Periconceptual Maternal Nutrition Affects Fetal Liver Programming of Energy- and Lipid-Related Genes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Model, Experimental Design, and Tissue Collection
2.3. RNA Isolation, Library Construction, Sequencing, and Data Processing
2.4. Differential Gene Expression, k-Means Clustering, and Functional Over-Representation Analyses
3. Results
3.1. Fetal Liver Growth and Mineral Concentration Is Affected by Maternal Diet
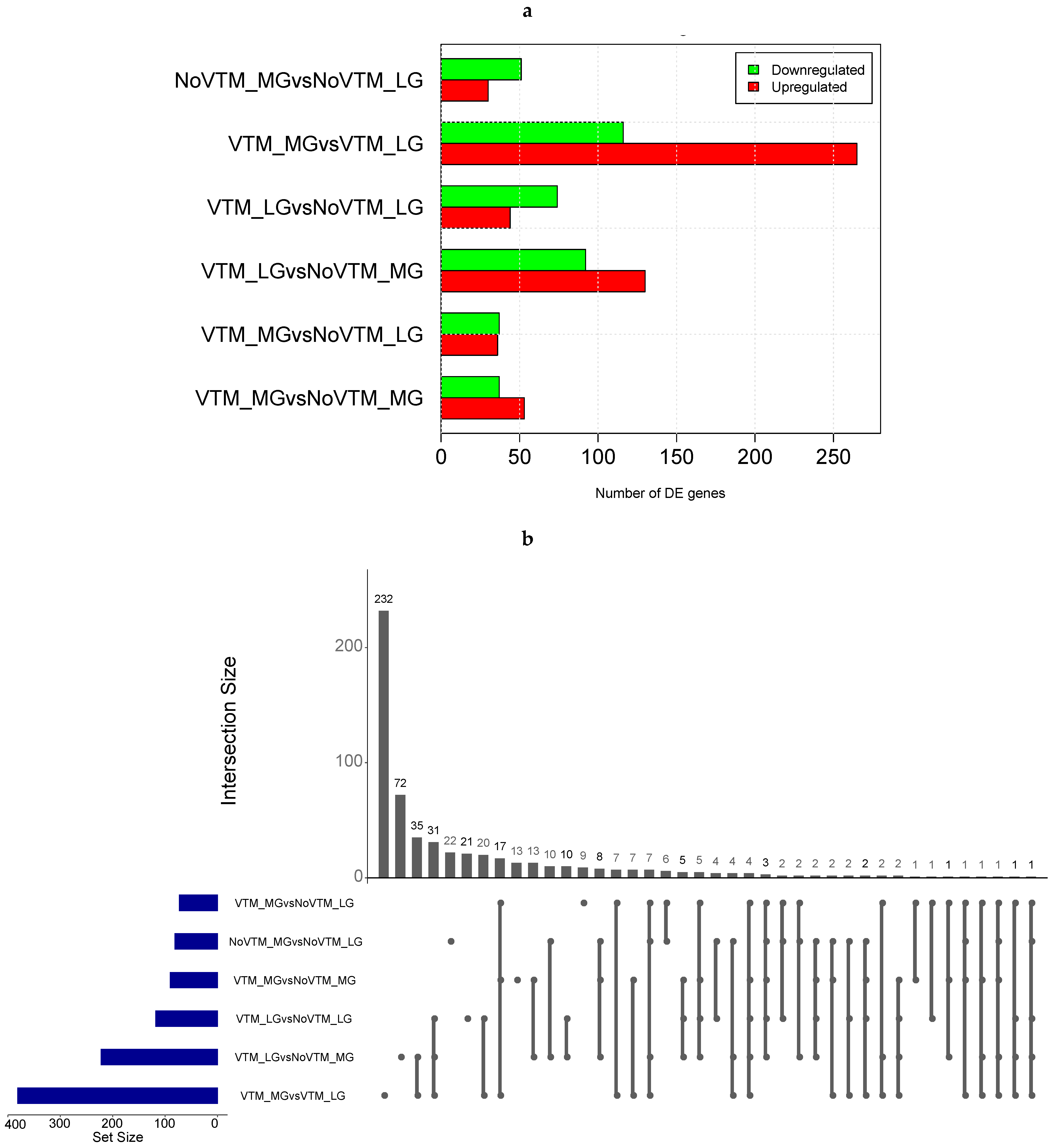
3.2. Maternal Diet Affects Fetal Liver Gene Expression
3.3. Hepatic Metabolic Pathways, Mineral Absorption, and Amino Acid-Transport Genes Are Affected by Maternal Diet
4. Discussion
4.1. Maternal Body-Weight Gain during Early Pregnancy Affects the Expression of Hepatic Fetal Genes Involved with Lipid and Energy Metabolism
4.2. Periconceptual Maternal Mineral and Vitamin Supplementation Affect the Expression of Hepatic Fetal Genes Involved with Mineral Homeostasis and Amino Acid Transport
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Hammer, C.J.; Maddock Carlin, K.R.; Grazul-Bilska, A.T.; Redmer, D.A. Developmental programming: The concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 2010, 88, 61–72. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in pregnancy and lactation: A review article. J. Clin. Diagnostic Res. 2017, 11, QE01–QE05. [Google Scholar] [CrossRef]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J.P. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344–1352. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Haque, C.G.B.M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8, e66318. [Google Scholar] [CrossRef]
- Maloney, C.A.; Rees, W.D. Gene-nutrient interactions during fetal development. Reproduction 2005, 130, 401–410. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Ward, A.K.; Caton, J.S. Epigenetics and developmental programming in ruminants: Long-term impacts on growth and development. In Biology of Domestic Animals; Scanes, C.G., Hill, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 85–120. ISBN 9781315152080. [Google Scholar]
- Caton, J.S.; Crouse, M.S.; McLean, K.J.; Dahlen, C.R.; Ward, A.K.; Cushman, R.A.; Grazul-Bilska, A.T.; Neville, B.W.; Borowicz, P.P.; Reynolds, L.P. Maternal periconceptual nutrition, early pregnancy, and developmental outcomes in beef cattle. J. Anim. Sci. 2020, 98, skaa358. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Budge, H.; Symonds, M.E. Early developmental influences on hepatic organogenesis. Organogenesis 2008, 4, 170–175. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 176, 177–197. [Google Scholar] [CrossRef]
- Sookoian, S.; Gianotti, T.F.; Burgueño, A.L.; Pirola, C.J. Fetal metabolic programming and epigenetic modifications: A systems biology approach. Pediatr. Res. 2013, 73, 531–542. [Google Scholar] [CrossRef]
- Caton, J.S.; Crouse, M.S.; Reynolds, L.P.; Neville, T.L.; Dahlen, C.R.; Ward, A.K.; Swanson, K.C. Maternal nutrition and programming of offspring energy requirements. Transl. Anim. Sci. 2019, 3, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Vonnahme, K.A.; Lemley, C.O.; Caton, J.S.; Meyer, A.M. Impacts of maternal nutrition on vascularity of nutrient transferring tissues during gestation and lactation. Nutrients 2015, 7, 3497–3523. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.I.; Liefeld, A.; Vásquez-Hidalgo, M.A.; Vonnahme, K.A.; Grazul-Bilska, A.T.; Swanson, K.C.; Mishra, N.; Reed, S.A.; Zinn, S.A.; Govoni, K.E. Mid- to late-gestational maternal nutrient restriction followed by realimentation alters development and lipid composition of liver and skeletal muscles in ovine fetuses. J. Anim. Sci. 2021, 99, skab299. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Gardner, D.S.; Sebert, S.; Wilson, V.; Davidson, N.; Nigmatullina, Y.; Chan, L.L.Y.; Budge, H.; Symonds, M.E. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 2011, 141, 119–126. [Google Scholar] [CrossRef]
- Hyatt, M.A.; Gopalakrishnan, G.S.; Bispham, J.; Gentili, S.; McMillen, I.C.; Rhind, S.M.; Rae, M.T.; Kyle, C.E.; Brooks, A.N.; Jones, C.; et al. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J. Endocrinol. 2007, 192, 87–97. [Google Scholar] [CrossRef]
- Copping, K.J.; Hoare, A.; McMillen, I.C.; Rodgers, R.J.; Wallace, C.R.; Perry, V.E.A. Maternal periconceptional and first trimester protein restriction in beef heifers: Effects on maternal performance and early fetal growth. Reprod. Fertil. Dev. 2020, 32, 835–850. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Camacho, L.E.; Lemley, C.O.; Keomanivong, F.E.; Caton, J.S.; Vonnahme, K.A.; Swanson, K.C. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef]
- Crouse, M.S.; Caton, J.S.; Cushman, R.A.; McLean, K.J.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Ward, A.K. Moderate nutrient restriction of beef heifers alters expression of genes associated with tissue metabolism, accretion, and function in fetal liver, muscle, and cerebrum by day 50 of gestation. Transl. Anim. Sci. 2019, 3, 855–866. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Bobe, G.; Klopfenstein, J.J.; Gultekin, Y.; Davis, T.Z.; Ward, A.K.; Hall, J.A. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes 2021, 12, 1884. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.S.; Crouse, M.S.; Cushman, R.A.; McLean, K.J.; Caton, J.S.; Dahlen, C.R.; Reynolds, L.P.; Ward, A.K. Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy. Sci. Rep. 2021, 11, 2771. [Google Scholar] [CrossRef]
- Cammack, R.; Wrigglesworth, J.M.; Baum, H. Iron-dependent enzymes in mammalian systems. In Iron: Transport and Storage; Ponka, P., Schulman, H.M., Woodworth, R.C., Richter, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 17–39. [Google Scholar]
- Hostetler, C.E.; Kincaid, R.L.; Mirando, M.A. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 2003, 166, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.M.; Cooke, R.F.; Marques, R.D.S. Supplementing trace minerals to beef cows during gestation to enhance productive and health responses of the offspring. Animals 2021, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, C.R.; Reynolds, L.P.; Caton, J.S. Selenium supplementation and pregnancy outcomes. Front. Nutr. 2022, 9, 2576. [Google Scholar] [CrossRef]
- USDA Beef 2017. Beef Cow-Calf Management Practices in the United States, 2017, Report 1. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/nahms/NAHMS_Beef_CowCalf_Studies (accessed on 1 February 2021).
- Menezes, A.C.B.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers I: Effects on dam hormonal and metabolic status, fetal tissue and organ mass, and concentration of glucose and fructose in fetal fluids at d 83 of gestation. Animals 2022, 12, 1757. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Menezes, A.C.B.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain in beef heifers II: Effects on concentration of trace minerals in maternal liver and fetal liver, muscle, allantoic, and amniotic fluids at day 83 of gestation. Animals 2022, 12, 1925. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Reynolds, L.P.; Borowicz, P.P.; Ward, A.K.; Sedivec, K.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; et al. Maternal vitamin and mineral supplementation and rate of maternal weight gain affects placental expression of energy metabolism and transport-related genes. Genes 2021, 12, 385. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Reynolds, L.P.; Ward, A.K.; Borowicz, P.P.; Sedivec, K.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; et al. Untangling the placentome gene network of beef heifers in early gestation. Genomics 2022, 114, 110274. [Google Scholar] [CrossRef]
- Crouse, M.S.; McCarthy, K.L.; Menezes, A.C.B.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; et al. Vitamin and mineral supplementation and rate of weight gain during the first trimester of gestation in beef heifers alters the fetal liver amino acid, carbohydrate, and energy profile at day 83 of gestation. Metabolites 2022, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar]
- Lamb, G.C.; Dahlen, C.R.; Larson, J.E.; Marquezini, G.; Stevenson, J.S. Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: A review. J. Anim. Sci. 2010, 88, E181–E192. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.C.; Dahlen, C.R.; Brown, D.R. Reproductive Ultrasonography for Monitoring Ovarian Structure Development, Fetal Development, Embryo Survival, and Twins in Beef Cows. Prof. Anim. Sci. 2003, 19, 135–143. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Utero-placental vascular development and placental function. J. Anim. Sci. 1995, 73, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88 (Suppl. 13), E51–E60. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.J.; Dahlen, C.R.; Borowicz, P.P.; Reynolds, L.P.; Crosswhite, M.R.; Neville, B.W.; Walden, S.D.; Caton, J.S. Technical note: A new surgical technique for ovariohysterectomy during early pregnancy in beef heifers. J. Anim. Sci. 2016, 94, 5089–5096. [Google Scholar] [CrossRef]
- Andrews, S.; FASTQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 February 2021).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.D.; Bickhart, D.M.; Schnabel, R.D.; Koren, S.; Elsik, C.G.; Tseng, E.; Rowan, T.N.; Low, W.Y.; Zimin, A.; Couldrey, C.; et al. De novo assembly of the cattle reference genome with single-molecule sequencing. Gigascience 2020, 9, giaa021. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Tarazona, S.; Furió-Tarí, P.; Turrà, D.; Di Pietro, A.; Nueda, M.J.; Ferrer, A.; Conesa, A. Data quality aware analysis of differential expression in RNA-seq with NOISeq R/Bioc package. Nucleic Acids Res. 2015, 43, e140. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Chen, Y.; Lun, A.T.L.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Research 2016, 5, 1438. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M. 147 Nutritional advances in fetal and neonatal development: Mineral nutrition. J. Anim. Sci. 2020, 98, 121. [Google Scholar] [CrossRef]
- Harvey, K.M.; Cooke, R.F.; Moriel, P. Impacts of nutritional management during early postnatal life on long-term physiological and productive responses of beef cattle. Front. Anim. Sci. 2021, 2, 44. [Google Scholar] [CrossRef]
- Reed, S.A.; Raja, J.S.; Hoffman, M.L.; Zinn, S.A.; Govoni, K.E. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotechnol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Thayer, Z.M.; Rutherford, J.; Kuzawa, C.W. Maternal nutritional buffering model: An evolutionary framework for pregnancy nutritional intervention. Evol. Med. Public Health 2020, 2020, 14–27. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Borowicz, P.P.; Johnson, M.L.; Minten, M.A.; Bilski, J.J.; Wroblewski, R.; Redmer, D.A.; Reynolds, L.P. Placental development during early pregnancy in sheep: Vascular growth and expression of angiogenic factors in maternal placenta. Reproduction 2010, 140, 165–174. [Google Scholar] [CrossRef]
- Trollmann, R.; Gassmann, M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain Dev. 2009, 31, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental adaptations to the maternal-fetal environment: Implications for fetal growth and developmental programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Dwyer, T.; Riley, M.; Cochrane, J.; Jones, G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur. J. Clin. Nutr. 2010, 64, 131–137. [Google Scholar] [CrossRef]
- Valdmanis, P.N.; Kim, H.K.; Chu, K.; Zhang, F.; Xu, J.; Munding, E.M.; Shen, J.; Kay, M.A. miR-122 removal in the liver activates imprinted microRNAs and enables more effective microRNA-mediated gene repression. Nat. Commun. 2018, 9, 5321. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; Dahlen, C.R.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; et al. Fetal Hepatic Lipidome Is More Greatly Affected by Maternal Rate of Gain Compared with Vitamin and Mineral Supplementation at Day 83 of Gestation. Metabolites 2023, 13, 175. [Google Scholar] [CrossRef]
- Fresno Vara, J.Á.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. P13K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Zhu, M.J.; Du, M.; Hess, B.W.; Nathanielsz, P.W.; Ford, S.P. Periconceptional nutrient restriction in the ewe alters MAPK/ERK1/2 and PI3K/Akt growth signaling pathways and vascularity in the placentome. Placenta 2007, 28, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Stangl, G.I.; Schwarz, F.J.; Kirchgessner, M. Cobalt deficiency effects on trace elements, hormones and enzymes involved in energy metabolism of cattle. Int. J. Vitam. Nutr. Res. 1999, 69, 120–126. [Google Scholar] [CrossRef]
- Da Silva Diniz, W.J.; Banerjee, P.; Regitano, L.C.A. Cross talk between mineral metabolism and meat quality: A systems biology overview. Physiol. Genomics 2019, 51, 529–538. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z. Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr. Metab. 2016, 13, 49. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Hornick, J.L.; Van Eenaeme, C.; Gérard, O.; Dufrasne, I.; Istasse, L. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 2000, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 2nd ed.; Elsevier: Wallingford, UK, 2003; ISBN 9780444513670. [Google Scholar]
- Beckett, E.L.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. The role of vitamins and minerals in modulating the expression of microRNA. Nutr. Res. Rev. 2014, 27, 94–106. [Google Scholar] [CrossRef] [PubMed]
- da Silva Diniz, W.J.; Coutinho, L.L.; Tizioto, P.C.; Cesar, A.S.M.; Gromboni, C.F.; Nogueira, A.R.A.; de Oliveira, P.S.N.; de Souza, M.M.; Regitano, L.C.D.A. Iron content affects lipogenic gene expression in the muscle of Nelore beef cattle. PLoS ONE 2016, 11, e0161160. [Google Scholar] [CrossRef]
- Afonso, J.; Fortes, M.R.S.; Reverter, A.; da Silva Diniz, W.J.; Cesar, A.S.M.; de Lima, A.O.; Petrini, J.; de Souza, M.M.; Coutinho, L.L.; Mourão, G.B.; et al. Genetic regulators of mineral amount in Nelore cattle muscle predicted by a new co-expression and regulatory impact factor approach. Sci. Rep. 2020, 10, 8436. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.; Neville, T.L.; Ward, A.K.; Borowicz, P.P.; Reynolds, L.P.; et al. Vitamin and mineral supplementation and rate of gain during the first trimester of gestation affect concentrations of amino acids in maternal serum and allantoic fluid of beef heifers. J. Anim. Sci. 2021, 99, skab024. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed]
- Bremner, I.; Beattie, J.H. Metallothionein and the trace minerals. Annu. Rev. Nutr. 1990, 10, 63–83. [Google Scholar] [CrossRef]
- Mercadante, C.J.; Prajapati, M.; Conboy, H.L.; Dash, M.E.; Herrera, C.; Pettiglio, M.A.; Cintron-Rivera, L.; Salesky, M.A.; Rao, D.B.; Bartnikas, T.B. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Investig. 2019, 129, 5442. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Metwally, E.; Yang, H.; Kalhoro, M.S.; Kalhoro, D.H.; Chughtai, M.I.; Yin, Y. Role of Dietary Amino Acids and Nutrient Sensing System in Pregnancy Associated Disorders. Front. Pharmacol. 2020, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
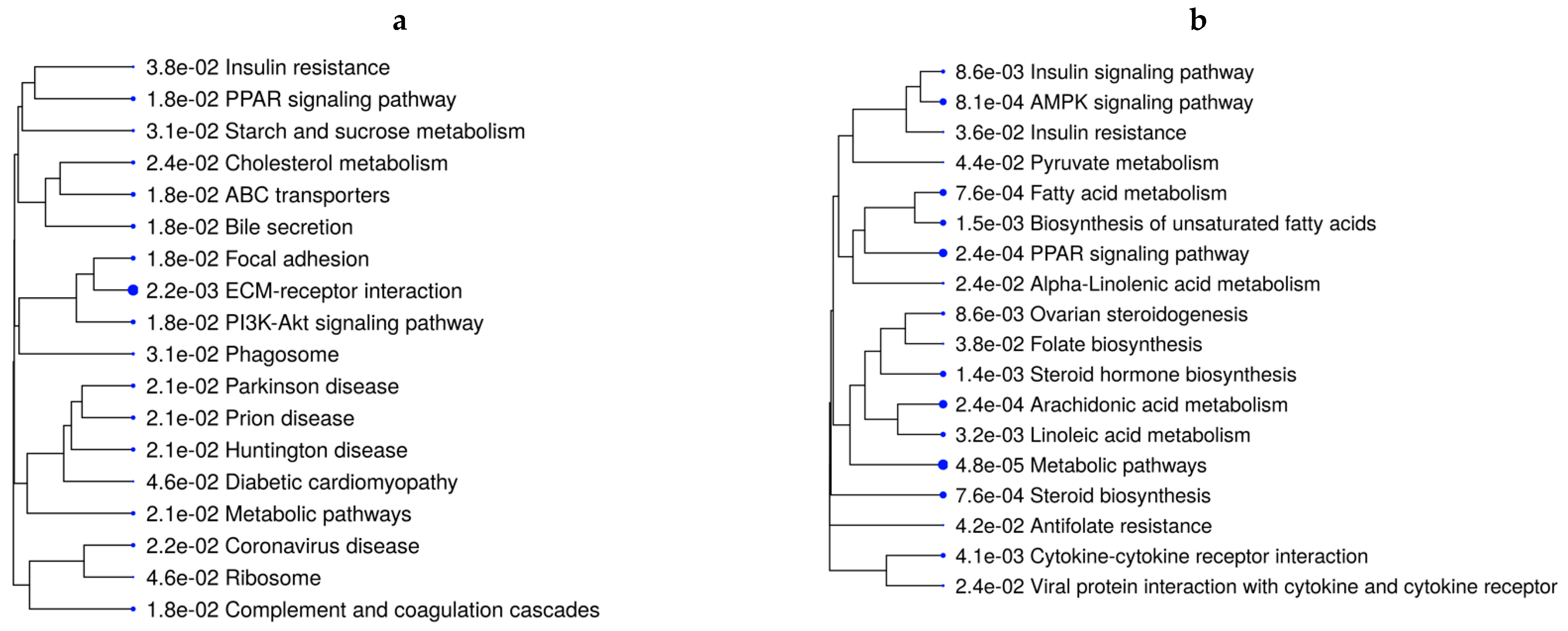

| Cluster | Number of Genes | Number of Pathways Over-Represented | Key Pathways * |
|---|---|---|---|
| A | 702 | 15 | Metabolic pathways Type I diabetes mellitus Retinol metabolism Cell adhesion molecules Arachidonic acid metabolism PPAR signaling pathway |
| B | 577 | 6 | ECM-receptor interaction Focal adhesion ABC transporters PI3K-Akt signaling pathway |
| C | 357 | – | – |
| D | 364 | 15 | Oxidative phosphorylation IL-17 signaling pathway Metabolic Ribosome Pathways of neurodegeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, W.J.S.; Ward, A.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Reynolds, L.P.; Borowicz, P.P.; Sedivec, K.K.; Kirsch, J.D.; Dorsam, S.T.; et al. Periconceptual Maternal Nutrition Affects Fetal Liver Programming of Energy- and Lipid-Related Genes. Animals 2023, 13, 600. https://doi.org/10.3390/ani13040600
Diniz WJS, Ward AK, McCarthy KL, Kassetas CJ, Baumgaertner F, Reynolds LP, Borowicz PP, Sedivec KK, Kirsch JD, Dorsam ST, et al. Periconceptual Maternal Nutrition Affects Fetal Liver Programming of Energy- and Lipid-Related Genes. Animals. 2023; 13(4):600. https://doi.org/10.3390/ani13040600
Chicago/Turabian StyleDiniz, Wellison J. S., Alison K. Ward, Kacie L. McCarthy, Cierrah J. Kassetas, Friederike Baumgaertner, Lawrence P. Reynolds, Pawel P. Borowicz, Kevin K. Sedivec, James D. Kirsch, Sheri T. Dorsam, and et al. 2023. "Periconceptual Maternal Nutrition Affects Fetal Liver Programming of Energy- and Lipid-Related Genes" Animals 13, no. 4: 600. https://doi.org/10.3390/ani13040600
APA StyleDiniz, W. J. S., Ward, A. K., McCarthy, K. L., Kassetas, C. J., Baumgaertner, F., Reynolds, L. P., Borowicz, P. P., Sedivec, K. K., Kirsch, J. D., Dorsam, S. T., Neville, T. L., Forcherio, J. C., Scott, R., Caton, J. S., & Dahlen, C. R. (2023). Periconceptual Maternal Nutrition Affects Fetal Liver Programming of Energy- and Lipid-Related Genes. Animals, 13(4), 600. https://doi.org/10.3390/ani13040600






