Presence, Tissue Localization, and Gene Expression of the Adiponectin Receptor 1 in Testis and Accessory Glands of Male Rams during the Non-Breeding Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Ram Reproductive Tissues
2.2. Immunohistochemistry
2.3. RNA Extraction and RT-qPCR
2.4. Statistical Analysis
3. Results
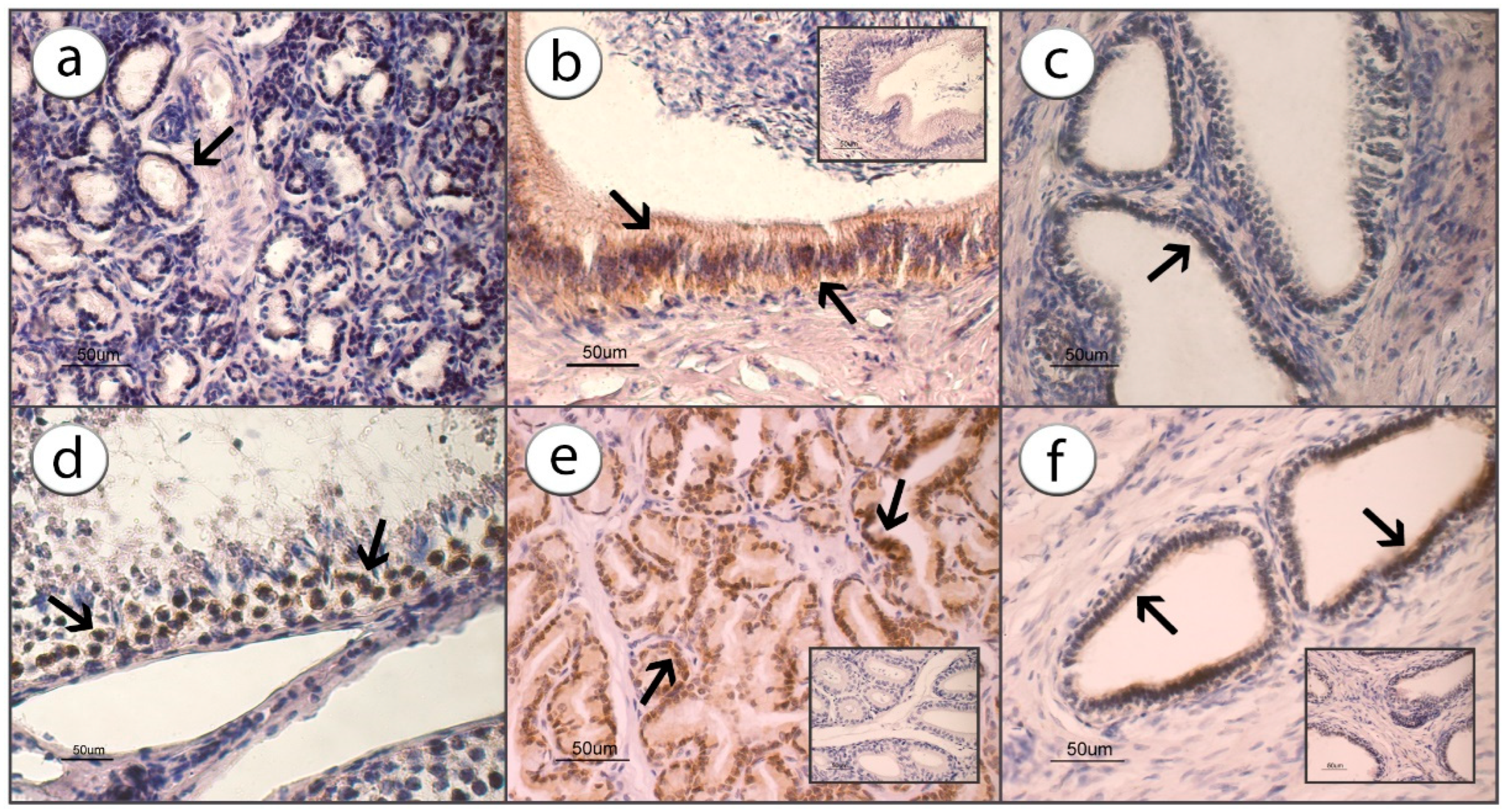
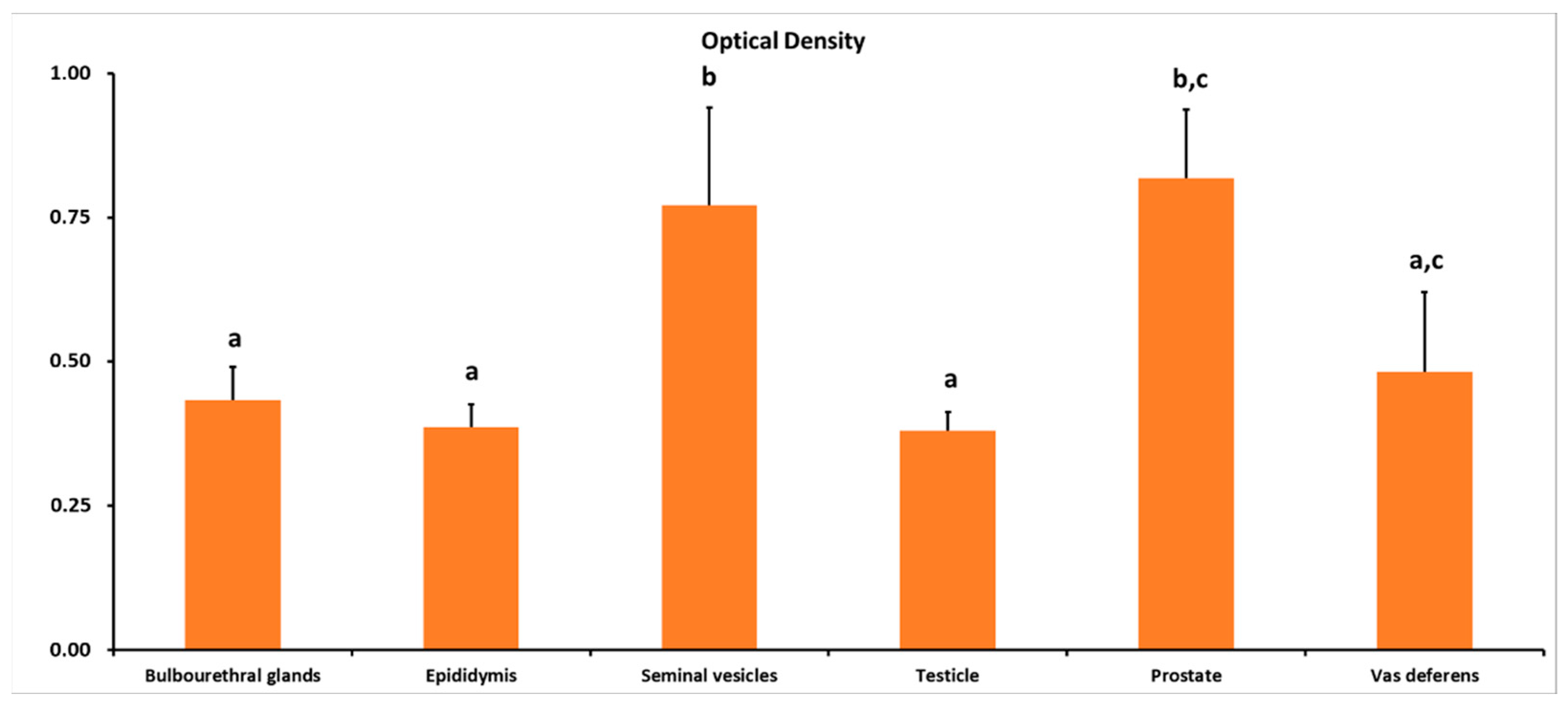
3.1. ADIPOR1 Immunolocalization
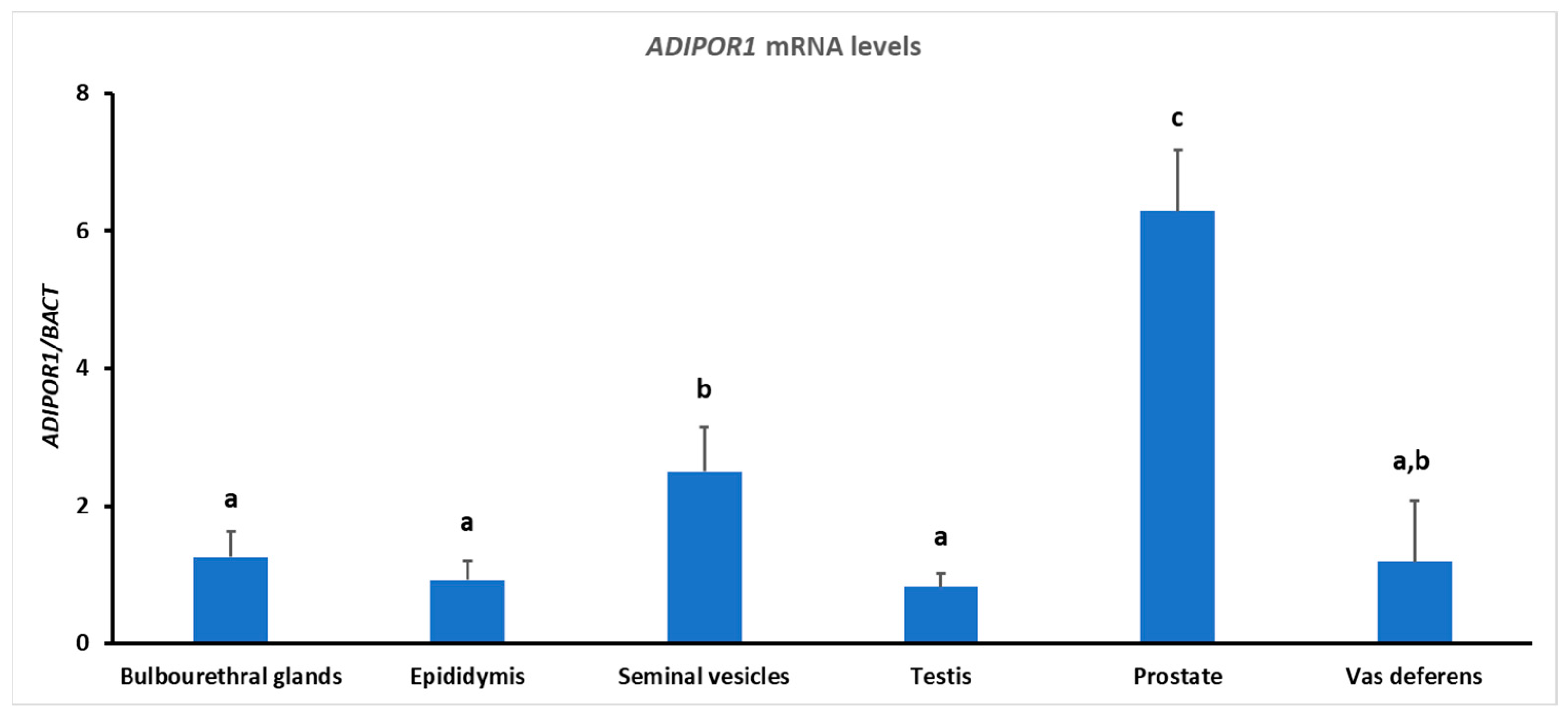
3.2. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef]
- Zhang, D.; Bai, J.; Ma, Z.; Ma, X.; Cao, X.; Li, F. Regulatory roles of adiponectin receptor 1 and 2 in sheep preadipocytes during adipocyte differentiation. Ital. J. Anim. Sci. 2019, 18, 704–712. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Al-Jumaili, W.S.; Kadhim, A.H.; AL-Thuwaini, T.M. Polymorphism of the ADIPOQ gene and its association with productive traits in Awassi Ewes. Mol. Biol. Rep. 2023, 50, 913–917. [Google Scholar] [CrossRef]
- Shapiro, L.; Scherer, P.E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 1998, 8, 335–338. [Google Scholar] [CrossRef]
- Fruebis, J.; Tsao, T.S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef]
- Budak, E.; Fernández Sánchez, M.; Bellver, J.; Cerveró, A.; Simón, C.; Pellicer, A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3-36 with the reproductive system. Fertil. Steril. 2006, 85, 1563–1581. [Google Scholar] [CrossRef]
- Martin, L.J. Implications of adiponectin in linking metabolism to testicular function. Endocrine 2014, 46, 16–28. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Ramachandran, R.; Ocon-Grove, O.M.; Metzger, S.L. Molecular cloning and tissue expression of chicken AdipoR1 and AdipoR2 complementary deoxyribonucleic acids. Domest. Anim. Endocrinol. 2007, 33, 19–31. [Google Scholar] [CrossRef]
- Lord, E.; Ledoux, S.; Murphy, B.D.; Beaudry, D.; Palin, M.F. Expression of adiponectin and its receptors in swine. J. Anim. Sci. 2005, 83, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.H.; Xia, T.; Zhang, G.D.; Chen, X.D.; Gan, L.; Feng, S.Q. Cloning, expression and chromosome localization of porcine adiponectin and adiponectin receptors genes. Domest. Anim. Endocrinol. 2006, 30, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh, M.R.; Hosseini, A.; Saeb, M.; Kafi, M.; Saeb, S. Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology 2010, 73, 659–669. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Li, J.; Zhang, N.; Li, Y.; Li, Y.; Li, H.; Yan, F.; Kang, X.; Liu, X.; et al. Expression and localization of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamic-pituitary-ovarian axis of laying hens. Theriogenology 2021, 159, 35–44. [Google Scholar] [CrossRef]
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Psilopanagioti, A.; Papadaki, H.; Kranioti, E.F.; Alexandrides, T.K.; Varakis, J.N. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology 2008, 89, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ocón-Grove, O.M.; Krzysik-Walker, S.M.; Maddineni, S.R.; Hendricks, G.L.; Ramachandran, R. Adiponectin and its receptors are expressed in the chicken testis: Influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance. Reproduction 2008, 136, 627–638. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Wang, F.; Yuan, S. Roles of AMP-Activated Protein Kinase (AMPK) in mammalian reproduction. Front. Cell Dev. Biol. 2020, 8, 1338. [Google Scholar] [CrossRef]
- Rahmanifar, F.; Tabandeh, M.R. Adiponectin and its receptors gene expression in the reproductive tract of ram. Small Rumin. Res. 2012, 105, 263–267. [Google Scholar]
- Kadivar, A.; Heidari Khoei, H.; Hassanpour, H.; Golestanfar, A.; Ghanaei, H. Correlation of Adiponectin mRNA abundance and its receptors with quantitative parameters of sperm motility in rams. Int. J. Fertil. Steril. 2016, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Dall’Aglio, C.; Maranesi, M.; Di Loria, A.; Piantedosi, D.; Ciaramella, P.; Alterisio, M.C.; Lepri, E.; Mercati, F. Effects of obesity on adiponectin system skin expression in dog: A comparative study. Animals 2021, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Boiti, C.; Guelfi, G.; Zerani, M.; Zampini, D.; Brecchia, G.; Gobbetti, A. Expression patterns of cytokines, p53 and nitric oxide synthase isoenzymes in corpora lutea of pseudopregnant rabbits during spontaneous luteolysis. Reproduction 2004, 127, 229–238. [Google Scholar] [CrossRef]
- Guelfi, G.; Zerani, M.; Brecchia, G.; Parillo, F.; Dall’Aglio, C.; Maranesi, M.; Boiti, C. Direct actions of ACTH on ovarian function of pseudopregnant rabbits. Mol. Cell Endocrinol. 2011, 339, 63–71. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Zerani, M.; Catone, G.; Maranesi, M.; Gobbetti, A.; Boiti, C.; Parillo, F. Gonadotropin-releasing hormone 1 directly affects corpora lutea lifespan in Mediterranean buffalo (Bubalus bubalis) during diestrus: Presence and in vitro effects on enzymatic and hormonal activities. Biol. Reprod. 2012, 87, 45. [Google Scholar] [CrossRef]
- Mercati, F.; Maranesi, M.; Dall’Aglio, C.; Petrucci, L.; Pasquariello, R.; Tardella, F.M.; De Felice, E.; Scocco, P. Apelin System in Mammary Gland of Sheep Reared in Semi-Natural Pastures of the Central Apennines. Animals 2018, 8, 223. [Google Scholar] [CrossRef]
- Dall’Aglio, C.; Polisca, A.; Boiti, C.; Ceccarelli, P. Immunolocalization of leptin and its receptor in the placenta of cats. Acta Histochem. 2012, 114, 719–722. [Google Scholar] [CrossRef]
- Caminos, J.E.; Nogueiras, R.; Gaytán, F.; Pineda, R.; González, C.R.; Barreiro, M.L.; Castaño, J.P.; Malagón, M.M.; Pinilla, L.; Toppari, J.; et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology 2008, 149, 3390–3402. [Google Scholar] [CrossRef]
- Ramachandran, R.; Maddineni, S.; Ocón-Grove, O.; Hendricks, G., 3rd; Vasilatos-Younken, R.; Hadley, J.A. Expression of adiponectin and its receptors in avian species. Gen. Comp. Endocrinol. 2013, 190, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, B.; Fan, W.; Zhu, X.; Wang, G.; Zhang, A. Adiponectin protects Adiponectin protects Leydig cells against proinflammatory cytokines by suppressing the nuclear factor-κB signaling pathway. FEBS J. 2013, 280, 3920–3927. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, Y.; Bastard, J.P.; McAvoy, C.; Fellahi, S.; Dupont, J.; Levy, R. Adipokines in semen: Physiopathology and effects on spermatozoas. Int. J. Endocrinol. 2018, 2018, 3906490. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Krishna, A. Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitam. Horm. 2021, 115, 611–634. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; Krishna, A. Direct actions of adiponectin on changes in reproductive, metabolic, and anti-oxidative enzymes status in the testis of adult mice. Gen. Comp. Endocrinol. 2019, 279, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; Martin, L.J.; Krishna, A. Role of adiponectin as a modulator of testicular function during aging in mice. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 413–427. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Krishna, A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie 2020, 168, 41–52. [Google Scholar] [CrossRef]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Maekawa, M.; Kamimura, K.; Nagano, T. Peritubular myoid cells in the testis: Their structure and function. Arch. Histol. Cytol. 1996, 59, 1–13. [Google Scholar] [CrossRef]
- Galdieri, M.; Ricci, G. Characterization of different cell populations isolated from rat testis peritubular cells. Differentiation 1998, 63, 13–19. [Google Scholar] [CrossRef]
- Tung, P.S.; Fritz, I.B. Interactions of Sertoli cells with myoid cells in vitro. Biol. Reprod. 1980, 23, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.C. Testicular macrophages. Int. Rev. Cytol. 1994, 149, 99–143. [Google Scholar] [PubMed]
- Cheng, X.B.; Wen, J.P.; Yang, J.; Yang, Y.; Ning, G.; Li, X.Y. GnRH secretion is inhibited by adiponectin through activation of amp-activated protein kinase and extracellular signal-regulated kinase. Endocrine 2011, 39, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Brown, R.; Imran, S.A.; Ur, E. Adipokine gene expression in brain and pituitary gland. Neuroendocrinology 2007, 86, 191–209. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Klerman, E.B. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog. Horm. Res. 1999, 54, 97–102. [Google Scholar]
- Moustafa, A. Effect of light-dark cycle misalignment on the hypothalamic-pituitary-gonadal axis, testicular oxidative stress, and expression of clock genes in adult male rats. J. Endocrinol. 2020, 2020, 1426846. [Google Scholar] [CrossRef]
- Taibi, N.; Dupont, J.; Bouguermouh, Z.; Froment, P.; Ramé, C.; Anane, A.; Amirat, Z.; Khammar, F. Expression of adenosine 5′-monophosphate—Activated protein kinase (AMPK) in ovine testis (Ovis aries): In vivo regulation by nutritional state. Anim. Reprod. Sci. 2017, 178, 9–22. [Google Scholar] [CrossRef]
- Wen, J.P.; Lv, W.S.; Yang, J.; Nie, A.F.; Cheng, X.B.; Yang, Y.; Ge, Y.; Li, X.Y.; Ning, G. Globular adiponectin inhibits GnRH secretion from GT1–7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem. Biophys. Res. Commun. 2008, 371, 756–761. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Biswas, A. Adiponectin in male reproduction and infertility. Asian Pac. J. Reprod. 2019, 8, 244. [Google Scholar] [CrossRef]
- Ledoux, S.; Campos, D.B.; Lopes, F.L.; Dobias-Goff, M.; Palin, M.F.; Murphy, B.D. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 2006, 147, 5178–5186. [Google Scholar] [CrossRef]
| Gene | NCBI Seq. Ref. | Primers | Bp | |
|---|---|---|---|---|
| ADIPOR1 | NM_001306110.1 | F | GGTGGTGTTCGGGATGTTCT | 128 |
| R | CGATCCCCGAATAGTCCAGC | |||
| ACTB | U39357.2 | F | CCTTAGCAACCATGCTGTGA | 130 |
| R | AAGCTGGTGCAGGTAGAGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Barbitta, M.; Maranesi, M.; Mercati, F.; Marini, D.; Anipchenko, P.; Grispoldi, L.; Cenci-Goga, B.T.; Zerani, M.; Dall’Aglio, C. Presence, Tissue Localization, and Gene Expression of the Adiponectin Receptor 1 in Testis and Accessory Glands of Male Rams during the Non-Breeding Season. Animals 2023, 13, 601. https://doi.org/10.3390/ani13040601
Martínez-Barbitta M, Maranesi M, Mercati F, Marini D, Anipchenko P, Grispoldi L, Cenci-Goga BT, Zerani M, Dall’Aglio C. Presence, Tissue Localization, and Gene Expression of the Adiponectin Receptor 1 in Testis and Accessory Glands of Male Rams during the Non-Breeding Season. Animals. 2023; 13(4):601. https://doi.org/10.3390/ani13040601
Chicago/Turabian StyleMartínez-Barbitta, Marcelo, Margherita Maranesi, Francesca Mercati, Daniele Marini, Polina Anipchenko, Luca Grispoldi, Beniamino T. Cenci-Goga, Massimo Zerani, and Cecilia Dall’Aglio. 2023. "Presence, Tissue Localization, and Gene Expression of the Adiponectin Receptor 1 in Testis and Accessory Glands of Male Rams during the Non-Breeding Season" Animals 13, no. 4: 601. https://doi.org/10.3390/ani13040601
APA StyleMartínez-Barbitta, M., Maranesi, M., Mercati, F., Marini, D., Anipchenko, P., Grispoldi, L., Cenci-Goga, B. T., Zerani, M., & Dall’Aglio, C. (2023). Presence, Tissue Localization, and Gene Expression of the Adiponectin Receptor 1 in Testis and Accessory Glands of Male Rams during the Non-Breeding Season. Animals, 13(4), 601. https://doi.org/10.3390/ani13040601










