Genetic Divergence of Thai Indigenous Pigs from Three Distinct Geographic Regions Revealed by Microsatellite Marker Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
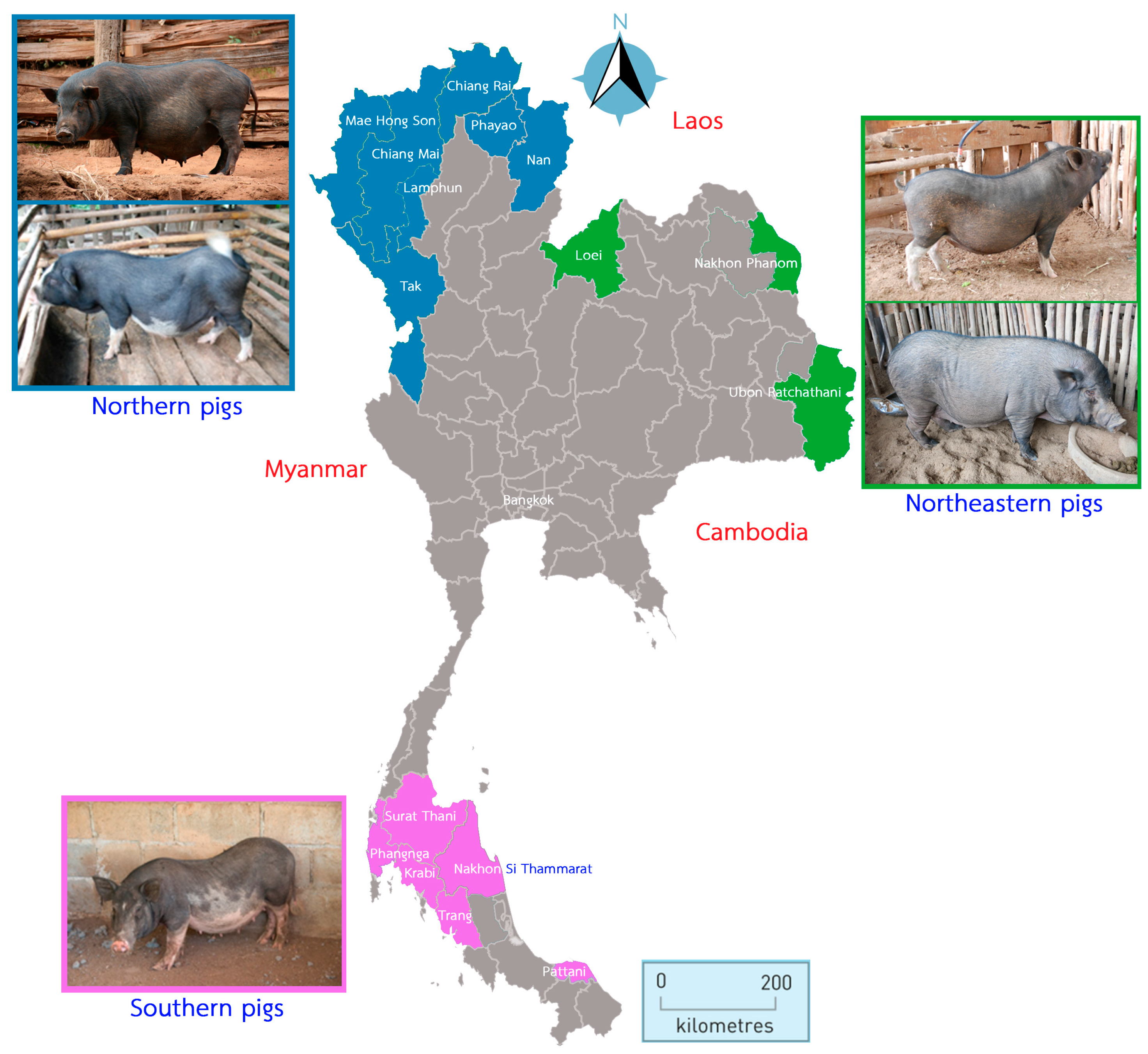
2.1. Sample Collection and Populations
2.2. Preparation of DNA Samples
2.3. Microsatellite Genotyping
2.4. Statistical Analysis
3. Results
3.1. Microsatellite Polymorphism
3.2. Genetic Diversity among Thai Indigenous Pig Populations
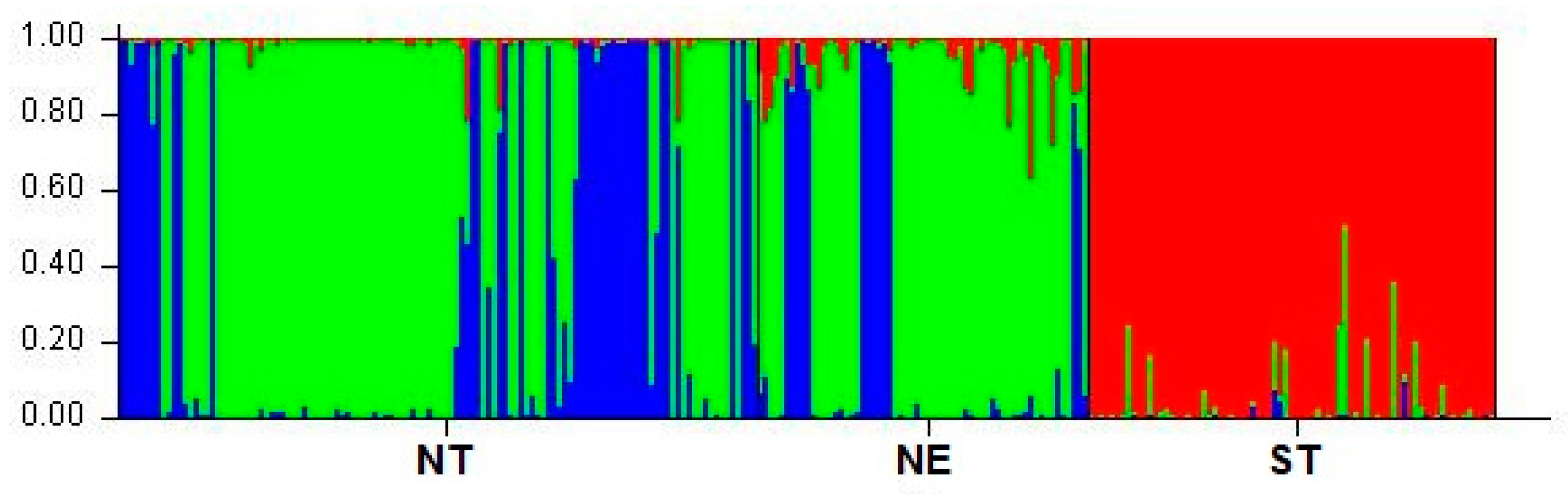
3.3. Population Cluster Analysis
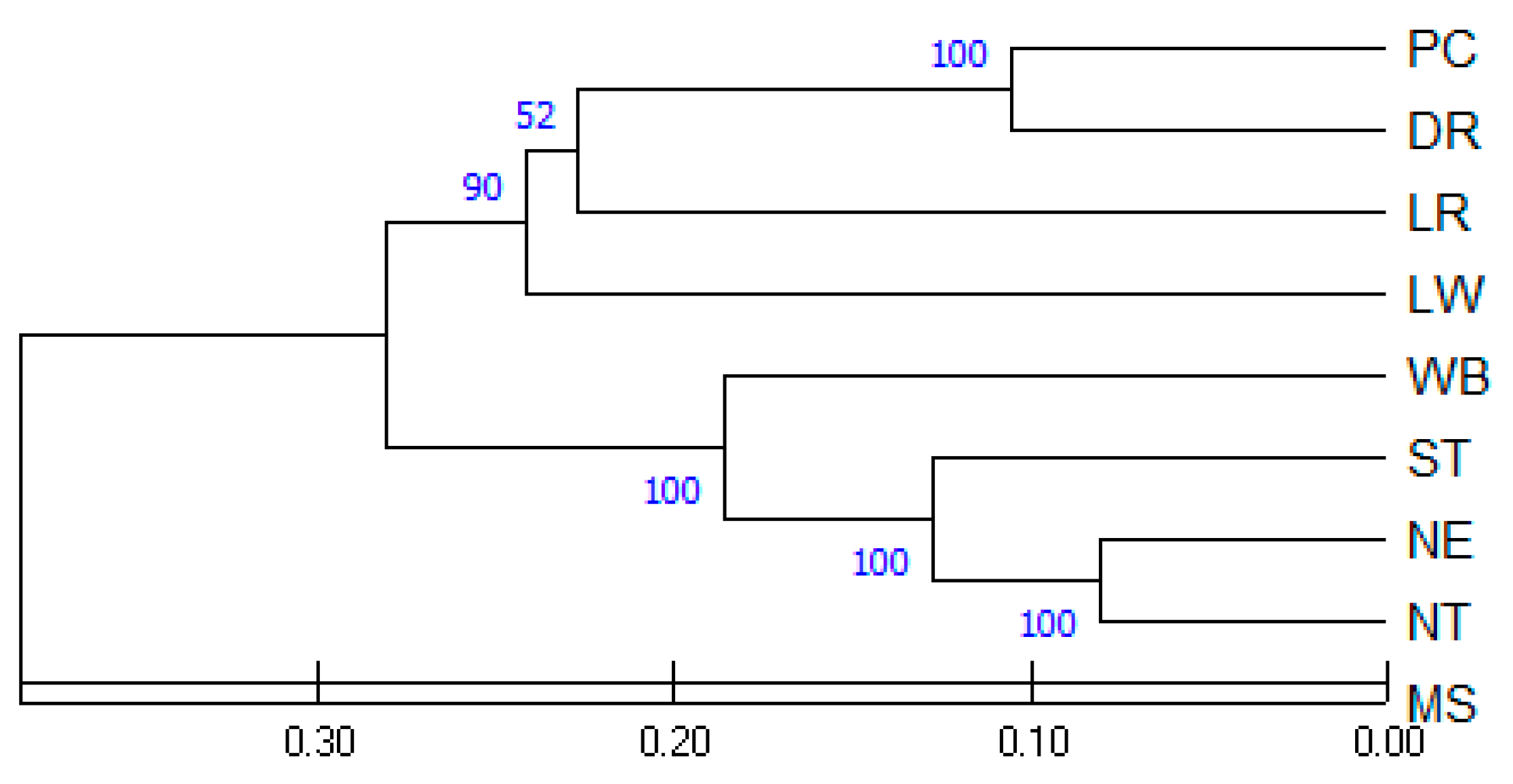
3.4. Genetic Relationship of Pig Populations
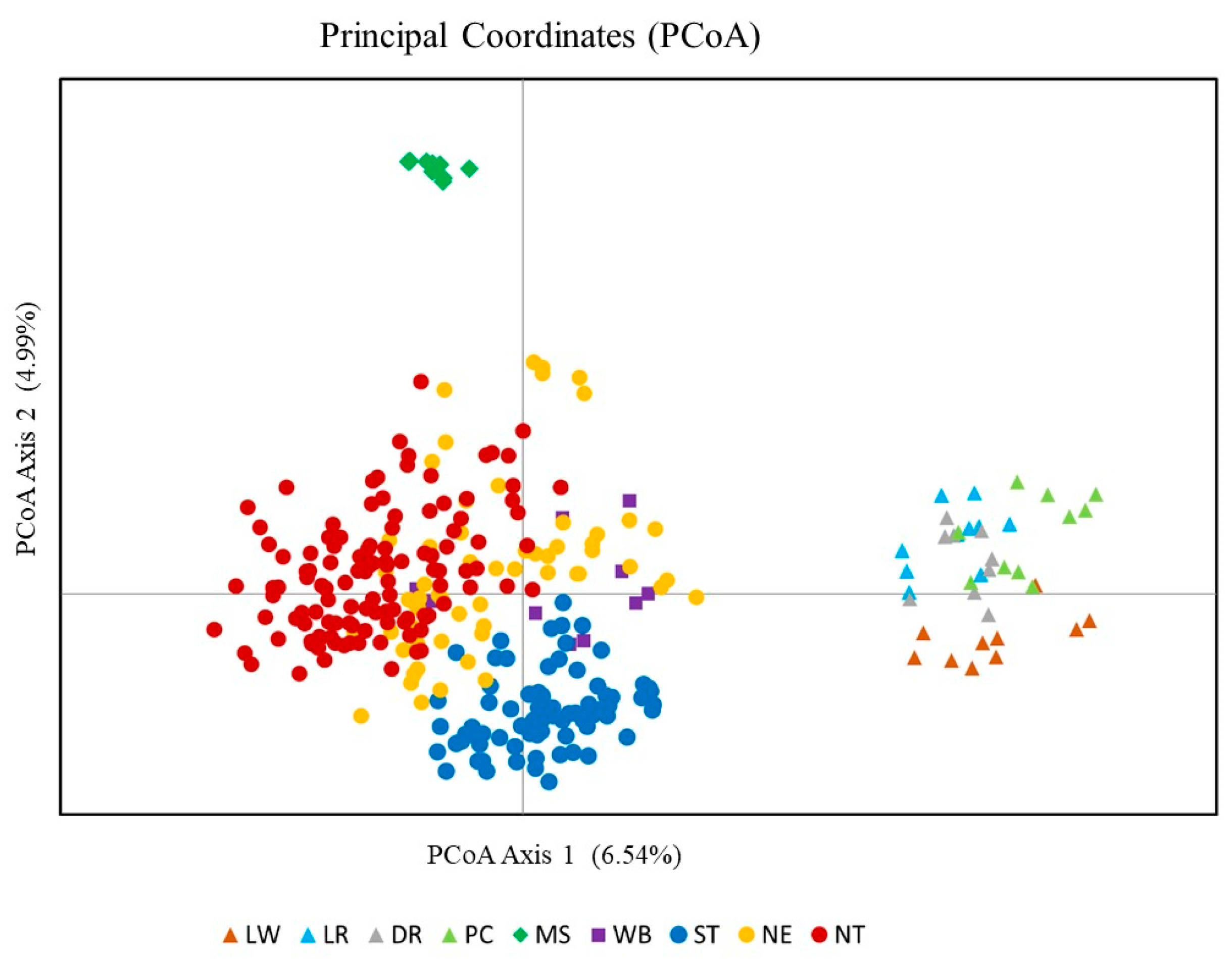
3.5. Principal Coordinate Analysis (PCoA)
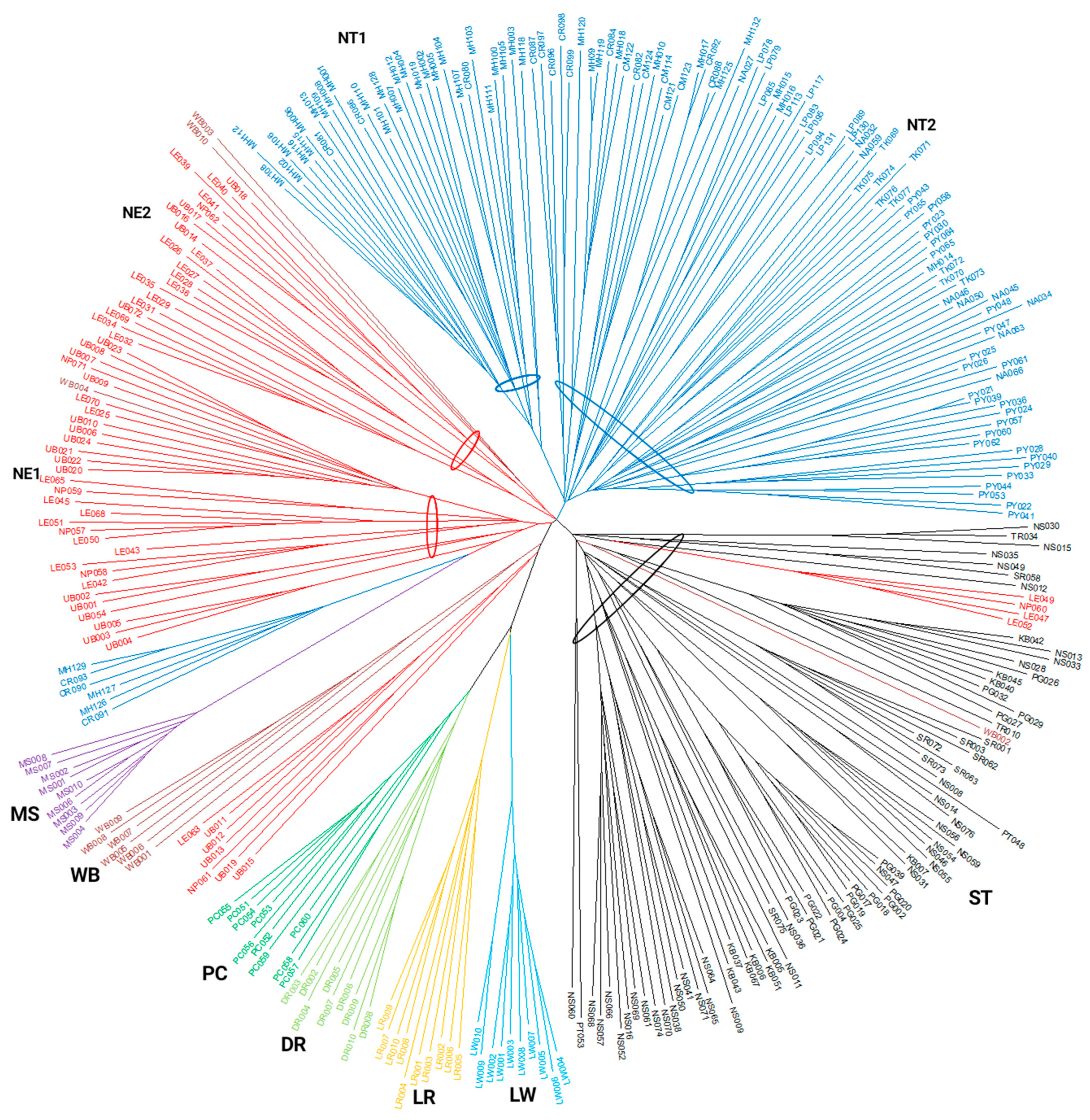
3.6. Phylogenetic Tree of Individual Pigs
4. Discussion
4.1. Characteristics of the Microsatellite Loci
4.2. Genetic Relatedness among TIPs from the Three Geographic Regions
4.3. Genetic Relationship of TIPs with Thai Wild Boars
4.4. Genetic Relationship of TIPs with Exotic Pigs
4.5. Implications and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higham, C. The Archaeology of Mmainland Southeast Asia: From 10,000 BC to the Fall of Angkor; Cambridge University Press: Cambridge, MA, USA, 1989; p. 404. [Google Scholar]
- Nakai, S. Research Report: Analysis of Pig Consumption by Smallholders in a Hillside Swidden Agriculture Society of Northern Thailand. Hum. Ecol. 2009, 37, 501–511. [Google Scholar] [CrossRef]
- Wannajuk, M.; Sangthong, P.; Natapintu, S.; Wonnapinij, P.; Vuttipongchaikij, S.; Kubera, A.; Won-In, K.; Mingmuang, M.; Surat, W. Ancient DNA of pigs in Thailand: Evidence of multiple origins of Thai pigs in the late Neolithic period. Sci. Asia 2013, 39, 456–465. [Google Scholar] [CrossRef]
- Rattanaronchart, S. Present Situation of Thai Native Pigs; Department of Animal Science, Faculty of Agriculture, Chiangmai University: Chiangmai, Thailand, 1994; p. 46. [Google Scholar]
- Falvey, L. Research on Native Pigs in Thailand. Wld Anim. 1981, 38, 16–22. [Google Scholar]
- Nakai, S. Decision-making on the use of diverse combinations of agricultural products and natural plants in pig feed: A case study of native pig smallholder in northern Thailand. Trop. Anim. Health Prod. 2008, 40, 201–208. [Google Scholar] [CrossRef]
- Vasupen, K.; Sarnklong, C.; Yuangklang, C.; Wongsuttravas, S.; Srinanuan, P.; Kesorn, P. Effect of dietary protein levels on growth performance of native swine in Sakon Nakhon province. In Proceedings of the Agricultural Seminar on Animal Science, Khon Kaen University, Khon Kaen, Thailand, 27–28 January 2004; pp. 599–605. [Google Scholar]
- Department of Livestock Development (DLD). Information of Pig Farmers, Fiscal Year 2021. Available online: https://ict.dld.go.th/webnew/images/stories/stat_web/yearly/2564/country/5-pig.pdf (accessed on 5 May 2022). (In Thai)
- Bunleangthong, S.; Na-Ayuthaya, J.N.; Sanbua, S.; Srisuriya, V.; Pichaironnarongsongkram, K. Economic Performance of Three-Bred Cross Swine with the Different Levels of Meishan Breed; Animal Breeding Division, Department of Livestock Development: Bangkok, Thailand, 1988; pp. 15–21. (In Thai)
- Kemp, S.J.; Hishida, O.; Wambugu, J.; Rink, A.; Longeri, M.L.; Ma, R.Z.; Da, Y.; Lewin, H.A.; Barendse, W.; Teale, A.J. A panel of polymorphic bovine, ovine and caprine microsatellite markers. Anim. Genet. 1995, 26, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lawson Handley, L.J.; Byrne, K.; Santucci, F.; Townsend, S.; Taylor, M.; Bruford, M.W.; Hewitt, G.M. Genetic structure of European sheep breeds. Heredity 2007, 99, 620–631. [Google Scholar] [CrossRef] [PubMed]
- MacHugh, D.E.; Loftus, R.T.; Cunningham, P.; Bradley, D.G. Genetic structure of seven European cattle breeds assessed using 20 microsatellite markers. Anim. Genet. 1998, 29, 333–340. [Google Scholar] [CrossRef]
- Nidup, K.; Moran, C. Genetic diversity of domestic pigs as revealed by microsatellites: A mini review. Genom. Quant. Genet. 2011, 2, 5–18. [Google Scholar]
- Wang, X.; Cao, H.H.; Geng, S.M.; Li, H.B. Genetic Diversity of 10 Indigenous Pig Breeds in China by Using Microsatellite Markers. Asian-Australas J. Anim. Sci. 2004, 17, 1219–1222. [Google Scholar] [CrossRef]
- Megens, H.-J.; Crooijmans, R.P.M.A.; Cristobal, M.S.; Hui, X.; Li, N.; Groenen, M.A.M. Biodiversity of pig breeds from China and Europe estimated from pooled DNA samples: Differences in microsatellite variation between two areas of domestication. Genet. Sel. Evol. 2008, 40, 103. [Google Scholar] [CrossRef]
- Wu, G.; Shen, W.; Xue, X.; Wang, L.; Ma, Y.; Zhou, J. A novel (ATC)(n) microsatellite locus is associated with litter size in an indigenous Chinese pig. Vet. Med. Sci. 2021, 7, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, R.; Li, H.; Yu, H.; Yang, Y. Genetic diversity analysis in Chinese miniature pigs using swine leukocyte antigen complex microsatellites. Anim. Biosci. 2021, 34, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Chu, H.P.; Jiang, Y.N.; Li, S.H.; Wang, Y.; Chen, C.H.; Chen, K.J.; Lin, C.Y.; Ju, Y.T. Genetic variation and phylogenetics of Lanyu and exotic pig breeds in Taiwan analyzed by nineteen microsatellite markers. J. Anim. Sci. 2009, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, K.S.; Choi, B.H.; Yoon, D.H.; Jang, G.W.; Lee, K.T.; Chung, H.Y.; Lee, H.Y.; Park, H.S.; Lee, J.W. Genetic structure of pig breeds from Korea and China using microsatellite loci analysis1. J. Anim. Sci. 2005, 83, 2255–2263. [Google Scholar] [CrossRef]
- Kaul, R.; Singh, A.; Vijh, R.K.; Tantia, M.S.; Behl, R. Evaluation of the genetic variability of 13 microsatellite markers in native Indian pigs. J. Genet. 2001, 80, 149–153. [Google Scholar] [CrossRef]
- Oh, J.-D.; Grace, R.; Cacho, C.; Choi, J.; Seo, J.; Song, K.-D.; Vega, R.; Santiago, R.; Octura, J.E.; Octura, R.; et al. Genetic Analysis of Philippine Native Pigs (Sus scrofa L.) Using Microsatellite Loci. Philipp. J. Sci. 2014, 143, 87–93. [Google Scholar]
- Thuy, N.T.; Melchinger-Wild, E.; Kuss, A.W.; Cuong, N.V.; Bartenschlager, H.; Geldermann, H. Comparison of Vietnamese and European pig breeds using microsatellites. J. Anim. Sci. 2006, 84, 2601–2608. [Google Scholar] [CrossRef]
- Chaiwatanasin, W.; Chantsawang, S.; Tabchareon, S. Genetic diversity of four pig breeds in Thailand based on microsatellite analysis. Kasetsart J. Nat. Sci. 2002, 36, 248–252. [Google Scholar]
- Yang, S.L.; Surintorn, B.; Pongchan, N.L.; Uthairat, N.N.; Shi, Z.H. Genetic Variation and Population Structure of Thai Indigenous Pig Populations Based on Mitochondrial and Microsatellite DNA Markers. J. Anim. Vet. Adv. 2012, 11, 509–516. [Google Scholar] [CrossRef]
- Charoensook, R.; Gatphayak, K.; Brenig, B.; Knorr, C. Genetic diversity analysis of Thai indigenous pig population using microsatellite markers. Asian-Australas J. Anim. Sci. 2019, 32, 1491–1500. [Google Scholar] [CrossRef]
- Gatphayak, K.; Chaisongkram, C.; Taitamthong, B.; Knorr, C. Diversity of northern thai native pigs determined by microsatellite analysis. Maejo Int. J. Sci. Technol. 2020, 14, 209–220. [Google Scholar]
- ISAG Conference 2014. Pig Genetics and Genomics. Available online: https://www.isag.us/docs/Pig%20Genetics%20Genomics%20and%20CT_2014.pdf (accessed on 5 January 2023).
- FAO. Molecular Genetic Characterization of Animal Genetic Resources. Available online: http://www.fao.org/3/i2413e/i2413e00.htm (accessed on 15 June 2016).
- Chaweewan, K.; Nakavisut, S.; Jumparat, V.; Srisuriya, V. Genetic Parameters of Selection for Economic Traits Over Five Generationsof Pakchong 5 Swine. In Proceedings of the 15th AAAP Animal Science Congress, Thammasat University, Rangsit, Bangkok, 26–30 November 2012; pp. 1347–1351. [Google Scholar]
- Myakishev, M.V.; Kapanadze, G.I.; Shaikhayev, G.O.; Georgiev, G.P.; Beritashvili, D.R. Extraction of DNA from the Whole Blood by Silica Gel; Institute of Gene Biology: Moscow, Russia, 1995. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar]
- Goudet, J. Fstat (ver. 2.9.4), a Program to Estimate and Test Population Genetics Parameters. 2002. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 1 June 2021).
- Raymond, M.; Rousset, F. GENEPOP (Version 1.2): Population Genetics Software for Exact Tests and Ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Takezaki, N.; Nei, M.; Tamura, K. POPTREEW: Web version of POPTREE for constructing population trees from allele frequency data and computing some other quantities. Mol. Biol. Evol. 2014, 31, 1622–1624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.L.; Burg, T.M.; Steeves, T.E. Sampling for Microsatellite-Based Population Genetic Studies: 25 to 30 Individuals per Population Is Enough to Accurately Estimate Allele Frequencies. PLoS ONE 2012, 7, e45170. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T. How many alleles per locus should be used to estimate genetic distances? Heredity 2002, 88, 62–65. [Google Scholar] [CrossRef]
- Zhao, J.; Li, T.; Zhu, C.; Jiang, X.; Zhao, Y.; Xu, Z.; Yang, S.; Chen, A. Selection and use of microsatellite markers for individual identification and meat traceability of six swine breeds in the Chinese market. Food Sci. Technol. Int. 2018, 24, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Maudet, C.; Luikart, G.; Taberlet, P. Genetic diversity and assignment tests among seven French cattle breeds based on microsatellite DNA analysis. J. Anim. Sci. 2002, 80, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Cooperatives (MOAC). Animal Epidemics act, B.E. 2558. Available online: https://www.moac.go.th/law_agri-files-422791791810 (accessed on 5 May 2022). (In Thai)
- Na-Lampang, P. Improvement of Carcass Characteristics of Thai Pigs by Crossbreeding with Wild Boars; Suranaree University of Technology: Nakhon Ratchasima, Thailand, 2013; p. 17. [Google Scholar]
- Gama, L.T.; Martínez, A.M.; Carolino, I.; Landi, V.; Delgado, J.V.; Vicente, A.A.; Vega-Pla, J.L.; Cortés, O.; Sousa, C.O.; Consortium, B. Genetic structure, relationships and admixture with wild relatives in native pig breeds from Iberia and its islands. Genet. Sel. Evol. 2013, 45, 18. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wang, Z.G.; Li, Y.J.; Zhao, X.L.; Liu, B.; Zhao, S.H.; Yu, M.; Li, M.H.; Chen, S.L.; Xiong, T.A.; et al. Genetic variation analysis within and among Chinese indigenous swine populations using microsatellite markers. Anim. Genet. 2002, 33, 422–427. [Google Scholar] [CrossRef]
- Kramarenko, S.; Lugovoy, S.; Kharzinova, V.R.; Lykhach, V.; Kramarenko, A.; Lykhach, A. Genetic diversity of Ukrainian local pig breeds based on microsatellite markers. Regul. Mech. Biosyst. 2018, 9, 177–182. [Google Scholar] [CrossRef]
- Laval, G.; Iannuccelli, N.; Legault, C.; Milan, D.; Groenen, M.A.; Giuffra, E.; Andersson, L.; Nissen, P.H.; Jørgensen, C.B.; Beeckmann, P.; et al. Genetic diversity of eleven European pig breeds. Genet. Sel. Evol. 2000, 32, 187–203. [Google Scholar] [CrossRef]
- Michailidou, S.; Kalivas, A.; Ganopoulos, I.; Stea, E.; Michailidis, G.; Tsaftaris, A.; Argiriou, A. A multi-farm assessment of Greek black pig genetic diversity using microsatellite molecular markers. Genet. Mol. Res. 2014, 13, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.A.; Carolino, M.I.; Sousa, M.C.; Ginja, C.; Silva, F.S.; Martinez, A.M.; Vega-Pla, J.L.; Carolino, N.; Gama, L.T. Genetic diversity in native and commercial breeds of pigs in Portugal assessed by microsatellites. J. Anim. Sci. 2008, 86, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
| Locus | SSC | Na | Ne | PIC | He | Ho | Fis | Fit | Fst | HWE |
|---|---|---|---|---|---|---|---|---|---|---|
| S0155 | 1q | 10.333 | 4.552 | 0.857 | 0.776 | 0.659 | 0.151 | 0.169 | 0.021 | *** |
| SW1828 | 1 | 10.333 | 4.826 | 0.867 | 0.768 | 0.599 | 0.219 | 0.280 | 0.077 | *** |
| S0226 | 2q | 12.000 | 4.129 | 0.847 | 0.757 | 0.570 | 0.247 | 0.273 | 0.035 | *** |
| SW240 | 2p | 13.000 | 5.010 | 0.868 | 0.770 | 0.618 | 0.197 | 0.239 | 0.053 | *** |
| S0002 | 3q | 19.333 | 11.305 | 0.958 | 0.905 | 0.740 | 0.182 | 0.208 | 0.033 | *** |
| SW72 | 3p | 16.000 | 7.409 | 0.923 | 0.853 | 0.796 | 0.067 | 0.114 | 0.050 | *** |
| S0097 | 4 | 13.000 | 4.661 | 0.854 | 0.765 | 0.598 | 0.218 | 0.241 | 0.029 | *** |
| SW445 | 4 | 15.333 | 7.152 | 0.931 | 0.837 | 0.765 | 0.086 | 0.150 | 0.070 | *** |
| IGF1 | 5 | 10.333 | 4.200 | 0.874 | 0.759 | 0.529 | 0.303 | 0.363 | 0.086 | *** |
| S0005 | 5 | 15.333 | 7.945 | 0.920 | 0.872 | 0.701 | 0.196 | 0.214 | 0.022 | *** |
| S0228 | 6 | 11.667 | 6.639 | 0.919 | 0.845 | 0.526 | 0.378 | 0.408 | 0.049 | *** |
| SW122 | 6 | 10.667 | 3.730 | 0.809 | 0.706 | 0.527 | 0.254 | 0.281 | 0.036 | *** |
| SW2406 | 6 | 11.333 | 5.291 | 0.895 | 0.789 | 0.611 | 0.226 | 0.278 | 0.068 | *** |
| S0101 | 7 | 8.667 | 3.099 | 0.775 | 0.667 | 0.465 | 0.303 | 0.327 | 0.035 | *** |
| SW632 | 7 | 9.667 | 3.319 | 0.798 | 0.698 | 0.528 | 0.244 | 0.259 | 0.019 | *** |
| S0178 | 8 | 14.000 | 6.717 | 0.917 | 0.845 | 0.614 | 0.273 | 0.307 | 0.046 | *** |
| SW2410 | 8 | 13.667 | 8.208 | 0.935 | 0.878 | 0.666 | 0.242 | 0.266 | 0.032 | *** |
| SW911 | 9 | 9.667 | 6.132 | 0.913 | 0.834 | 0.723 | 0.133 | 0.174 | 0.048 | *** |
| SW830 | 10 | 12.333 | 5.541 | 0.895 | 0.809 | 0.539 | 0.334 | 0.365 | 0.047 | *** |
| SW951 | 10 | 8.000 | 2.916 | 0.752 | 0.649 | 0.465 | 0.284 | 0.301 | 0.023 | *** |
| SW2008 | 11 | 7.667 | 3.888 | 0.826 | 0.731 | 0.469 | 0.358 | 0.397 | 0.061 | *** |
| S0090 | 12 | 6.667 | 4.477 | 0.847 | 0.776 | 0.466 | 0.399 | 0.407 | 0.013 | *** |
| S0143 | 12 | 10.667 | 4.605 | 0.836 | 0.752 | 0.629 | 0.163 | 0.205 | 0.051 | *** |
| S0068 | 13 | 15.667 | 7.455 | 0.932 | 0.862 | 0.724 | 0.160 | 0.198 | 0.046 | *** |
| SW857 | 14 | 9.667 | 5.597 | 0.888 | 0.817 | 0.639 | 0.217 | 0.235 | 0.023 | *** |
| SW936 | 15 | 13.333 | 7.563 | 0.924 | 0.867 | 0.614 | 0.292 | 0.312 | 0.028 | *** |
| S0026 | 16 | 9.667 | 3.768 | 0.873 | 0.685 | 0.376 | 0.451 | 0.519 | 0.123 | *** |
| SW24 | 17 | 13.667 | 5.117 | 0.890 | 0.799 | 0.680 | 0.149 | 0.192 | 0.051 | *** |
| S0218 | X | 12.000 | 4.168 | 0.835 | 0.743 | 0.416 | 0.440 | 0.467 | 0.049 | *** |
| Mean | 11.851 | 5.497 | 0.874 | 0.787 | 0.595 | 0.247 | 0.281 | 0.046 | *** |
| Population 1 | Na | Ne | He | Ho | Fis | HWE |
|---|---|---|---|---|---|---|
| NT | 12.655 | 5.399 | 0.778 | 0.574 | 0.260 | *** |
| NE | 11.759 | 6.014 | 0.801 | 0.628 | 0.221 | *** |
| ST | 11.241 | 5.095 | 0.781 | 0.586 | 0.254 | *** |
| Mean | 11.885 | 5.503 | 0.787 | 0.596 | 0.245 | *** |
| Locus | NT | NE | ST |
|---|---|---|---|
| S0155 | - | - | 153 (0.060) |
| SW1828 | - | - | - |
| S0226 | - | - | 209 (0.053) |
| SW240 | - | - | 130 (0.107) |
| S0002 | - | - | - |
| SW72 | - | - | 98 (0.207) |
| SW445 | - | - | - |
| S0097 | 223 (0.052) | 210 (0.057) | - |
| IGF1 | - | - | - |
| S0005 | - | 203 (0.092), 222 (0.057) | 229 (0.053), 239 (0.080) |
| S0228 | - | 236 (0.189) | 248 (0.073) |
| SW2406 | - | - | 242 (0.080) |
| SW122 | - | - | - |
| SW632 | - | - | - |
| S0101 | 224 (0.085) | - | - |
| S0178 | - | 115 (0.139) | 121 (0.333) |
| SW2410 | - | - | 113 (0.053) |
| SW911 | - | - | - |
| SW951 | - | - | - |
| SW830 | 200 (0.097) | - | 185 (0.053), 189 (0.067) |
| SW2008 | - | - | 110 (0.060) |
| S0090 | - | - | - |
| S0143 | - | - | 167 (0.107) |
| S0068 | - | - | - |
| SW857 | - | - | 133 (0.053) |
| SW936 | - | - | 123 (0.080) |
| S0026 | 97 (0.161), 107 (0.081) | - | - |
| SW24 | - | - | - |
| S0218 | - | - | 178 (0.060) |
| Total | 5 | 5 | 17 |
| Population 1 | Cluster | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| NT | 0.012 | 0.655 | 0.333 |
| NE | 0.053 | 0.737 | 0.210 |
| ST | 0.957 | 0.037 | 0.007 |
| Population 1 | NT | NE | ST | LW | LR | DR | PC | MS | WB |
|---|---|---|---|---|---|---|---|---|---|
| NT | - | ||||||||
| NE | 0.162 | - | |||||||
| ST | 0.261 | 0.250 | - | ||||||
| LW | 0.587 | 0.593 | 0.516 | - | |||||
| LR | 0.595 | 0.568 | 0.566 | 0.482 | - | ||||
| DR | 0.540 | 0.477 | 0.522 | 0.504 | 0.487 | - | |||
| PC | 0.548 | 0.474 | 0.506 | 0.461 | 0.421 | 0.211 | - | ||
| MS | 0.575 | 0.625 | 0.769 | 0.909 | 0.835 | 0.860 | 0.825 | - | |
| WB | 0.383 | 0.319 | 0.413 | 0.652 | 0.639 | 0.589 | 0.611 | 0.726 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaweewan, K.; Mahinchai, P.; Kongsook, S.; Soponchit, S.; Weerasamith, P.; Awiruttapanich, W.; Prapawat, P.; Jamparat, W.; Chanthaworn, T.; Rattanamahavichai, N.; et al. Genetic Divergence of Thai Indigenous Pigs from Three Distinct Geographic Regions Revealed by Microsatellite Marker Analysis. Animals 2023, 13, 625. https://doi.org/10.3390/ani13040625
Chaweewan K, Mahinchai P, Kongsook S, Soponchit S, Weerasamith P, Awiruttapanich W, Prapawat P, Jamparat W, Chanthaworn T, Rattanamahavichai N, et al. Genetic Divergence of Thai Indigenous Pigs from Three Distinct Geographic Regions Revealed by Microsatellite Marker Analysis. Animals. 2023; 13(4):625. https://doi.org/10.3390/ani13040625
Chicago/Turabian StyleChaweewan, Kamon, Prapas Mahinchai, Sornchai Kongsook, Surasak Soponchit, Phuree Weerasamith, Wiranphat Awiruttapanich, Pakhawan Prapawat, Warocha Jamparat, Thitawat Chanthaworn, Natinee Rattanamahavichai, and et al. 2023. "Genetic Divergence of Thai Indigenous Pigs from Three Distinct Geographic Regions Revealed by Microsatellite Marker Analysis" Animals 13, no. 4: 625. https://doi.org/10.3390/ani13040625
APA StyleChaweewan, K., Mahinchai, P., Kongsook, S., Soponchit, S., Weerasamith, P., Awiruttapanich, W., Prapawat, P., Jamparat, W., Chanthaworn, T., Rattanamahavichai, N., Weangchanok, S., Arikit, S., Duangjinda, M., Tuntivisoottikul, K., Chaosap, C., & Jirajaroenrat, K. (2023). Genetic Divergence of Thai Indigenous Pigs from Three Distinct Geographic Regions Revealed by Microsatellite Marker Analysis. Animals, 13(4), 625. https://doi.org/10.3390/ani13040625





