Using QTL to Identify Genes and Pathways Underlying the Regulation and Production of Milk Components in Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. QTL for Major Pathways Involved in Milk Production
2.1. Milk Proteins
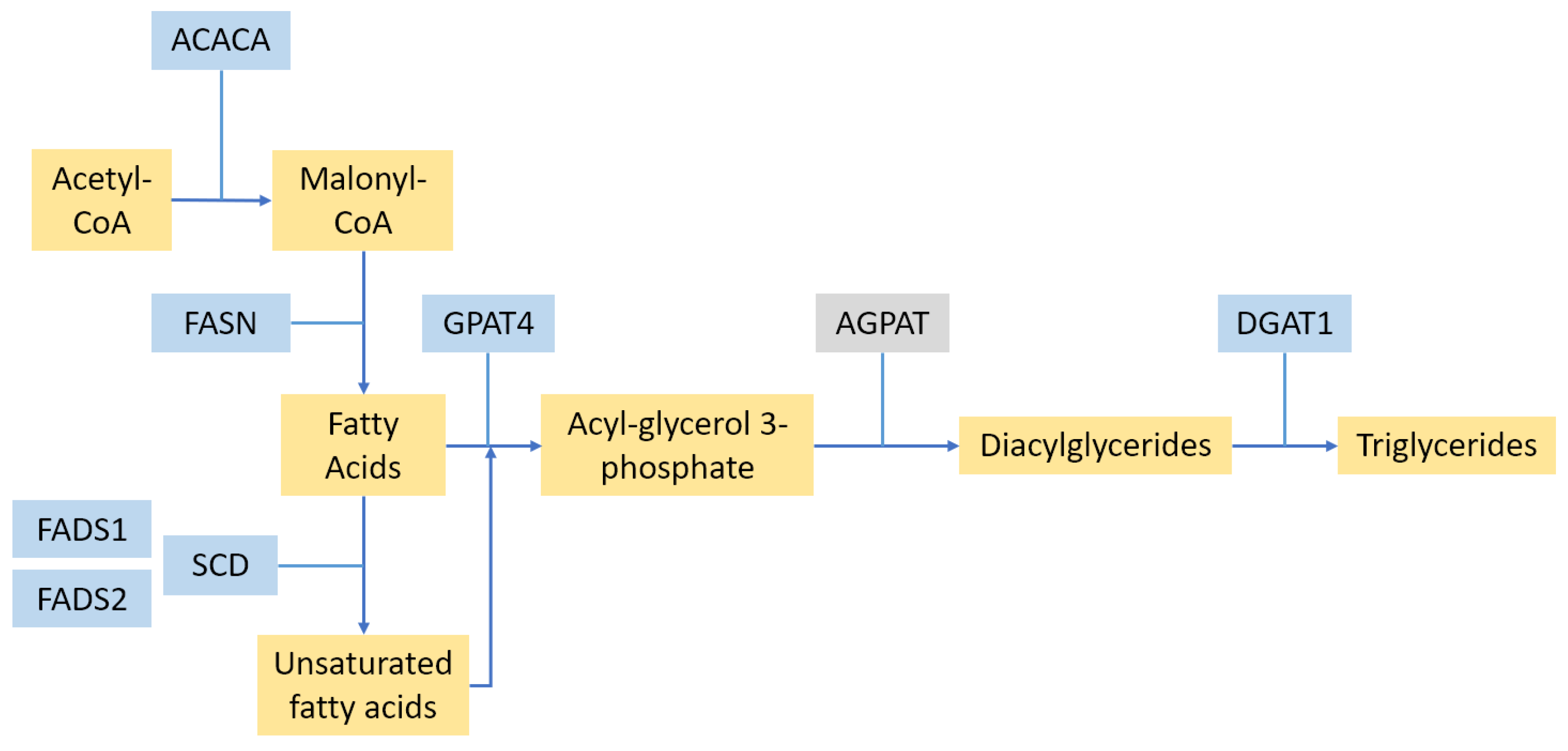
2.2. Fat Synthesis Pathways
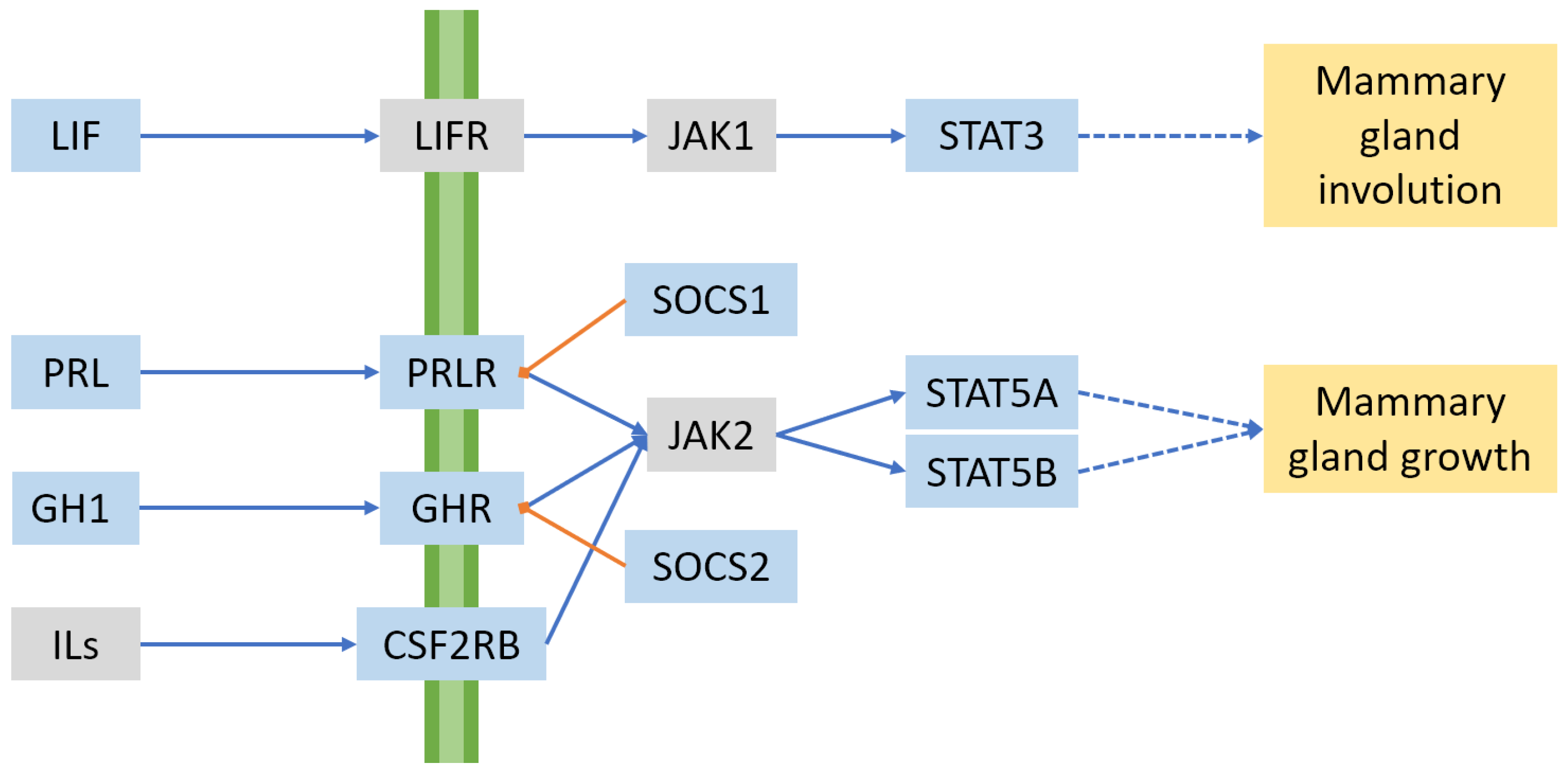
2.3. Hormones and Signalling
2.4. Transporters and Ion Channels
3. Identifying Candidate Causative Genes
3.1. Molecular Phenotypes
3.2. Chromatin Structure Phenotypes
3.3. From Candidate Genes to Causative Variants
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTA | Bos taurus (cow) chromosome |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| eQTL | Expression quantitative trait locus |
| FT-MIR | Fourier-transform mid-infrared |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GWAS | Genome-wide association study |
| HDR | Homology-directed repair |
| HPLC | High-performance liquid chromatography |
| IL | Interleukin |
| LD | Linkage disequilibrium |
| Mbp | Million base pairs |
| MFG | Milk fat globules |
| MPRA | Massively parallel reporter assay |
| NHEJ | Non-homologous end joining |
| OAR | Ovis aries (sheep) chromosome |
| qPCR | Quantitative polymerase chain reaction |
| QTL | Quantitative trait locus |
| TDT | Transmission disequilibrium test |
| TFBS | Transcription factor binding site |
| TWAS | Transcriptome-wide association scan |
References
- Jenness, R. Composition of Milk. In Fundamentals of Dairy Chemistry; Wong, N.P., Jenness, R., Keeney, M., Marth, E.H., Eds.; Van Nostrand Reinhold Company: Boston, MA, USA, 1988; Chapter 1; pp. 1–38. [Google Scholar] [CrossRef]
- Oftedal, O.T.; Iverson, S.J. Comparative Analysis of Nonhuman Milks. In Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: San Diego, CA, USA, 1995; Chapter 10; pp. 749–789. [Google Scholar]
- Hu, Z.L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2022, 50, D956–D961. [Google Scholar] [CrossRef]
- Vegarud, G.E.; Langsrud, T.; Svenning, C. Mineral-binding milk proteins and peptides; occurrence, biochemical and technological characteristics. Br. J. Nutr. 2000, 84, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Shekar, P.C.; Goel, S.; Rani, S.D.S.; Sarathi, D.P.; Alex, J.L.; Singh, S.; Kumar, S. κ-Casein-deficient mice fail to lactate. Proc. Natl. Acad. Sci. USA 2006, 103, 8000–8005. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Chessa, S.; Erhardt, G. Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, H.; Olsen, H.G.; Hayes, B.; Sehested, E.; Svendsen, M.; Nome, T.; Meuwissen, T.; Lien, S. Casein haplotypes and their association with milk production traits in Norwegian Red cattle. Genet. Sel. Evol. 2009, 41, 24. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.E.; Reich, C.M.; Bowman, P.J.; Vander Jagt, C.J.; Chamberlain, A.J.; Mason, B.A.; Hayes, B.J.; Goddard, M.E. Improved precision of QTL mapping using a nonlinear Bayesian method in a multi-breed population leads to greater accuracy of across-breed genomic predictions. Genet. Sel. Evol. 2015, 47, 29. [Google Scholar] [CrossRef] [PubMed]
- Pausch, H.; Emmerling, R.; Gredler-Grandl, B.; Fries, R.; Daetwyler, H.D.; Goddard, M.E. Meta-analysis of sequence-based association studies across three cattle breeds reveals 25 QTL for fat and protein percentages in milk at nucleotide resolution. BMC Genom. 2017, 18, 853. [Google Scholar] [CrossRef]
- MacLeod, I.; Bowman, P.; Vander Jagt, C.; Haile-Mariam, M.; Kemper, K.; Chamberlain, A.; Schrooten, C.; Hayes, B.; Goddard, M. Exploiting biological priors and sequence variants enhances QTL discovery and genomic prediction of complex traits. BMC Genom. 2016, 17, 1–21. [Google Scholar] [CrossRef]
- Olsen, H.G.; Knutsen, T.M.; Lewandowska-Sabat, A.M.; Grove, H.; Nome, T.; Svendsen, M.; Arnyasi, M.; Sodeland, M.; Sundsaasen, K.K.; Dahl, S.R.; et al. Fine mapping of a QTL on bovine chromosome 6 using imputed full sequence data suggests a key role for the group-specific component (GC) gene in clinical mastitis and milk production. Genet. Sel. Evol. 2016, 48, 79. [Google Scholar] [CrossRef]
- Vordenbäumen, S.; Braukmann, A.; Petermann, K.; Scharf, A.; Bleck, E.; von Mikecz, A.; Jose, J.; Schneider, M. Casein α s1 is expressed by human monocytes and upregulates the production of GM-CSF via p38 MAPK. J. Immunol. 2011, 186, 592–601. [Google Scholar] [CrossRef]
- Vordenbäumen, S.; Braukmann, A.; Altendorfer, I.; Bleck, E.; Jose, J.; Schneider, M. Human casein alpha s1 (CSN1S1) skews in vitro differentiation of monocytes towards macrophages. BMC Immunol. 2013, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Bovenhuis, H.; Weller, J.I. Mapping and analysis of dairy cattle quantitative trait loci by maximum likelihood methodology using milk protein genes as genetic markers. Genetics 1994, 137, 267–280. [Google Scholar] [CrossRef]
- Berry, S.; Lopez-Villalobos, N.; Beattie, E.; Davis, S.; Adams, L.; Thomas, N.; Ankersmit-Udy, A.; Stanfield, A.; Lehnert, K.; Ward, H.; et al. Mapping a quantitative trait locus for the concentration of β-lactoglobulin in milk, and the effect of β-lactoglobulin genetic variants on the composition of milk from Holstein-Friesian x Jersey crossbred cows. N. Zeal. Vet. J. 2010, 58, 1–5. [Google Scholar] [CrossRef]
- Farrell, H., Jr.; Jimenez-Flores, R.; Bleck, G.; Brown, E.; Butler, J.; Creamer, L.; Hicks, C.; Hollar, C.; Ng-Kwai-Hang, K.; Swaisgood, H. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [PubMed]
- Bedere, N.; Bovenhuis, H. Characterizing a region on BTA11 affecting β-lactoglobulin content of milk using high-density genotyping and haplotype grouping. BMC Genet. 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, M.; Leeb, T. Aberrant low expression level of bovine β-lactoglobulin is associated with a C to A transversion in the BLG promoter region. J. Dairy Sci. 2006, 89, 4414–4419. [Google Scholar] [CrossRef]
- Davis, S.R.; Ward, H.E.; Kelly, V.; Palmer, D.; Ankersmit-Udy, A.E.; Lopdell, T.J.; Berry, S.D.; Littlejohn, M.D.; Tiplady, K.; Adams, L.F.; et al. Screening for phenotypic outliers identifies an unusually low concentration of a β-lactoglobulin B protein isoform in bovine milk caused by a synonymous SNP. Genet. Sel. Evol. 2022, 54, 22. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Schenkel, F.S.; Sargolzaei, M.; Robinson, J.A.B. A genome scan to detect quantitative trait loci for economically important traits in Holstein cattle using two methods and a dense single nucleotide polymorphism map. J. Dairy Sci. 2008, 91, 3225–3236. [Google Scholar] [CrossRef]
- Kemper, K.; Vander Jagt, C.; Bowman, P.; Reich, C.; Mason, B.; Hayes, B.; Goddard, M. Mapping QTL in Australian dairy cattle using Genomic Selection Methodologies. In Proceedings of the 10th World Congress on Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- van den Berg, I.; Xiang, R.; Jenko, J.; Pausch, H.; Boussaha, M.; Schrooten, C.; Tribout, T.; Gjuvsland, A.B.; Boichard, D.; Nordbø, Ø.; et al. Meta-analysis for milk fat and protein percentage using imputed sequence variant genotypes in 94,321 cattle from eight cattle breeds. Genet. Sel. Evol. 2020, 52, 37. [Google Scholar] [CrossRef]
- Pedrosa, V.B.; Schenkel, F.S.; Chen, S.Y.; Oliveira, H.R.; Casey, T.M.; Melka, M.G.; Brito, L.F. Genomewide Association Analyses of Lactation Persistency and Milk Production Traits in Holstein Cattle Based on Imputed Whole-Genome Sequence Data. Genes 2021, 12, 1830. [Google Scholar] [CrossRef]
- Lopdell, T.J.; Tiplady, K.; Struchalin, M.; Johnson, T.J.; Keehan, M.; Sherlock, R.; Couldrey, C.; Davis, S.R.; Snell, R.G.; Spelman, R.J.; et al. DNA and RNA-sequence based GWAS highlights membrane-transport genes as key modulators of milk lactose content. BMC Genom. 2017, 18, 968. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkia, H.R.; Aminlari, M.; Cravador, A. Association of polymorphism of the β (1, 4)-galactosyltransferase-I gene with milk production traits in Holsteins. Mol. Biol. Rep. 2012, 39, 6715–6721. [Google Scholar] [CrossRef] [PubMed]
- Stinnakre, M.; Vilotte, J.; Soulier, S.; Mercier, J. Creation and phenotypic analysis of α-lactalbumin-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 6544–6548. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A.; Zhivotovsky, B.; Orrenius, S.; SabharwAl, H.; Svanborg, C. Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. USA 1995, 92, 8064–8068. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, J.; Bu, D.; Liu, G.; Zhang, C.; Wei, H.; Zhou, L.; Wang, J. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef]
- Villavicencio, A.; Rueda, M.S.; Turin, C.G.; Ochoa, T.J. Factors affecting lactoferrin concentration in human milk: How much do we know? Biochem. Cell Biol. 2017, 95, 12–21. [Google Scholar] [CrossRef]
- Prieur, D.J. Tissue specific deficiency of lysozyme in ruminants. Comp. Biochem. Physiol. B Comp. Biochem. 1986, 85, 349–353. [Google Scholar] [CrossRef]
- Seyfert, H.M. Lactoferrin and lysozyme-two mammalian antibiotics. Arch. Tierz. 1999, 42, 22–27. [Google Scholar]
- Carlsson, Å.; Björck, L.; Persson, K. Lactoferrin and lysozyme in milk during acute mastitis and their inhibitory effect in Delvotest P. J. Dairy Sci. 1989, 72, 3166–3175. [Google Scholar] [CrossRef]
- Gaunt, S.; Raffio, N.; Kingsbury, E.; Damon, R., Jr.; Johnson, W.; Mitchell, B. Variation of lactoferrin and mastitis and their heritabilities. J. Dairy Sci. 1980, 63, 1874–1880. [Google Scholar] [CrossRef]
- Arnould, V.R.; Soyeurt, H.; Gengler, N.; Colinet, F.; Georges, M.; Bertozzi, C.; Portetelle, D.; Renaville, R. Genetic analysis of lactoferrin content in bovine milk. J. Dairy Sci. 2009, 92, 2151–2158. [Google Scholar] [CrossRef]
- Cecchinato, A.; Ribeca, C.; Chessa, S.; Cipolat-Gotet, C.; Maretto, F.; Casellas, J.; Bittante, G. Candidate gene association analysis for milk yield, composition, urea nitrogen and somatic cell scores in Brown Swiss cows. Animal 2014, 8, 1062–1070. [Google Scholar] [CrossRef]
- Gothefors, L.; Marklund, S. Lactoperoxidase activity in human milk and in saliva of newborn infants. Infect. Immun. 1975, 11, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Littlejohn, M.; Lopdell, T.; Hayes, B.; Bennett, L.; Williams, R.; Xu, X.; Visscher, P.M.; Carrick, M.; Goddard, M. Leveraging genetically simple traits to identify small-effect variants for complex phenotypes. BMC Genom. 2016, 17, 858. [Google Scholar] [CrossRef] [PubMed]
- Cavaletto, M.; Giuffrida, M.G.; Conti, A. Milk fat globule membrane components—A proteomic approach. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; Chapter II; pp. 129–142. [Google Scholar]
- Robenek, H.; Hofnagel, O.; Buers, I.; Lorkowski, S.; Schnoor, M.; Robenek, M.J.; Heid, H.; Troyer, D.; Severs, N.J. Butyrophilin controls milk fat globule secretion. Proc. Natl. Acad. Sci. USA 2006, 103, 10385–10390. [Google Scholar] [CrossRef] [PubMed]
- Vorbach, C.; Scriven, A.; Capecchi, M.R. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: Gene sharing in the lactating mammary gland. Genes Dev. 2002, 16, 3223–3235. [Google Scholar] [CrossRef]
- Reynolds, E.G.; Neeley, C.; Lopdell, T.J.; Keehan, M.; Dittmer, K.; Harland, C.S.; Couldrey, C.; Johnson, T.J.; Tiplady, K.; Worth, G.; et al. Non-additive association analysis using proxy phenotypes identifies novel cattle syndromes. Nat. Genet. 2021, 53, 949–954. [Google Scholar] [CrossRef]
- Grisart, B.; Coppieters, W.; Farnir, F.; Karim, L.; Ford, C.; Berzi, P.; Cambisano, N.; Mni, M.; Reid, S.; Simon, P.; et al. Positional candidate cloning of a QTL in dairy cattle: Identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 2002, 12, 222–231. [Google Scholar] [CrossRef]
- Yen, C.L.E.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef]
- Fink, T.; Lopdell, T.J.; Tiplady, K.; Handley, R.; Johnson, T.J.; Spelman, R.J.; Davis, S.R.; Snell, R.G.; Littlejohn, M.D. A new mechanism for a familiar mutation—Bovine DGAT1 K232A modulates gene expression through multi-junction exon splice enhancement. BMC Genom. 2020, 21, 591. [Google Scholar] [CrossRef]
- Littlejohn, M.D.; Tiplady, K.; Lopdell, T.; Law, T.A.; Scott, A.; Harland, C.; Sherlock, R.; Henty, K.; Obolonkin, V.; Lehnert, K.; et al. Expression variants of the lipogenic AGPAT6 gene affect diverse milk composition phenotypes in Bos taurus. PLoS ONE 2014, 9, e85757. [Google Scholar] [CrossRef]
- Reue, K.; Brindley, D.N. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 2008, 49, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yuan, Y.; Liang, R.; Li, Y.; Liu, L.; Sun, D. Genetic effects of LPIN1 polymorphisms on milk production traits in dairy cattle. Genes 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sasaki, K.; Bessho, T.; Kobayashi, E.; Abe, T.; Sasazaki, S.; Oyama, K.; Mannen, H. The SNPs in the ACACA gene are effective on fatty acid composition in Holstein milk. Mol. Biol. Rep. 2012, 39, 8637–8644. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Y.P.P.; MacLeod, I.M.; Pryce, J.E.; Goddard, M.E.; Hayes, B.J. Application of a Bayesian non-linear model hybrid scheme to sequence data for genomic prediction and QTL mapping. BMC Genom. 2017, 18, 618. [Google Scholar] [CrossRef] [PubMed]
- Schennink, A.; Bovenhuis, H.; Léon-Kloosterziel, K.M.; Van Arendonk, J.A.; Visker, M.H. Effect of polymorphisms in the FASN, OLR1, PPARGC1A, PRL and STAT5A genes on bovine milk-fat composition. Anim. Genet. 2009, 40, 909–916. [Google Scholar] [CrossRef]
- Cho, H.P.; Nakamura, M.; Clarke, S.D. Cloning, expression, and fatty acid regulation of the human Δ-5 desaturase. J. Biol. Chem. 1999, 274, 37335–37339. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Δ8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Akwanji, K.A.; Beaudoin, F.; Zhao, X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- Golan, Y.; Assaraf, Y.G. Genetic and physiological factors affecting human milk production and composition. Nutrients 2020, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Kawabata, T.; Shoji, K.; Ogata, H.; Kagawa, Y.; Nakayama, K.; Yanagisawa, Y.; Iwamoto, S.; Tatsuta, N.; Asato, K.; et al. Investigation of Maternal Diet and FADS1 Polymorphism Associated with Long-Chain Polyunsaturated Fatty Acid Compositions in Human Milk. Nutrients 2022, 14, 2160. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Kim, Y.C.; Gray-Keller, M.P.; Attie, A.D.; Ntambi, J.M. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 2000, 275, 30132–30138. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Dobrzyn, P.; Lee, S.H.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 11110–11115. [Google Scholar] [CrossRef]
- Banos, G.; Woolliams, J.; Woodward, B.; Forbes, A.; Coffey, M. Impact of single nucleotide polymorphisms in leptin, leptin receptor, growth hormone receptor, and diacylglycerol acyltransferase (DGAT1) gene loci on milk production, feed, and body energy traits of UK dairy cows. J. Dairy Sci. 2008, 91, 3190–3200. [Google Scholar] [CrossRef]
- Shi, T.; Xu, Y.; Yang, M.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Yang, X.; Chen, H. Copy number variations at LEPR gene locus associated with gene expression and phenotypic traits in Chinese cattle. Anim. Sci. J. 2016, 87, 336–343. [Google Scholar] [CrossRef]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef]
- Caro, J.F.; Sinha, M.K.; Kolaczynski, J.W.; Zhang, P.L.; Considine, R.V. Leptin: The tale of an obesity gene. Diabetes 1996, 45, 1455–1463. [Google Scholar] [CrossRef]
- Loos, R.J.; Yeo, G.S. The bigger picture of FTO—The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Han, Z.; Niu, T.; Chang, J.; Lei, X.; Zhao, M.; Wang, Q.; Cheng, W.; Wang, J.; Feng, Y.; Chai, J. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature 2010, 464, 1205–1209. [Google Scholar] [CrossRef]
- Wei, J.; Liu, F.; Lu, Z.; Fei, Q.; Ai, Y.; He, P.C.; Shi, H.; Cui, X.; Su, R.; Klungland, A.; et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell 2018, 71, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Y.; Yao, Y.; Zhao, Y.; Bi, Z.; Jiang, Q.; Liu, Q.; Cai, M.; Wang, F.; Wang, Y.; et al. FTO regulates adipogenesis by controlling cell cycle progression via m6A-YTHDF2 dependent mechanism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1323–1330. [Google Scholar] [CrossRef]
- Zielke, L.G.; Bortfeldt, R.H.; Reissmann, M.; Tetens, J.; Thaller, G.; Brockmann, G.A. Impact of variation at the FTO locus on milk fat yield in Holstein dairy cattle. PLoS ONE 2013, 8, e63406. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, M.D.; Henty, K.M.; Tiplady, K.; Johnson, T.; Harland, C.; Lopdell, T.; Sherlock, R.G.; Li, W.; Lukefahr, S.D.; Shanks, B.C.; et al. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Stanford, P.M.; Sutherland, K.; Oakes, S.R.; Naylor, M.J.; Robertson, F.G.; Blazek, K.D.; Kazlauskas, M.; Hilton, H.N.; Wittlin, S.; et al. Socs2 and Elf5 mediate prolactin-induced mammary gland development. Mol. Endocrinol. 2006, 20, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.H.; Watson, C.J. Making milk: A new link between STAT5 and Akt1. JAK-STAT 2013, 2, e23228. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Hadsell, D.L.; Haymond, M.W. Gene regulation of UDP-galactose synthesis and transport: Potential rate-limiting processes in initiation of milk production in humans. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E365–E376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Boxer, R.B.; Stairs, D.B.; Portocarrero, C.P.; Horton, R.H.; Alvarez, J.V.; Birnbaum, M.J.; Chodosh, L.A. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res. 2010, 12, 1–14. [Google Scholar] [CrossRef]
- Boxer, R.B.; Stairs, D.B.; Dugan, K.D.; Notarfrancesco, K.L.; Portocarrero, C.P.; Keister, B.A.; Belka, G.K.; Cho, H.; Rathmell, J.C.; Thompson, C.B.; et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006, 4, 475–490. [Google Scholar] [CrossRef]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 2007, 9, 204. [Google Scholar] [CrossRef]
- Chen, C.C.; Stairs, D.B.; Boxer, R.B.; Belka, G.K.; Horseman, N.D.; Alvarez, J.V.; Chodosh, L.A. Autocrine prolactin induced by the Pten–Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes Dev. 2012, 26, 2154–2168. [Google Scholar] [CrossRef]
- Lü, A.; Hu, X.; Chen, H.; Dong, Y.; Zhang, Y.; Wang, X. Novel SNPs of the bovine PRLR gene associated with milk production traits. Biochem. Genet. 2011, 49, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Meredith, B.K.; Kearney, F.J.; Finlay, E.K.; Bradley, D.G.; Fahey, A.G.; Berry, D.P.; Lynn, D.J. Genome-wide associations for milk production and somatic cell score in Holstein-Friesian cattle in Ireland. BMC Genet. 2012, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Tiplady, K.M.; Lopdell, T.J.; Reynolds, E.; Sherlock, R.G.; Keehan, M.; Johnson, T.J.; Pryce, J.E.; Davis, S.R.; Spelman, R.J.; Harris, B.L.; et al. Sequence-based genome-wide association study of individual milk mid-infrared wavenumbers in mixed-breed dairy cattle. Genet. Sel. Evol. 2021, 53, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wallis, M. The molecular evolution of pituitary growth hormone prolactin and placental lactogen: A protein family showing variable rates of evolution. J. Mol. Evol. 1981, 17, 10–18. [Google Scholar] [CrossRef]
- Bauman, D.E.; Currie, W.B. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef] [PubMed]
- Shingu, H.; Hodate, K.; Kushibiki, S.; Ueda, Y.; Touno, E.; Shinoda, M.; Ohashi, S. Hormonal and lactational responses to growth hormone-releasing hormone treatment in lactating Japanese Black cows. J. Dairy Sci. 2004, 87, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Blott, S.; Kim, J.J.; Moisio, S.; Schmidt-Küntzel, A.; Cornet, A.; Berzi, P.; Cambisano, N.; Ford, C.; Grisart, B.; Johnson, D.; et al. Molecular dissection of a quantitative trait locus: A phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics 2003, 163, 253–266. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, L.; Prakapenka, D.; VanRaden, P.M.; Cole, J.B.; Da, Y. A large-scale genome-wide association study in US Holstein cattle. Front. Genet. 2019, 10, 412. [Google Scholar] [CrossRef]
- Cai, Z.; Dusza, M.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Distinguishing pleiotropy from linked QTL between milk production traits and mastitis resistance in Nordic Holstein cattle. Genet. Sel. Evol. 2020, 52, 19. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Raven, L.A.; Cocks, B.G.; Kemper, K.E.; Chamberlain, A.J.; Vander Jagt, C.J.; Goddard, M.E.; Hayes, B.J. Targeted imputation of sequence variants and gene expression profiling identifies twelve candidate genes associated with lactation volume, composition and calving interval in dairy cattle. Mamm. Genome 2016, 27, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Lopdell, T.J.; Tiplady, K.; Couldrey, C.; Johnson, T.J.; Keehan, M.; Davis, S.R.; Harris, B.L.; Spelman, R.J.; Snell, R.G.; Littlejohn, M.D. Multiple QTL underlie milk phenotypes at the CSF2RB locus. Genet. Sel. Evol. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Robinson, G.; Bar-Peled, U.; Wagner, K.U.; Young, W.S.; Hennighausen, L.; Furth, P.A. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. USA 1997, 94, 3425–3430. [Google Scholar] [CrossRef]
- Kritikou, E.A.; Sharkey, A.; Abell, K.; Came, P.J.; Anderson, E.; Clarkson, R.W.; Watson, C.J. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 2003, 130, 3459–3468. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Boland, M.P.; Kritikou, E.A.; Lee, J.M.; Freeman, T.C.; Tiffen, P.G.; Watson, C.J. The genes induced by signal transducer and activators of transcription (STAT)3 and STAT5 in mammary epithelial cells define the roles of these STATs in mammary development. Mol. Endocrinol. 2006, 20, 675–685. [Google Scholar] [CrossRef]
- Cooper Phyn, C.V. Regulation of Tight Junction Proteins during Engorgement of the Mammary Gland. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2006. [Google Scholar]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Cobanoglu, O.; Zaitoun, I.; Chang, Y.; Shook, G.; Khatib, H. Effects of the signal transducer and activator of transcription 1 (STAT1) gene on milk production traits in Holstein dairy cattle. J. Dairy Sci. 2006, 89, 4433–4437. [Google Scholar] [CrossRef]
- Arun, S.J.; Thomson, P.C.; Sheehy, P.A.; Khatkar, M.S.; Raadsma, H.W.; Williamson, P. Targeted analysis reveals an important role of JAK-STAT-SOCS genes for milk production traits in Australian dairy cattle. Front. Genet. 2015, 6, 342. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, C.J.; Rico-Bautista, E.; Lorentzon, M.; Thaus, A.L.; Morgan, P.O.; Willson, T.A.; Zervoudakis, P.; Metcalf, D.; Street, I.; Nicola, N.A.; et al. SOCS2 negatively regulates growth hormone action in vitro and in vivo. J. Clin. Investig. 2005, 115, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Rupp, R.; Senin, P.; Sarry, J.; Allain, C.; Tasca, C.; Ligat, L.; Portes, D.; Woloszyn, F.; Bouchez, O.; Tabouret, G.; et al. A point mutation in suppressor of cytokine signalling 2 (Socs2) increases the susceptibility to inflammation of the mammary gland while associated with higher body weight and size and higher milk production in a sheep model. PLoS Genet. 2015, 11, e1005629. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Chen, S.Y.; Jun, H.S.; Lin, S.R.; Mansfield, B.C.; Chou, J.Y. SLC37A1 and SLC37A2 are phosphate-linked, glucose-6-phosphate antiporters. PLoS ONE 2011, 6, e23157. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Govignon-Gion, A.; Croiseau, P.; Fritz, S.; Hozé, C.; Miranda, G.; Martin, P.; Barbat-Leterrier, A.; Letaïef, R.; Rocha, D.; et al. Within-breed and multi-breed GWAS on imputed whole-genome sequence variants reveal candidate mutations affecting milk protein composition in dairy cattle. Genet. Sel. Evol. 2017, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, J.; Monika, S.; Ankita, S.; Umesh, K.; Amit, K.; Ashok, M.; Mishra, B.; Sandeep, M.; Kataria, R.; Kaushik, J.; et al. Expression analysis of solute carrier (SLC2A) genes in milk derived mammary epithelial cells during different stages of lactation in sahiwal (Bos indicus) cows. J. Adv. Dairy Res. 2014, 2, 117. [Google Scholar] [CrossRef]
- Zwierzchowski, L.; Ostrowska, M.; Żelazowska, B.; Bagnicka, E. Single nucleotide polymorphisms in the bovine SLC2A12 and SLC5A1 glucose transporter genes–the effect on gene expression and milk traits of Holstein Friesian cows. Anim. Biotechnol. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Voss, F.K.; Ullrich, F.; Münch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef]
- Syeda, R.; Qiu, Z.; Dubin, A.E.; Murthy, S.E.; Florendo, M.N.; Mason, D.E.; Mathur, J.; Cahalan, S.M.; Peters, E.C.; Montal, M.; et al. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell 2016, 164, 499–511. [Google Scholar] [CrossRef]
- Tominaga, K.; Kondo, C.; Kagata, T.; Hishida, T.; Nishizuka, M.; Imagawa, M. The novel gene fad158, having a transmembrane domain and leucine-rich repeat, stimulates adipocyte differentiation. J. Biol. Chem. 2004, 279, 34840–34848. [Google Scholar] [CrossRef]
- Lu, J.; Argov-Argaman, N.; Anggrek, J.; Boeren, S.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K.A. The protein and lipid composition of the membrane of milk fat globules depends on their size. J. Dairy Sci. 2016, 99, 4726–4738. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Machida, N.; Tajika, Y.; Ablimit, A.; Suzuki, T.; Aoki, T.; Hagiwara, H.; Takata, K. Expression and immunolocalization of water-channel aquaporins in the rat and mouse mammary gland. Histochem. Cell Biol. 2005, 123, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.; Maxwell, J.; German, A.; Marples, D.; Royal, M.; Mobasheri, A. Immunohistochemical localisation of aquaporin water channels in the bovine mammary gland. In Proceedings of the British Society of Animal Science; Cambridge University Press: Cambridge, UK, 2007; Volume 2007, p. 1. [Google Scholar]
- Mobasheri, A.; Barrett-Jolley, R. Aquaporin water channels in the mammary gland: From physiology to pathophysiology and neoplasia. J. Mammary Gland. Biol. Neoplasia 2014, 19, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kaihoko, Y.; Tsugami, Y.; Suzuki, N.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Distinct expression patterns of aquaporin 3 and 5 in ductal and alveolar epithelial cells in mouse mammary glands before and after parturition. Cell Tissue Res. 2020, 380, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Sutera, A.M.; Tolone, M.; Mastrangelo, S.; Di Gerlando, R.; Sardina, M.T.; Portolano, B.; Pong-Wong, R.; Riggio, V. Detection of genomic regions underlying milk production traits in Valle del Belice dairy sheep using regional heritability mapping. J. Anim. Breed. Genet. 2021, 138, 552–561. [Google Scholar] [CrossRef]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; Mckinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stühmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef]
- Kubo, Y.; Adelman, J.P.; Clapham, D.E.; Jan, L.Y.; Karschin, A.; Kurachi, Y.; Lazdunski, M.; Nichols, C.G.; Seino, S.; Vandenberg, C.A. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 2005, 57, 509–526. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Bayliss, D.A.; Kim, D.; Lesage, F.; Plant, L.D.; Rajan, S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 2005, 57, 527–540. [Google Scholar] [CrossRef]
- Kaczmarek, L.K.; Aldrich, R.W.; Chandy, K.G.; Grissmer, S.; Wei, A.D.; Wulff, H. International union of basic and clinical pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol. Rev. 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef]
- Gonzalez-Perez, V.; Xia, X.M.; Lingle, C.J. Functional regulation of BK potassium channels by γ1 auxiliary subunits. Proc. Natl. Acad. Sci. USA 2014, 111, 4868–4873. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed]
- van Herwaarden, A.E.; Wagenaar, E.; Merino, G.; Jonker, J.W.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 2007, 27, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Zinder, M.; Seroussi, E.; Larkin, D.M.; Loor, J.J.; Everts-Van Der Wind, A.; Lee, J.H.; Drackley, J.K.; Band, M.R.; Hernandez, A.; Shani, M.; et al. Identification of a missense mutation in the bovine ABCG2 gene with a major effect on the QTL on chromosome 6 affecting milk yield and composition in Holstein cattle. Genome Res. 2005, 15, 936–944. [Google Scholar] [CrossRef]
- Leonard, S.; Khatib, H.; Schutzkus, V.; Chang, Y.; Maltecca, C. Effects of the osteopontin gene variants on milk production traits in dairy cattle. J. Dairy Sci. 2005, 88, 4083–4086. [Google Scholar] [CrossRef]
- Schnabel, R.D.; Kim, J.J.; Ashwell, M.S.; Sonstegard, T.S.; Van Tassell, C.P.; Connor, E.E.; Taylor, J.F. Fine-mapping milk production quantitative trait loci on BTA6: Analysis of the bovine osteopontin gene. Proc. Natl. Acad. Sci. USA 2005, 102, 6896–6901. [Google Scholar] [CrossRef]
- Fatehi, M.; Carter, C.R.; Youssef, N.; Hunter, B.E.; Holt, A.; Light, P.E. Molecular determinants of ATP-sensitive potassium channel MgATPase activity: Diabetes risk variants and diazoxide sensitivity. Biosci. Rep. 2015, 35, e00238. [Google Scholar] [CrossRef]
- Campbell, J.D.; Sansom, M.S.; Ashcroft, F.M. Potassium channel regulation: Structural insights into the function of the nucleotide-binding domains of the human sulphonylurea receptor. EMBO Rep. 2003, 4, 1038–1042. [Google Scholar] [CrossRef]
- Ashcroft, F.; Gribble, F. ATP-sensitive K+ channels and insulin secretion: Their role in health and disease. Diabetologia 1999, 42, 903. [Google Scholar] [CrossRef] [PubMed]
- VanHouten, J.N.; Neville, M.C.; Wysolmerski, J.J. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: A mechanism for calcium-regulated calcium transport into milk. Endocrinology 2007, 148, 5943–5954. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 2000, 289, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Vijen, S.; Hawes, C.; Runions, J.; Russell, R.G.G.; Wordsworth, B.P.; Carr, A.J.; Pink, R.C.; Zhang, Y. Differences in intracellular localisation of ANKH mutants that relate to mechanisms of calcium pyrophosphate deposition disease and craniometaphyseal dysplasia. Sci. Rep. 2020, 10, 7408. [Google Scholar] [CrossRef]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; van de Wetering, K. The mineralization regulator ANKH mediates cellular efflux of ATP, not pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef]
- Seifert, W.; Posor, Y.; Schu, P.; Stenbeck, G.; Mundlos, S.; Klaassen, S.; Nürnberg, P.; Haucke, V.; Kornak, U.; Kühnisch, J. The progressive ankylosis protein ANK facilitates clathrin-and adaptor-mediated membrane traffic at the trans-Golgi network-to-endosome interface. Hum. Mol. Genet. 2016, 25, 3836–3848. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, J.C.; Fan, E.Y.; Flajolet, M.; Greengard, P. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc. Natl. Acad. Sci. USA 2013, 110, 17071–17076. [Google Scholar] [CrossRef]
- Mehla, K.; Singh, P.K. MUC1: A novel metabolic master regulator. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1845, 126–135. [Google Scholar] [CrossRef]
- Stoop, W.; Van Arendonk, J.; Heck, J.; Van Valenberg, H.; Bovenhuis, H. Genetic parameters for major milk fatty acids and milk production traits of Dutch Holstein-Friesians. J. Dairy Sci. 2008, 91, 385–394. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Lognay, G.; Veselko, D.; Marlier, M.; Bertozzi, C.; Mayeres, P.; Gengler, N. Estimating fatty acid content in cow milk using mid-infrared spectrometry. J. Dairy Sci. 2006, 89, 3690–3695. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Ferrand, M.; Gelé, M.; Pourchet, D.; Miranda, G.; Martin, P.; Brochard, M.; Boichard, D. Genetic parameters for milk protein composition predicted using mid-infrared spectroscopy in the French Montbéliarde, Normande, and Holstein dairy cattle breeds. J. Dairy Sci. 2017, 100, 6371–6375. [Google Scholar] [CrossRef] [PubMed]
- Vanlierde, A.; Soyeurt, H.; Gengler, N.; Colinet, F.G.; Froidmont, E.; Kreuzer, M.; Grandl, F.; Bell, M.; Lund, P.; Olijhoek, D.; et al. Development of an equation for estimating methane emissions of dairy cows from milk Fourier transform mid-infrared spectra by using reference data obtained exclusively from respiration chambers. J. Dairy Sci. 2018, 101, 7618–7624. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Bonfatti, V.; Luke, T.; Pryce, J. Classifying the fertility of dairy cows using milk mid-infrared spectroscopy. J. Dairy Sci. 2019, 102, 10460–10470. [Google Scholar] [CrossRef]
- Tiplady, K.M.; Lopdell, T.J.; Sherlock, R.G.; Johnson, T.J.; Spelman, R.J.; Harris, B.L.; Davis, S.R.; Littlejohn, M.D.; Garrick, D.J. Comparison of the genetic characteristics of directly measured and Fourier-transform mid-infrared-predicted bovine milk fatty acids and proteins. J. Dairy Sci. 2022, 105, 9763–9791. [Google Scholar] [CrossRef] [PubMed]
- Prowse-Wilkins, C.P.; Lopdell, T.J.; Xiang, R.; Vander Jagt, C.J.; Littlejohn, M.D.; Chamberlain, A.J.; Goddard, M.E. Genetic variation in histone modifications and gene expression identifies regulatory variants in the mammary gland of cattle. BMC Genom. 2022, 23, 815. [Google Scholar] [CrossRef]
- Van Den Berg, I.; Hayes, B.; Chamberlain, A.; Goddard, M. Overlap between eQTL and QTL associated with production traits and fertility in dairy cattle. BMC Genom. 2019, 20, 291. [Google Scholar] [CrossRef]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.; Jansen, R.; De Geus, E.J.; Boomsma, D.I.; Wright, F.A.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, Y.; Zang, T.; Wang, Y. Integrate GWAS, eQTL, and mQTL data to identify Alzheimer’s disease-related genes. Front. Genet. 2019, 10, 1021. [Google Scholar] [CrossRef]
- Hormozdiari, F.; Van De Bunt, M.; Segre, A.V.; Li, X.; Joo, J.W.J.; Bilow, M.; Sul, J.H.; Sankararaman, S.; Pasaniuc, B.; Eskin, E. Colocalization of GWAS and eQTL signals detects target genes. Am. J. Hum. Genet. 2016, 99, 1245–1260. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wurmser, C.; Pausch, H.; Jung, S.; Reinhardt, F.; Tetens, J.; Thaller, G.; Fries, R. Identification and dissection of four major QTL affecting milk fat content in the German Holstein-Friesian population. PLoS ONE 2012, 7, e40711. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, M.D.; Tiplady, K.; Fink, T.A.; Lehnert, K.; Lopdell, T.; Johnson, T.; Couldrey, C.; Keehan, M.; Sherlock, R.G.; Harland, C.; et al. Sequence-based association analysis reveals an MGST1 eQTL with pleiotropic effects on bovine milk composition. Sci. Rep. 2016, 6, 25376. [Google Scholar] [CrossRef] [PubMed]
- Hebbes, T.R.; Thorne, A.W.; Crane-Robinson, C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988, 7, 1395–1402. [Google Scholar] [CrossRef]
- Zhou, V.W.; Goren, A.; Bernstein, B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011, 12, 7–18. [Google Scholar] [CrossRef]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.K.; Koche, R.P.; et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 2017, 6, e21856. [Google Scholar] [CrossRef]
- Boyle, A.P.; Davis, S.; Shulha, H.P.; Meltzer, P.; Margulies, E.H.; Weng, Z.; Furey, T.S.; Crawford, G.E. High-resolution mapping and characterization of open chromatin across the genome. Cell 2008, 132, 311–322. [Google Scholar] [CrossRef]
- Schones, D.E.; Cui, K.; Cuddapah, S.; Roh, T.Y.; Barski, A.; Wang, Z.; Wei, G.; Zhao, K. Dynamic regulation of nucleosome positioning in the human genome. Cell 2008, 132, 887–898. [Google Scholar] [CrossRef]
- Kuan, P.F.; Huebert, D.; Gasch, A.; Keles, S. A non-homogeneous hidden-state model on first order differences for automatic detection of nucleosome positions. Stat. Appl. Genet. Mol. Biol. 2009, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of native chromatin for multimodal regulatory analysis and personal epigenomics. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Tang, Q.; Wan, M.; Cui, K.; Zhang, Y.; Ren, G.; Ni, B.; Sklar, J.; Przytycka, T.M.; Childs, R.; et al. Genome-wide detection of DNase I) hypersensitive sites in single cells and FFPE tissue samples. Nature 2015, 528, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Wu, B.; Litzenburger, U.M.; Ruff, D.; Gonzales, M.L.; Snyder, M.P.; Chang, H.Y.; Greenleaf, W.J. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 2015, 523, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Sproul, D.; Gilbert, N.; Bickmore, W.A. The role of chromatin structure in regulating the expression of clustered genes. Nat. Rev. Genet. 2005, 6, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Rippe, K.; Dekker, M.; Kleckner, N. Capturing chromosome conformation. Science 2002, 295, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Simonis, M.; Klous, P.; Splinter, E.; Moshkin, Y.; Willemsen, R.; De Wit, E.; Van Steensel, B.; De Laat, W. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 2006, 38, 1348–1354. [Google Scholar] [CrossRef]
- Dostie, J.; Richmond, T.A.; Arnaout, R.A.; Selzer, R.R.; Lee, W.L.; Honan, T.A.; Rubio, E.D.; Krumm, A.; Lamb, J.; Nusbaum, C.; et al. Chromosome Conformation Capture Carbon Copy (5C): A massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006, 16, 1299–1309. [Google Scholar] [CrossRef]
- Lieberman-Aiden, E.; Van Berkum, N.L.; Williams, L.; Imakaev, M.; Ragoczy, T.; Telling, A.; Amit, I.; Lajoie, B.R.; Sabo, P.J.; Dorschner, M.O.; et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009, 326, 289–293. [Google Scholar] [CrossRef]
- Belaghzal, H.; Dekker, J.; Gibcus, J.H. Hi-C 2.0: An optimized Hi-C procedure for high-resolution genome-wide mapping of chromosome conformation. Methods 2017, 123, 56–65. [Google Scholar] [CrossRef]
- Lafontaine, D.L.; Yang, L.; Dekker, J.; Gibcus, J.H. Hi-C 3.0: Improved protocol for genome-wide chromosome conformation capture. Curr. Protoc. 2021, 1, e198. [Google Scholar] [CrossRef]
- Meuwissen, T.; Hayes, B.; MacLeod, I.; Goddard, M. Identification of Genomic Variants Causing Variation in Quantitative Traits: A Review. Agriculture 2022, 12, 1713. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl variant effect predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Dietze, P.; Karas, H.; Knüppel, R. TRANSFAC: A database on transcription factors and their DNA binding sites. Nucleic Acids Res. 1996, 24, 238–241. [Google Scholar] [CrossRef]
- Sandelin, A.; Alkema, W.; Engström, P.; Wasserman, W.W.; Lenhard, B. JASPAR: An open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004, 32, D91–D94. [Google Scholar] [CrossRef]
- Tewhey, R.; Kotliar, D.; Park, D.S.; Liu, B.; Winnicki, S.; Reilly, S.K.; Andersen, K.G.; Mikkelsen, T.S.; Lander, E.S.; Schaffner, S.F.; et al. Direct identification of hundreds of expression-modulating variants using a multiplexed reporter assay. Cell 2016, 165, 1519–1529. [Google Scholar] [CrossRef]
- Dixit, A.; Parnas, O.; Li, B.; Chen, J.; Fulco, C.P.; Jerby-Arnon, L.; Marjanovic, N.D.; Dionne, D.; Burks, T.; Raychowdhury, R.; et al. Perturb-Seq: Dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 2016, 167, 1853–1866. [Google Scholar] [CrossRef]
- Datlinger, P.; Rendeiro, A.F.; Schmidl, C.; Krausgruber, T.; Traxler, P.; Klughammer, J.; Schuster, L.C.; Kuchler, A.; Alpar, D.; Bock, C. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 2017, 14, 297–301. [Google Scholar] [CrossRef]
- Reilly, S.K.; Gosai, S.J.; Gutierrez, A.; Mackay-Smith, A.; Ulirsch, J.C.; Kanai, M.; Mouri, K.; Berenzy, D.; Kales, S.; Butler, G.M.; et al. Direct characterization of cis-regulatory elements and functional dissection of complex genetic associations using HCR–FlowFISH. Nat. Genet. 2021, 53, 1166–1176. [Google Scholar] [CrossRef]

| Trait | Number of QTL Variants |
|---|---|
| Milk fat percentage | 11,911 |
| Milk protein percentage | 9958 |
| Milk fat yield | 9255 |
| Milk yield | 7383 |
| Milk C14 index | 4847 |
| Milk kappa-casein percentage | 4275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopdell, T.J. Using QTL to Identify Genes and Pathways Underlying the Regulation and Production of Milk Components in Cattle. Animals 2023, 13, 911. https://doi.org/10.3390/ani13050911
Lopdell TJ. Using QTL to Identify Genes and Pathways Underlying the Regulation and Production of Milk Components in Cattle. Animals. 2023; 13(5):911. https://doi.org/10.3390/ani13050911
Chicago/Turabian StyleLopdell, Thomas John. 2023. "Using QTL to Identify Genes and Pathways Underlying the Regulation and Production of Milk Components in Cattle" Animals 13, no. 5: 911. https://doi.org/10.3390/ani13050911
APA StyleLopdell, T. J. (2023). Using QTL to Identify Genes and Pathways Underlying the Regulation and Production of Milk Components in Cattle. Animals, 13(5), 911. https://doi.org/10.3390/ani13050911






