, a more desirable realization of the Pyraloidea mitogenome requires an extension of the genome and taxon samplings, especially in the Orybina and Chrysauginae.
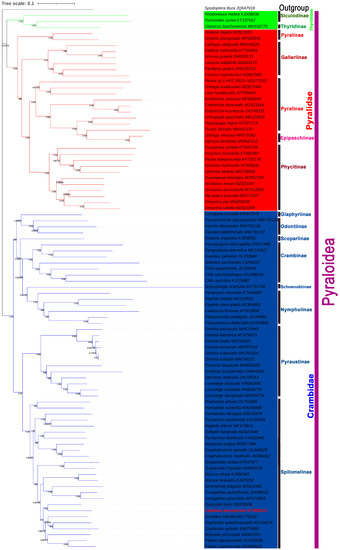
Figure 6.
Phylogenetic tree derived for Pyraloidea using Bayesian inference (BI) and maximum likelihood (ML) analyses based on nucleotide (NT). Bootstrap value (BP) and Bayesian posterior probability (BPP) of each node are shown such as BPP based on the NT dataset/BP based on the NT dataset, 1.00/100.
4. Conclusions
In this study, we reported a complete mitogenome of Cydalima perspectalis, and the phylogenetic analyses of C. perspectalis were inferred using nucleotide sequence. The arrangement of a gene in the C. perspectalis mitogenome is similar to that of the Pyraloidea mitogenome. All of the PCGs were initiated by ATN codons, except for cox1, which was undertaken by CGA. Five genes had incomplete stop codons that contain only ‘T’. All tRNA genes displayed a typical cloverleaf structure of mitochondrial tRNA, except for trnS1 (AGN). The control region contained an ‘ATAGG(A)’-like motif followed by a poly-T stretch. Phylogenetic analysis within Pyraloidea was constructed using the BI and ML methods. The results showed that C. perspectalis is more closely related to Pygospila tyres within Spilomelinae than those of Crambidae and Pyraloidea. These molecular-based phylogenies support the morphological classification of the relationships within the Pyraloidea species.
Author Contributions
Conceptualization, Q.L. and B.T.; methodology, Y.G.; software, Y.G., Q.W. and J.Z.; validation, Y.G., Q.L. and B.T.; formal analysis, Y.G.; investigation, Q.L.; resources, Q.L.; data curation, Y.G., and Q.L.; writing—original draft preparation, Y.G.; writing—review and editing, Q.L.; visualization, Y.G.; supervision, Q.L.; project administration, Q.L. and B.T.; funding acquisition, B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32270487).
Institutional Review Board Statement
The Committee of the Yancheng Teachers University and Nanjing University of Chinese Medicine approved the animal protocols, and all experiments were performed under the applicable standards, with access no. YCTU-2020007 and SP-2020003, respectively.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study can be found in the GenBank accession no. KY865331.
Conflicts of Interest
The authors declare no competing interests.
References
- Timmermans, M.J.; Lees, D.C.; Simonsen, T.J. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phylogenet. Evol. 2014, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- van Nieukerken, E.J.; Kaila, L.; Kaila, I.J.; Kitching, N.P.; Kristensen, D.C.; Lees, J.; Minet, C.; Mitter, M.; Mutanen, J.C.; Regier, T.J.; et al. Order Lepidoptera Linnaeus, 1758. Zootaxa 2011, 3148, 212–221. [Google Scholar] [CrossRef]
- Janzen, D.; Hallwachs, W.; Blandin, P.; Burns, J.M.; Cadiou, J.-M.; Chacon, I.; Dapkey, T.; Deans, A.R.; Epstein, M.E.; Espinoza, B.; et al. Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol. Ecol. Resour. 2009, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.L.; Min, S.F.; Wu, W.J.; Liang, G.W.; Zeng, L. Estimating index of population trend by re-sampling techniques (jackknife and bootstrap) and its application to the life table study of the rice leaf roller, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Insect Sci. 2008, 15, 153–161. [Google Scholar] [CrossRef]
- Fuji, T.; Ito, K.; Tatematsu, M.; Shimada, T.; Katsuma, S.; Ishikawa, Y. Sex pheromone desaturase functioning in a primitive Ostrinia moth is cryptically conserved in congeners’ genomes. Proc. Nat. Acad. Sci. USA 2011, 108, 7102–7106. [Google Scholar] [CrossRef]
- Liu, Q.N.; Bian, D.D.; Zhou, C.L.; Tang, B.P. Molecular cloning and expression analysis of a myosin light chain 1 (MLC-1) gene from Indian meal moth Plodia interpunctella (Lepidoptera: Pyralidae). Entomol. Res. 2015, 45, 305–313. [Google Scholar] [CrossRef]
- Liu, Q.N.; Bian, D.D.; Ge, B.M.; Zhou, C.L.; Tang, B.P. Molecular characterization of a 14-3-3 zeta gene from Plodia interpunctella: A potential marker for phylogenetic inference. Biochem. System. Ecol. 2015, 60, 171–176. [Google Scholar] [CrossRef]
- Mally, R.; Nuss, M. Phylogeny and nomenclature of the box tree moth, Cydalima perspectalis (Walker, 1859) comb. n., which was recently introduced into Europe (Lepidoptera: Pyraloidea: Crambidae: Spilomelinae). Eur. J. Entomol. 2010, 107, 393–400. [Google Scholar] [CrossRef]
- Barr, C.M.; Neiman, M.; Taylor, D.R. Inheritance and recombination of mitochondrial genomes in plants, fungi and animals. New Phytol. 2005, 168, 39–50. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]
- Curole, J.P.; Kocher, T.D. Mitogenomics: Digging deeper with complete mitochondrial genomes. Trends Ecol. Evol. 1999, 14, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, E.V.; Caterino, M.S.; Sperling, F.A.H. Molecular phylogeny, historical biogeography, and divergence time estimates for swallowtail butterflies of the genus Papilio (Lepidoptera: Papilionidae). Syst. Biol. 2004, 53, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, A.; Gebauer-Jung, S.; Blaxter, M.L.; McMillan, W.O.; Jiggins, C.D. ButterflyBase: A platform for lepidopteran genomics. Nucleic Acids Res. 2008, 36, D582–D587. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, C.; Liu, Q.; Zou, T.; Qiao, G.; Huang, X. Insights into the evolution of Aphid mitogenome features from new data and comparative analysis. Animals 2022, 12, 1970. [Google Scholar] [CrossRef]
- Liu, Q.N.; Zhu, B.J.; Dai, L.S.; Wei, G.Q.; Liu, C.L. The complete mitochondrial genome of the wild silkworm moth, Actias selene. Gene 2012, 505, 291–299. [Google Scholar] [CrossRef]
- Liu, Q.N.; Bian, D.D.; Jiang, S.H.; Ge, B.M.; Zhou, C.L.; Tang, B.P. Characterization of the complete mitochondrial genome of the Oriental armyworm, Mythimna separate (Lepidoptera: Noctuidae). Eur. J. Entomol. 2015, 112, 399–408. [Google Scholar] [CrossRef]
- Liu, Q.N.; Chai, X.Y.; Zhou, C.L.; Tang, B.P. The complete mitochondrial genome of Plodia interpunctella (Lepidoptera: Pyralidae) and comparison with other Pyraloidea insects. Genome 2016, 59, 37–49. [Google Scholar] [CrossRef]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 2461. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Nylander, J. MrModeltest v2; Program distributed by the author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004; Volume 2. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xin, Z.Z.; Wang, Y.; Zhang, H.B.; Zhang, D.Z.; Wang, Z.F.; Zhou, C.L.; Tang, B.P.; Liu, Q.N. The complete mitochondrial genome of Clostera anachoreta (Lepidoptera: Notodontidae) and phylogenetic implications for Noctuoidea species. Genomics 2017, 109, 221–226. [Google Scholar] [CrossRef]
- Xin, Z.Z.; Liu, Y.; Zhu, X.Y.; Wang, Y.; Zhang, H.B.; Zhang, D.Z.; Zhou, C.L.; Tang, B.P.; Liu, Q.N. Mitochondrial genomes of two Bombycoidea insects and implications for their phylogeny. Sci. Rep. 2017, 7, 6544. [Google Scholar] [CrossRef]
- Dai, L.S.; Kausar, S.; Abbas, M.N.; Wang, T.T. Complete sequence and characterization of the Ectropis oblique mitochondrial genome and its phylogenetic implications. Int. J. Biol. Macromol. 2018, 107, 1142–1150. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997, 25, 99–120. [Google Scholar] [CrossRef]
- Taanman, J.W. The mitochondrial genome: Structure, transcription, translation and replication. Biochim. Biophys. Acta 1999, 1410, 103–123. [Google Scholar] [CrossRef]
- Fusté, J.M.; Wanrooij, S.; Jemt, E. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell 2010, 37, 67–78. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Ma, C.; Chen, J.Y.; Yang, D.R. The complete mitochondrial genomes of two ghost moths, Thitarodes renzhiensis and Thitarodes yunnanensis: The ancestral gene arrangement in Lepidoptera. BMC Genom. 2012, 13, 276. [Google Scholar] [CrossRef]
- Juhling, F.; Putz, J.; Bernt, M.; Donath, A.; Middendorf, M.; Florentz, C.; Stadler, P.F. Improved systematic tRNA gene annotation allows new insights into the evolution of mitochondrial tRNA structures and into the mechanisms of mitochondrial genome rearrangements. Nucleic Acids Res. 2012, 40, 2833–2845. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.J.; Daehler, L.L.; Moritz, C.C.; Brown, W.M. Sequences with the potential to form stem-and-loop structures are associated with coding-region duplications in animal mitochondrial DNA. Genetics 1994, 137, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Zheng, Y.; Murphy, R.W. Intraspecific rearrangement of mitochondrial genome suggests the prevalence of the tandem duplication-random loss (TDLR) mechanism in Quasipaa boulengeri. BMC Genom. 2016, 17, 965. [Google Scholar]
- Liu, Q.N.; Xin, Z.Z.; Zhu, X.Y.; Chai, X.Y.; Zhao, X.M.; Zhou, C.L.; Tang, B.P. A transfer RNA gene rearrangement in the lepidopteran mitochondrial genome. Biochem. Bioph. Res. Commun. 2017, 489, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yan, J.; Song, J.; You, P. The first mitochondrial genomes for Pyralinae (Pyralidae) and Glaphyriinae (Crambidae), with phylogenetic implications of Pyraloidea. PLoS ONE 2018, 13, e0194672. [Google Scholar] [CrossRef] [PubMed]
- Regier, J.C.; Mitter, C.; Solis, M.A.; Hayden, J.E.; Landry, B.; Nuss, M.; Simonsen, T.J.; Yen, S.H.; Zwick, A.; Cummings, M.P. A molecular phylogeny for the pyraloid moths (Lepidoptera: Pyraloidea) and its implications for higher-level classification. Syst. Entomol. 2012, 37, 635–656. [Google Scholar] [CrossRef]
- Yang, M.; Song, L.; Mao, J.; Shi, Y.; Wu, C.; Zhang, Y.; Huang, L.; Peng, W.; Liu, X. Complete mitochondrial genome of the soybean leaffolder, Omiodes indicata (Lepidoptera: Pyraloidea: Crambidae), and phylogenetic analysis for Pyraloidea. Int. J. Biol. Macromol. 2018, 115, 53–60. [Google Scholar] [CrossRef]
- Cao, S.S.; Yu, W.W.; Sun, M.; Du, Y.Z. Characterization of the complete mitochondrial genome of Tryporyza incertulas, in comparison with seven other Pyraloidea moths. Gene 2014, 533, 356–365. [Google Scholar]
- Chen, Q.; Chen, L.; Liao, C.Q.; Wang, X.; Wang, M.; Huang, G.H. Comparative mitochondrial genome analysis and phylogenetic relationship among lepidopteran species. Gene 2022, 830, 146516. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhao, H.; Yu, F.; Zhang, A.; Li, H. The First Mitogenomes of the Subfamily Odontiinae (Lepidoptera, Crambidae) and Phylogenetic Analysis of Pyraloidea. Insects 2021, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, M.; Xu, H.; Wu, Z.; Hu, L.; Yang, M.; Li, H. Nine Mitochondrial Genomes of the Pyraloidea and Their Phylogenetic Implications (Lepidoptera). Insects 2021, 12, 1039. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhang, Y.; Fang, H.; Lu, J.; Wang, J. Characterization of four mitochondrial genomes of Crambidae (Lepidoptera, Pyraloidea) and phylogenetic implications. Arch. Insect Biochem. Physiol. 2023, 112, e21914. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).