Identification of Personality-Related Candidate Genes in Thoroughbred Racehorses Using a Bioinformatics-Based Approach Involving Functionally Annotated Human Genes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
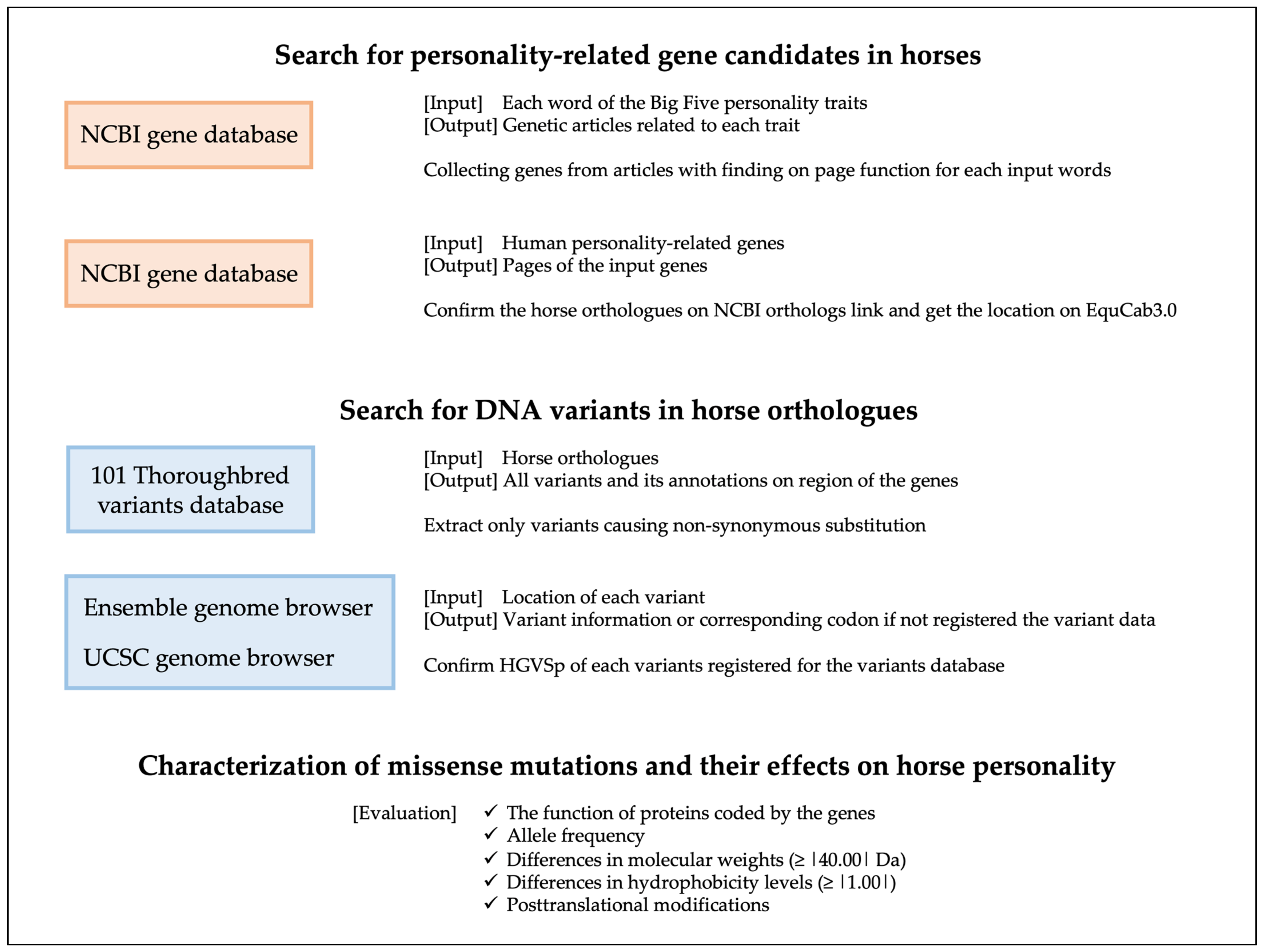
2.1. Search for Personality-Related Candidate Genes in Horses
2.2. Search for DNA Variants in Equine Personality-Related Gene Candidates
2.3. Characterization of Missense Mutations and Their Effects on Equine Personality
3. Results
3.1. Search for Personality-Related Candidate Genes in Horses
3.2. Search for DNA Variants in Equine Personality-Related Gene Candidates
3.3. Characterization of Missense Mutations and Their Effects on Equine Personality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holcomb, K.E.; Stull, C.L.; Kass, P.H. Characteristics of relinquishing and adoptive owners of horses associated with US nonprofit equine rescue organizations. J. Appl. Anim. Welf. Sci. 2012, 15, 21–31. [Google Scholar] [CrossRef]
- Holcomb, K.E.; Stull, C.L.; Kass, P.H. Unwanted horses: The role of nonprofit equine rescue and sanctuary organizations. J. Anim. Sci. 2010, 88, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.L.; Finnane, A.; Greer, R.M.; Phillips, C.J.; Woldeyohannes, S.M.; Perkins, N.R.; Ahern, B.J. Appraising the welfare of Thoroughbred racehorses in training in Queensland, Australia: The incidence, risk factors and outcomes for horses after retirement from racing. Animals 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Burattini, B.; Fenner, K.; Anzulewicz, A.; Romness, N.; McKenzie, J.; Wilson, B.; McGreevy, P. Age-related changes in the behaviour of domestic horses as reported by owners. Animals 2020, 10, 2321. [Google Scholar] [CrossRef] [PubMed]
- Valenchon, M.; Lévy, F.; Prunier, A.; Moussu, C.; Calandreau, L.; Lansade, L. Stress modulates instrumental learning performances in horses (Equus caballus) in interaction with temperament. PLoS ONE 2013, 8, e62324. [Google Scholar] [CrossRef]
- Lansade, L.; Coutureau, E.; Marchand, A.; Baranger, G.; Valenchon, M.; Calandreau, L. Dimensions of temperament modulate cue-controlled behavior: A study on Pavlovian to instrumental transfer in horses (Equus caballus). PLoS ONE 2013, 8, e64853. [Google Scholar] [CrossRef]
- Swanberg, J.E.; Clouser, J.M.; Westneat, S.C.; Marsh, M.W.; Reed, D.B. Occupational injuries on Thoroughbred horse farms: A description of Latino and non-Latino workers’ experiences. Int. J. Environ. Res. Public Health 2013, 10, 6500–6516. [Google Scholar] [CrossRef]
- Swanberg, J.E.; Clouser, J.M.; Bush, A.; Westneat, S. From the horse worker’s mouth: A detailed account of injuries experienced by Latino horse workers. J. Immigr. Minor. Health 2016, 18, 513–521. [Google Scholar] [CrossRef]
- Sallis, H.; Davey Smith, G.; Munafò, M.R. Genetics of biologically based psychological differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170162. [Google Scholar] [CrossRef]
- Persson, B. Genotype-Environment Correlation and Its Relation to Personality—A Twin and Family Study. Twin Res. Hum. Genet. 2020, 23, 228–234. [Google Scholar] [CrossRef]
- Heilbronner, U.; Papiol, S.; Budde, M.; Andlauer, T.F.M.; Strohmaier, J.; Streit, F.; Frank, J.; Degenhardt, F.; Heilmann-Heimbach, S.; Witt, S.H.; et al. “The Heidelberg Five” personality dimensions: Genome-wide associations, polygenic risk for neuroticism, and psychopathology 20 years after assessment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2021, 186, 77–89. [Google Scholar] [CrossRef]
- Le Scolan, N.; Hausberger, M.; Wolff, A. Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behav. Process. 1997, 41, 257–266. [Google Scholar] [CrossRef]
- Hausberger, M.; Bruderer, C.; Le Scolan, N.; Pierre, J.S. Interplay between environmental and genetic factors in temperament/personality traits in horses (Equus caballus). J. Comp. Psychol. 2004, 118, 434–446. [Google Scholar] [CrossRef]
- Jacobs, L.N.; Staiger, E.A.; Albright, J.D.; Brooks, S.A. The MC1R and ASIP Coat Color Loci May Impact Behavior in the Horse. J. Hered. 2016, 107, 214–219. [Google Scholar] [CrossRef]
- Momozawa, Y.; Takeuchi, Y.; Kusunose, R.; Kikusui, T.; Mori, Y. Association between equine temperament and polymorphisms in dopamine D4 receptor gene. Mamm. Genome 2005, 16, 538–544. [Google Scholar] [CrossRef]
- Roberts, K.; Hemmings, A.J.; Moore-Colyer, M.; Parker, M.O.; McBride, S.D. Neural modulators of temperament: A multivariate approach to personality trait identification in the horse. Physiol. Behav. 2016, 167, 125–131. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Kim, E.J.; Jung, H.; Yoon, M. Relationship between oxytocin and serotonin and the fearfulness, dominance, and trainability of horses. J. Anim. Sci. Technol. 2021, 63, 453–460. [Google Scholar] [CrossRef]
- Momozawa, Y.; Takeuchi, Y.; Tozaki, T.; Kikusui, T.; Hasegawa, T.; Raudsepp, T.; Chowdhary, B.P.; Kusunose, R.; Mori, Y. SNP detection and radiation hybrid mapping in horses of nine candidate genes for temperament. Anim. Genet. 2007, 38, 81–83. [Google Scholar] [CrossRef]
- Hori, Y.; Tozaki, T.; Nambo, Y.; Sato, F.; Ishimaru, M.; Inoue-Murayama, M.; Fujita, K. Evidence for the effect of serotonin receptor 1A gene (HTR1A) polymorphism on tractability in Thoroughbred horses. Anim. Genet. 2016, 47, 62–67. [Google Scholar] [CrossRef]
- Fenner, K.; Dashper, K.; Serpell, J.; McLean, A.; Wilkins, C.; Klinck, M.; Wilson, B.; McGreevy, P. The development of a novel questionnaire approach to the investigation of horse training, management, and behaviour. Animals 2020, 10, 1960. [Google Scholar] [CrossRef]
- Tozaki, T.; Ohnuma, A.; Kikuchi, M.; Ishige, T.; Kakoi, H.; Hirota, K.I.; Kusano, K.; Nagata, S.I. Rare and common variant discovery by whole-genome sequencing of 101 Thoroughbred racehorses. Sci. Rep. 2021, 11, 16057. [Google Scholar] [CrossRef] [PubMed]
- Gene. National Library of Medicine (US), National Center for Biotechnology Information: Bethesda, MD, USA. 2004. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 5 October 2022).
- Jang, K.L.; Livesley, W.J.; Vernon, P.A. Heritability of the big five personality dimensions and their facets: A twin study. J. Pers. 1996, 64, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Kalbfleisch, T.S.; Rice, E.S.; DePriest, M.S., Jr.; Walenz, B.P.; Hestand, M.S.; Vermeesch, J.R.; O’Connell, B.L.; Fiddes, I.T.; Vershinina, A.O.; Saremi, N.F.; et al. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun. Biol. 2018, 1, 197. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Eisenberg, D.S.; Schwarz, E.E.; Komaromy, M.; Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 1984, 179, 125–142. [Google Scholar] [CrossRef]
- Martin, G.G.; McIntosh, A.L.; Huang, H.; Gupta, S.; Atshaves, B.P.; Landrock, K.K.; Landrock, D.; Kier, A.B.; Schroeder, F. The human liver fatty acid binding protein T94A variant alters the structure, stability, and interaction with fibrates. Biochemistry 2013, 52, 9347–9357. [Google Scholar] [CrossRef]
- Taketomi, T.; Yasuda, T.; Morita, R.; Kim, J.; Shigeta, Y.; Eroglu, C.; Harada, R.; Tsuruta, F. Autism-associated mutation in Hevin/Sparcl1 induces endoplasmic reticulum stress through structural instability. Sci. Rep. 2022, 12, 11891. [Google Scholar] [CrossRef]
- Fujikawa, K.; Nakahara, K.; Takasugi, N.; Nishiya, T.; Ito, A.; Uchida, K.; Uehara, T. S-Nitrosylation at the active site decreases the ubiquitin-conjugating activity of ubiquitin-conjugating enzyme E2 D1 (UBE2D1), an ERAD-associated protein. Biochem. Biophys. Res. Commun. 2020, 524, 910–915. [Google Scholar] [CrossRef]
- Hirata, T.; Kizuka, Y. N-Glycosylation. Adv. Exp. Med. Biol. 2021, 1325, 3–24. [Google Scholar] [CrossRef]
- Hunter, T. Why nature chose phosphate to modify proteins. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2513–2516. [Google Scholar] [CrossRef]
- Terracciano, A.; Aschwanden, D.; Passamonti, L.; Toschi, N.; Stephan, Y.; Luchetti, M.; Lee, J.H.; Sesker, A.; O’Súilleabháin, P.S.; Sutin, A.R. Is neuroticism differentially associated with risk of Alzheimer’s disease, vascular dementia, and frontotemporal dementia? J. Psychiatr. Res. 2021, 138, 34–40. [Google Scholar] [CrossRef]
- Adams, M.J.; Howard, D.M.; Luciano, M.; Clarke, T.K.; Davies, G.; Hill, W.D.; Smith, D.; Deary, I.J.; 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; et al. Genetic stratification of depression by neuroticism: Revisiting a diagnostic tradition. Psychol. Med. 2020, 50, 2526–2535. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Holmans, P.; Pocklington, A.J.; Escott-Price, V.; Ripke, S.; Carrera, N.; Legge, S.E.; Bishop, S.; Cameron, D.; Hamshere, M.L.; et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 2018, 50, 381–389. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Ansari, A.; Siddiqui, Z.; Ashraf, J.M.; Abdullah, S.M.; et al. Protein oxidation: An overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. Schol. Ed. 2017, 9, 71–87. [Google Scholar] [CrossRef]
- Eley, T.C.; Tahir, E.; Angleitner, A.; Harriss, K.; McClay, J.; Plomin, R.; Riemann, R.; Spinath, F.; Craig, I. Association analysis of MAOA and COMT with neuroticism assessed by peers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 120, 90–96. [Google Scholar] [CrossRef]
- Lloyd, A.S.; Martin, J.E.; Bornett-Gauci, H.L.I.; Wilkinson, R.G. Horse personality: Variation between breeds. Appl. Anim. Behav. Sci. 2008, 112, 369–383. [Google Scholar] [CrossRef]
- Christensen, J.W.; Rundgren, M.; Olsson, K. Training methods for horses: Habituation to a frightening stimulus. Equine Vet. J. 2006, 38, 439–443. [Google Scholar] [CrossRef]
- Riva, M.G.; Dai, F.; Huhtinen, M.; Minero, M.; Barbieri, S.; Dalla Costa, E. The Impact of Noise Anxiety on Behavior and Welfare of Horses from UK and US Owner’s Perspective. Animals 2022, 12, 1319. [Google Scholar] [CrossRef]
- Lee, K.E.; Kim, J.G.; Lee, H.; Kim, B.S. Behavioral and cardiac responses in mature horses exposed to a novel object. J. Anim. Sci. Technol. 2021, 63, 651–661. [Google Scholar] [CrossRef]
- Lansade, L.; Bouissou, M.-F. Reactivity to Humans: A Temperament Trait of Horses Which Is Stable across Time and Situations. Appl. Anim. Behav. Sci. 2008, 114, 492–508. [Google Scholar] [CrossRef]
| Biological Function | Gene | ||
|---|---|---|---|
| Intracellular signaling pathway | DGKH, FAAH | ||
| Neurotransmission pathway | Monoamine neurotransmission | Dopaminergic signaling system | ANKK1, DRD2 |
| Serotonergic signaling system | HTR2A, SLC6A4 | ||
| Monoamine inactivation enzyme | COMT, MAOA | ||
| Amino acid neurotransmission | GABAergic signaling system | GABRA6 | |
| Endogenous cannabinoid signaling system | CNR1 | ||
| Peptide neurotransmission | LEP, NPY | ||
| Purinergic signaling system | P2RX7 | ||
| Hypothalamic–pituitary–adrenal axis | HSD11B1 | ||
| Neurotrophic-related factor | APOE, BDNF | ||
| Intercellular connections | CDH13 | ||
| Circadian rhythm | PER3 | ||
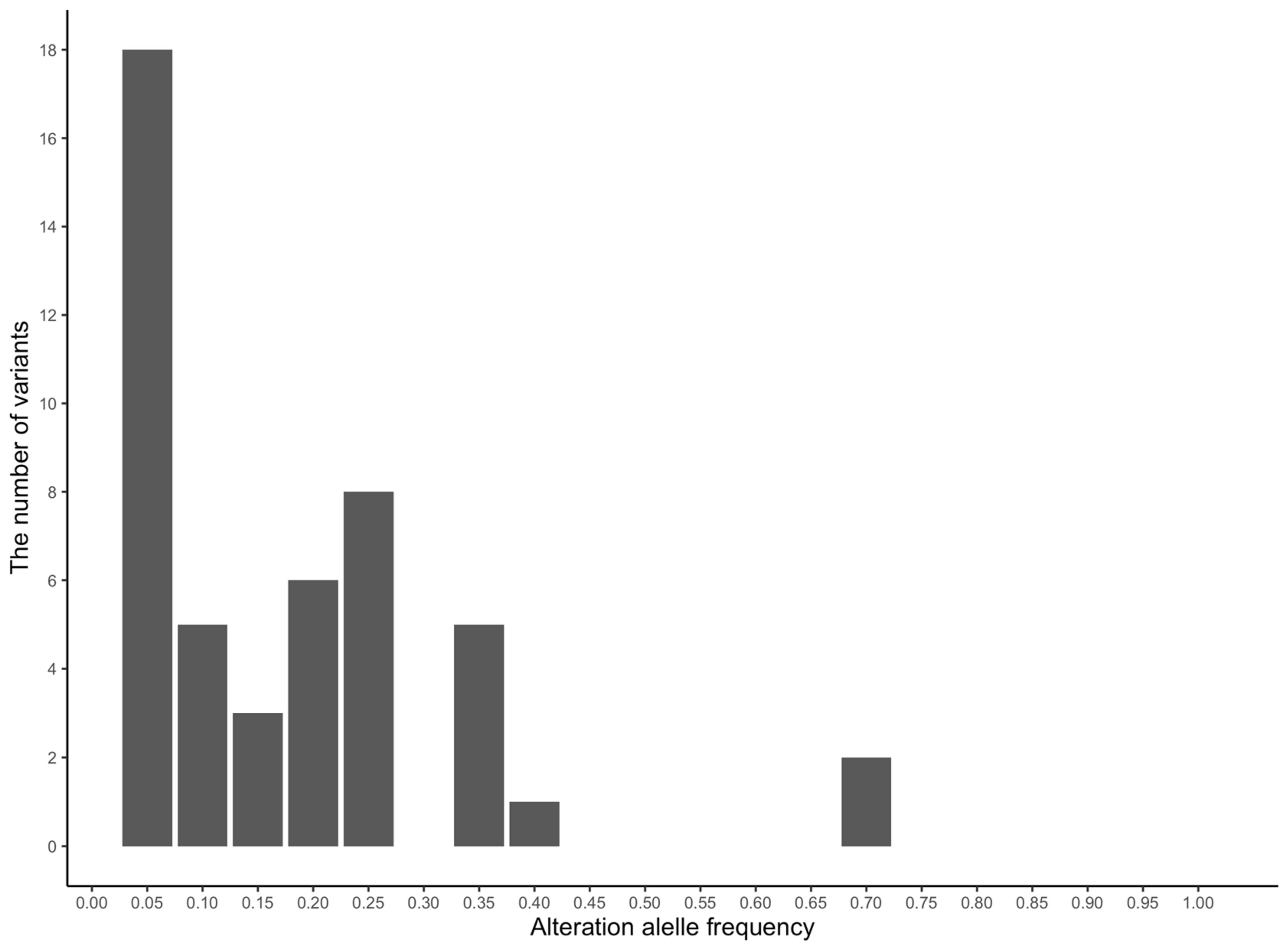
| Gene | Chromosome | Position | Reference Allele | Alteration Allele | Reference Allele Frequency | Alteration Allele Frequency |
|---|---|---|---|---|---|---|
| FAAH | 2 | 12167952 | G | A | 0.995 | 0.005 |
| 12174477 | G | A | 0.936 | 0.064 | ||
| 12174492 | G | T | 0.955 | 0.045 | ||
| PER3 | 2 | 43209639 | A | G | 0.683 | 0.317 |
| 43231315 | A | G | 0.678 | 0.322 | ||
| 43240108 | C | A | 0.822 | 0.178 | ||
| CDH13 | 3 | 31079056 | T | C | 0.911 | 0.089 |
| 31230472 | G | A | 0.955 | 0.045 | ||
| 31382147 | C | T | 0.757 | 0.243 | ||
| 31845727 | C | T | 0.995 | 0.005 | ||
| NPY | 4 | 55899514 | C | G | 0.861 | 0.139 |
| LEP | 4 | 83529565 | C | T | 0.891 | 0.109 |
| HSD11B1 | 5 | 25786262 | T | G | 0.767 | 0.233 |
| ANKK1 | 7 | 22319266 | A | G | 0.782 | 0.218 |
| 22319267 | T | A | 0.787 | 0.213 | ||
| 22319365 | G | C | 0.772 | 0.228 | ||
| 22319396 | T | C | 0.995 | 0.005 | ||
| 22319430 | G | T | 0.995 | 0.005 | ||
| 22319446 | A | G | 0.782 | 0.218 | ||
| 22319470 | G | A | 0.787 | 0.213 | ||
| 22326586 | A | G | 0.698 | 0.302 | ||
| 22327351 | G | A | 0.698 | 0.302 | ||
| 22329824 | C | T | 0.975 | 0.025 | ||
| 22330242 | G | A | 0.995 | 0.005 | ||
| 22331004 | C | A | 0.960 | 0.040 | ||
| 22331042 | G | A | 0.941 | 0.059 | ||
| DRD2 | 7 | 22348064 | T | A | 0.995 | 0.005 |
| BDNF | 7 | 96310373 | A | T | 0.812 | 0.188 |
| COMT | 8 | 432351 | T | C | 0.842 | 0.158 |
| 434481 | A | G | 0.886 | 0.114 | ||
| P2RX7 | 8 | 24214858 | A | G | 0.644 | 0.356 |
| APOE | 10 | 15713778 | A | G | 0.995 | 0.005 |
| 15714427 | A | G | 0.832 | 0.168 | ||
| 15714824 | T | G | 0.995 | 0.005 | ||
| CNR1 | 10 | 41805559 | C | T | 0.946 | 0.054 |
| SLC6A4 | 11 | 44188160 | A | G | 0.332 | 0.668 |
| 44188161 | C | T | 0.332 | 0.668 | ||
| 44192165 | T | C | 0.985 | 0.015 | ||
| 44200439 | A | C | 0.970 | 0.030 | ||
| GABRA6 | 14 | 17329705 | A | C | 0.950 | 0.050 |
| 17329709 | C | T | 0.842 | 0.158 | ||
| 17329851 | A | T | 0.777 | 0.223 | ||
| 17329886 | T | C | 0.985 | 0.015 | ||
| 17338413 | A | G | 0.673 | 0.327 | ||
| 17343960 | C | T | 0.926 | 0.074 | ||
| HTR2A | 17 | 23797786 | A | G | 0.990 | 0.010 |
| DGKH | 17 | 27918092 | G | T | 0.832 | 0.168 |
| 28048492 | A | G | 0.985 | 0.015 | ||
| MAOA | X | 36799409 | T | C | NA | NA |
| 36799613 | G | A | NA | NA | ||
| 36800118 | C | G | NA | NA | ||
| 36800211 | T | C | NA | NA | ||
| 36800238 | C | * | NA | NA | ||
| 36800403 | G | T | NA | NA | ||
| 36801932 | A | C | NA | NA |
| Gene | Chromosome | Position | HGVSp | Annotation | Molecular Weight Difference | Hydrophobicity Index Difference |
|---|---|---|---|---|---|---|
| FAAH | 2 | 12167952 | A96V | non-synonymous coding | 28.06 | 0.46 |
| 12174477 | A13V | non-synonymous coding | 28.06 | 0.46 | ||
| 12174492 | A8D | non-synonymous coding | 44.01 | −1.52 | ||
| PER3 | 2 | 43209639 | L837S | non-synonymous coding | −26.08 | −1.24 |
| 43231315 | V465A | non-synonymous coding | −28.06 | −0.46 | ||
| 43240108 | K241N | non-synonymous coding | −14.07 | 0.72 | ||
| CDH13 | 3 | 31079056 | Y20H | non-synonymous coding | −26.04 | −0.66 |
| 31230472 | V95I | non-synonymous coding | 14.02 | 0.3 | ||
| 31382147 | R173W R134W | non-synonymous coding | 30.03 | 3.34 | ||
| 31845727 | S696F S657F | non-synonymous coding | 60.1 | 1.37 | ||
| NPY | 4 | 55899514 | T139S | non-synonymous coding | −14.03 | −0.13 |
| LEP | 4 | 83529565 | T70M | splice site region non-synonymous coding | 30.09 | 0.69 |
| HSD11B1 | 5 | 25786262 | Y88S | non-synonymous coding | −76.1 | −0.44 |
| ANKK1 | 7 | 22319266 | I10V | non-synonymous coding | −14.02 | −0.3 |
| 22319267 | I10N | non-synonymous coding | 0.95 | −2.16 | ||
| 22319365 | E43Q | non-synonymous coding | −0.98 | 1.22 | ||
| 22319396 | L53P | non-synonymous coding | −16.04 | −0.94 | ||
| 22319430 | E64D | non-synonymous coding | −14.03 | −0.16 | ||
| 22319446 | T70A | non-synonymous coding | −30.03 | 0.67 | ||
| 22319470 | E78K | non-synonymous coding | −0.94 | −0.76 | ||
| 22326586 | I287V | non-synonymous coding | −14.02 | −0.3 | ||
| 22327351 | R337H | non-synonymous coding | −19.05 | 2.13 | ||
| 22329824 | R411W | non-synonymous coding | 30.03 | 3.34 | ||
| 22330242 | R550Q | non-synonymous coding | −28.05 | 3.01 | ||
| 22331004 | A804D | non-synonymous coding | 44.01 | −1.52 | ||
| 22331042 | E817K | non-synonymous coding | −0.94 | −0.76 | ||
| DRD2 | 7 | 22348064 | K101I | non-synonymous coding | −15.02 | 2.88 |
| BDNF | 7 | 96310373 | H14Q | non-synonymous coding | −9 | 0.88 |
| COMT | 8 | 432351 | T231A | non-synonymous coding | −30.03 | 0.67 |
| 434481 | M159T | non-synonymous coding | −30.09 | −0.69 | ||
| P2RX7 | 8 | 24214858 | S589G | non-synonymous coding | −30.02 | 0.66 |
| APOE | 10 | 15713778 | Q17R | non-synonymous coding | 28.05 | −3.01 |
| 15714427 | M100V | non-synonymous coding | −32.06 | 0.44 | ||
| 15714824 | V232G | non-synonymous coding | −42.08 | −0.6 | ||
| CNR1 | 10 | 41805559 | V263I | non-synonymous coding | 14.02 | 0.3 |
| SLC6A4 | 11 | 44188160 | V539A | non-synonymous coding | −28.06 | −0.46 |
| 44188161 | V539I | non-synonymous coding | 14.02 | 0.3 | ||
| 44192165 | I408V | non-synonymous coding | −14.02 | −0.3 | ||
| 44200439 | D36E | non-synonymous coding | 14.03 | 0.16 | ||
| GABRA6 | 14 | 17329705 | I423M | non-synonymous coding | 18.04 | −0.74 |
| 17329709 | R422Q | non-synonymous coding | −28.05 | 3.01 | ||
| 17329851 | S375T | non-synonymous coding | 14.03 | 0.13 | ||
| 17329886 | H363R | splice site region non-synonymous coding | 19.05 | −2.13 | ||
| 17338413 | V352A | non-synonymous coding | −28.06 | −0.46 | ||
| 17343960 | E30K | non-synonymous coding | −0.94 | −0.76 | ||
| HTR2A | 17 | 23797786 | T39A | non-synonymous coding | −30.03 | 0.67 |
| DGKH | 17 | 27918092 | S631Y | non-synonymous coding | 76.1 | 0.44 |
| 28048492 | V12A | non-synonymous coding | −28.06 | −0.46 | ||
| MAOA | X | 36799409 | C24R | non-synonymous coding | 53.04 | −2.82 |
| 36799613 | G92S | non-synonymous coding | 30.02 | −0.66 | ||
| 36800118 | A142G A260G | non-synonymous coding | −14.02 | −0.14 | ||
| 36800211 | L173P L291P | non-synonymous coding | −16.04 | −0.94 | ||
| 36800238 | * | codon insertion | NA | NA | ||
| 36800403 | W237L W355L | non-synonymous coding | −73.06 | 0.25 | ||
| 36801932 | N307T | non-synonymous coding | −13 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokomori, T.; Ohnuma, A.; Tozaki, T.; Segawa, T.; Itou, T. Identification of Personality-Related Candidate Genes in Thoroughbred Racehorses Using a Bioinformatics-Based Approach Involving Functionally Annotated Human Genes. Animals 2023, 13, 769. https://doi.org/10.3390/ani13040769
Yokomori T, Ohnuma A, Tozaki T, Segawa T, Itou T. Identification of Personality-Related Candidate Genes in Thoroughbred Racehorses Using a Bioinformatics-Based Approach Involving Functionally Annotated Human Genes. Animals. 2023; 13(4):769. https://doi.org/10.3390/ani13040769
Chicago/Turabian StyleYokomori, Tamu, Aoi Ohnuma, Teruaki Tozaki, Takao Segawa, and Takuya Itou. 2023. "Identification of Personality-Related Candidate Genes in Thoroughbred Racehorses Using a Bioinformatics-Based Approach Involving Functionally Annotated Human Genes" Animals 13, no. 4: 769. https://doi.org/10.3390/ani13040769
APA StyleYokomori, T., Ohnuma, A., Tozaki, T., Segawa, T., & Itou, T. (2023). Identification of Personality-Related Candidate Genes in Thoroughbred Racehorses Using a Bioinformatics-Based Approach Involving Functionally Annotated Human Genes. Animals, 13(4), 769. https://doi.org/10.3390/ani13040769








