Genetic Diversity and Fine-Scale Genetic Structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China Based on Microsatellite Markers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. PCR Amplification and Genotyping
2.3. Statistical Analyses
2.3.1. Genetic Diversity
2.3.2. Genetic Structure and Population Differentiation
2.3.3. Gene Flow
3. Results
3.1. Genetic Diversity of Populations
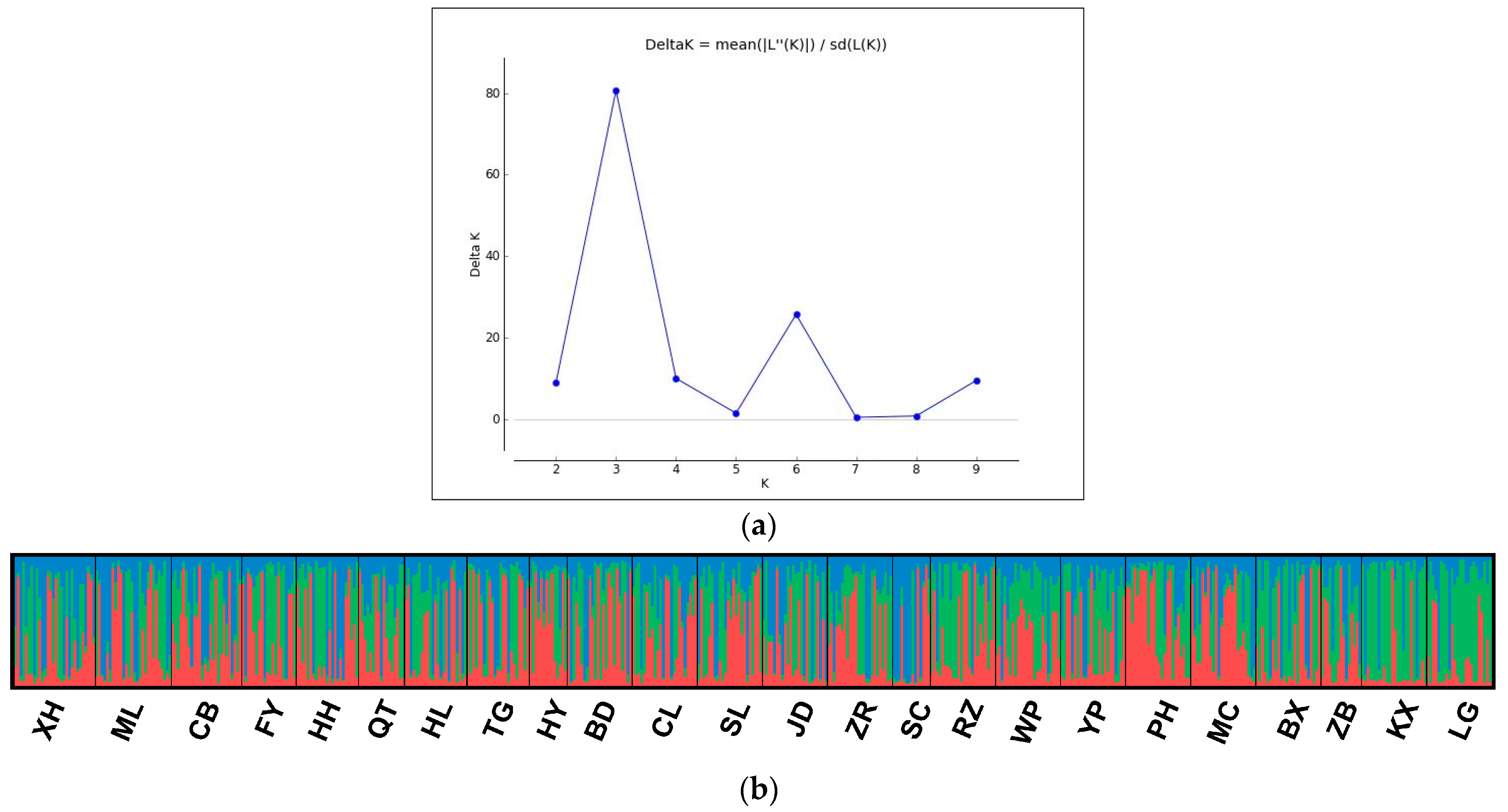
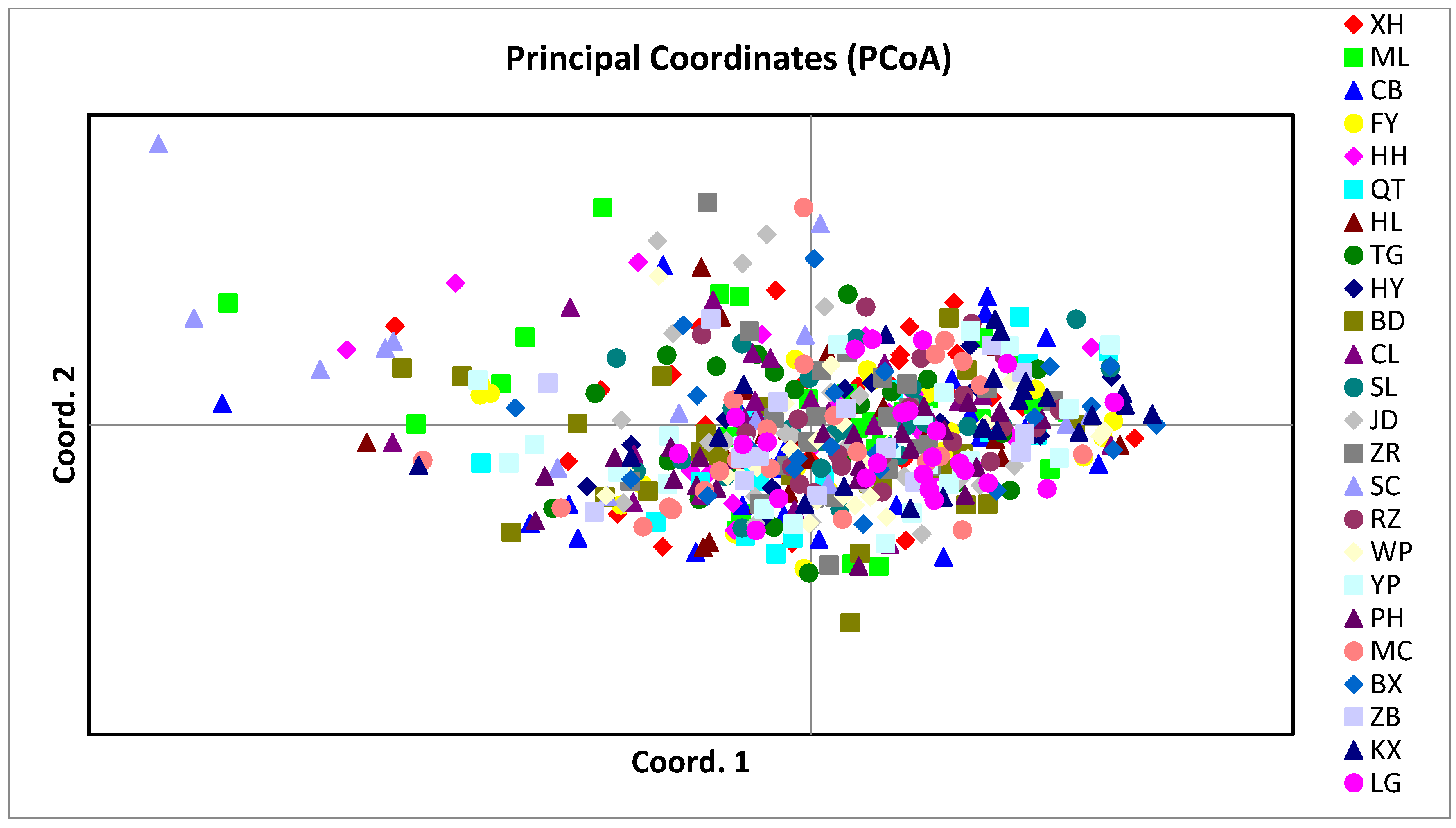
3.2. Population Genetic Structure
3.3. Population Differentiation
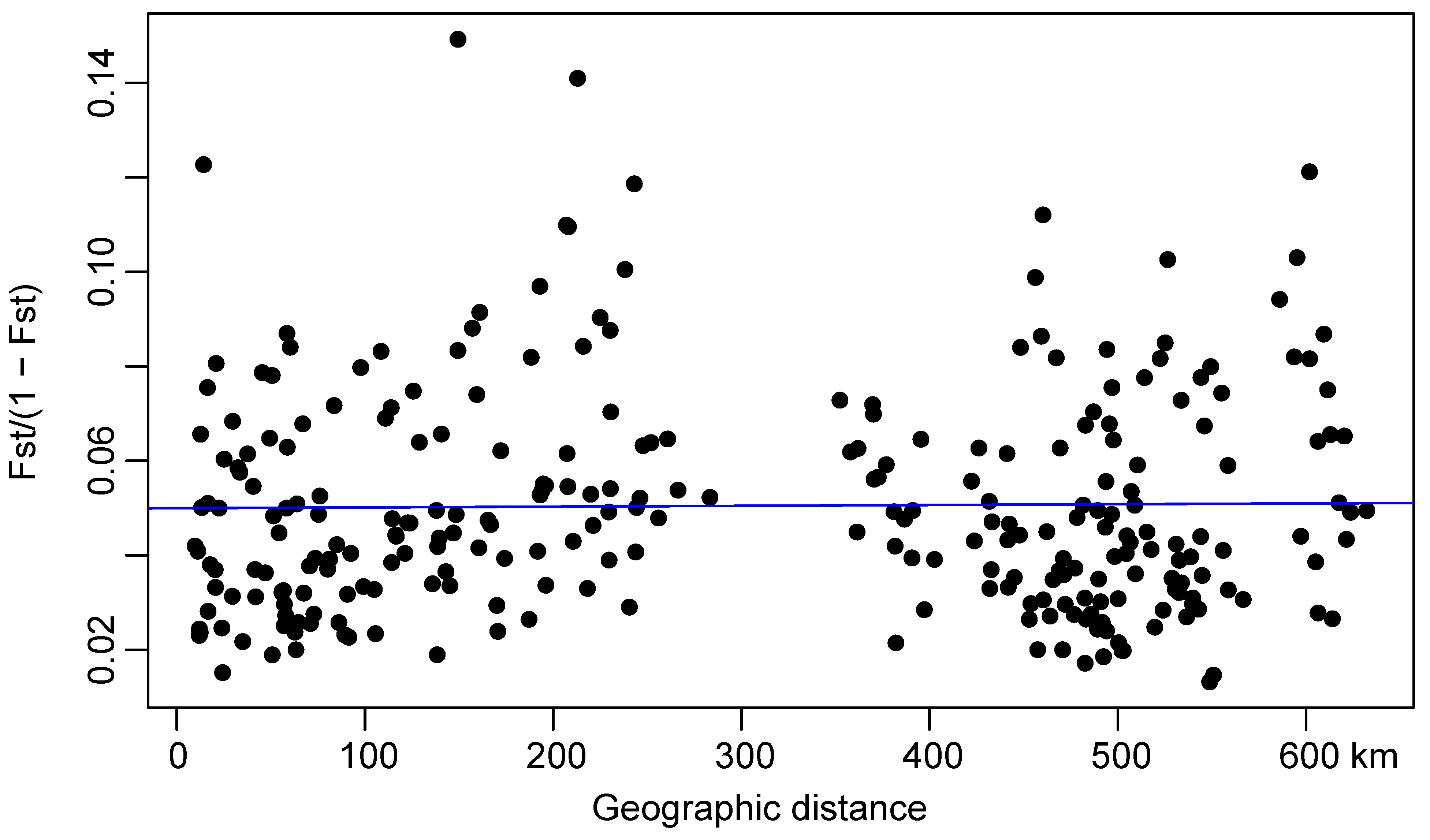
3.4. Gene Flow
4. Discussion
4.1. Genetic Diversity
4.2. Genetic Structure and Population Differentiation
4.3. Gene Flow
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting FST. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef]
- Qu, R.-Z.; Hou, L.; Lü, H.-L.; Li, H.-Y. The gene flow of population genetic structure. Hereditas 2004, 26, 377–382. [Google Scholar]
- Sciaretta, A.; Trematerra, P. Geostatistical characterization of the spatial distribution of Grapholita molesta and Anarsia Lineatella males in an agricultural landscape. J. Appl. Entomol. 2006, 130, 73–83. [Google Scholar] [CrossRef]
- Thaler, R.; Brandstitter, A.; Meraner, A.; Chabicovski, M.; Parson, W.; Zelger, R.; Dalla, V.J.; Dalinger, R. Molecular phylogeny and population structure of the codling moth (Cydia pomonella) in central Europe: I. AFLP analysis reflects human-aided local adaptation of a global pest species. Mol. Phylogenet Evol. 2008, 48, 838–849. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Jiang, S.; Zhao, Y.; Xu, X.; Wu, J. Microsatellite-based analysis of genetic structure and gene flow of Mythimna separata (Walker) (Lepidoptera: Noctuidae) in China. Ecol. Evol. 2019, 9, 13426–13437. [Google Scholar] [CrossRef]
- Yang, F.; Liu, N.; Crossley, M.S.; Wang, P.; Ma, Z.; Guo, J.; Zhang, R. Cropland connectivity affects genetic divergence of Colorado potato beetle along an invasion front. Evol. Appl. 2021, 14, 553–565. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhou, L.; Wyckhuys, K.A.; Jiang, S.; Van Liem, N.; Vi, L.X.; Ali, A.; Wu, K. Population genetics unveils large-scale migration dynamics and population turnover of Spodoptera exigua. Pest Manag. Sci. 2021, 78, 612–625. [Google Scholar] [CrossRef]
- Kim, K.S.; Jones, G.D.; Westbrook, J.K.; Sappington, T.W. Multidisciplinary fingerprints: Forensic reconstruction of an insect reinvasion. J. R. Soc. Interface 2010, 7, 677–686. [Google Scholar] [CrossRef]
- Kim, K.S.; Sappington, T.W. Molecular genetic variation of boll weevil populations in North America estimated with microsatellites: Implications for patterns of dispersal. Genetica 2006, 127, 143–161. [Google Scholar] [CrossRef]
- Schulman, A.H. Molecular markers to assess genetic diversity. Euphytica 2007, 158, 313–321. [Google Scholar] [CrossRef]
- Zane, L.; Bargelloni, L.; Patarnello, T. Strategies for microsatellite isolation: A review. Mol. Ecol. 2002, 11, 1–16. [Google Scholar] [CrossRef]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genetic microsatellite markers in plants: Features and applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, X.Y.; Wang, X.Q. Genetic variation and population genetic structure of the small brown planthopper, Laod-elphax striatellus (Hemiptera: Delphacidae), in Northeast China based on microsatellite markers. Acta Entomol. Sin. 2020, 63, 73–84. [Google Scholar]
- Matsuura, H.; Naito, A. Studies on the Cold-Hardiness and Overwintering of Spodoptera litura F. (Lepidoptera: Noctuidae): VI. Possible Overwintering Areas Predicted from Meteorological Data in Japan. Appl. Èntomol. Zool. 1997, 32, 167–177. [Google Scholar] [CrossRef]
- Bajpai, N.K.; Ballal, C.R.; Rao, N.S.; Singh, S.P.; Bhaskaran, T.V. Competitive interaction between two ichneumonid parasitoids of Spodoptera litura. BioControl 2006, 51, 419–438. [Google Scholar] [CrossRef]
- Wan, X.; Li, J.; Kim, M.J.; Park, H.C.; Kim, S.-S.; Kim, I. DNA Sequence Variation of the Tobacco Cutworm, Spodoptera litura (Lepidoptera: Noctuidae), Determined by Mitochondrial A+T-rich Region and Nuclear ITS2 Sequences. Biochem. Genet. 2011, 49, 760–787. [Google Scholar] [CrossRef]
- Maqsood, S.; Afzal, M.; Aqeel, A.; Raza, A.B.M.; Wakil, W. Influence of weather factors on population dynamics of armyworm, Spodoptera litura f. on cauliflower, brassica oleracea in Punjab. Pak. J. Zool. 2016, 48, 1311–1315. [Google Scholar]
- Zhang, S.M.; Zhao, Y.X. The Geographical Distribution of Agricultural and Forestry Insects in China; China Agriculture Press: Beijing, China, 1996; 143p. [Google Scholar]
- Qing, H.G.; Wang, D.D.; Ding, J.; Huang, R.H.; Ye, Z.X. Host plants of Spodoptera litura. Acta Agric. Jiangxi 2006, 18, 51–58. [Google Scholar]
- Fu, X.; Zhao, X.; Xie, B.; Ali, A.; Wu, K. Seasonal Pattern of Spodoptera litura (Lepidoptera: Noctuidae) Migration across the Bohai Strait in Northern China. J. Econ. Èntomol. 2015, 108, 525–538. [Google Scholar] [CrossRef]
- Gandhi, B.K.; Patil, R.H. Genetic diversity in Spodoptera litura (Fab.) from major soybean growing states of India. Legume Res. 2017, 40, 1119–1125. [Google Scholar]
- Wu, H.H. Study on Trapped Dynamics and Genetic Structure among Different Geographic Populations of Spodoptera litura. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Wu, H.-H.; Wan, P.; Huang, M.-S.; Lei, C.-L. Microsatellites reveal strong genetic structure in the common cutworm, Spodoptera litura. J. Integr. Agric. 2019, 18, 636–643. [Google Scholar] [CrossRef]
- Hulce, D.; Li, X.; Snyder-Leiby, T.; Liu, C.J. GeneMarker® Genotyping Software: Tools to Increase the Statistical Power of DNA Fragment Analysis. J. Biomol. Tech. 2011, 22, S35–S36. [Google Scholar]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Guo, S.W.; Thompson, E.A. Performing the exact test of Hardy-Weinberg proposition for multiple alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Goudet, J. Hierfstat, a package for r to compute and test hierarchical F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef]
- Rosenberg, N.A. Distruct: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT, Version 2.9.3, a Program to Estimate and Test Gene Diversities and Fixation Indices; Lausanne University: Lausanne, Switzerland, 2001. [Google Scholar]
- Yeh, F.C.; Boyle, T.; Yang, R.C. POPGENE, the User Friendly Shareware for Population Genetic Analysis; Version 1.31; University of Alberta and Centre for International Forestry Research: Edmonton, AB, Canada, 1999. [Google Scholar]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [CrossRef] [PubMed]
- Adamack, A.T.; Gruber, B. PopGenReport: Simplifying basic population genetic analyses in R. Methods Ecol. Evol. 2014, 5, 384–387. [Google Scholar] [CrossRef]
- Wilson, G.A.; Rannala, B. Bayesian Inference of Recent Migration Rates Using Multilocus Genotypes. Genetics 2003, 163, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Barbara, T.C.; Palma-Silva, G.M.; Paggi, F.; Bered, M.F.F.; Lexer, C. Cross-species transfer of nuclear icrosatellite markers: Potential and limitations. Mol. Ecol. 2007, 16, 3759–3767. [Google Scholar] [CrossRef]
- Duan, X.; Peng, X.; Qiao, X.; Chen, M. Life cycle and population genetics of bird cherry-oat aphids Rhopalosiphum padi in China: An important pest on wheat crops. J. Pest Sci. 2016, 90, 103–116. [Google Scholar] [CrossRef]
- Eriksson, G.; Namkoong, G.; Roberds, J.H. Dynamic gene conservation for uncertain futures. For. Ecol. Manag. 1993, 62, 15–37. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, S.Q.; Meng, W.; Jiang, C.; Jiang, X.F.; Fu, X.W.; Wang, X.Q.; Wang, X.Y. Genetic variation and population genetic structure of the beet armyworm, Spodoptera exigua, (Lepidoptera: Noctuidae), in Liaoning, based on microsatellite marker variation. Chin. J. Appl. Entomol. 2021, 58, 1143–1151. [Google Scholar]
- Niu, C.W.; Zhang, Q.W.; Ye, Z.H.; Luo, L.Z. Analysis of genetic diversity in different geographic populations of the beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) with AFLP technique. Acta Entomol. Sin. 2006, 49, 867–873. [Google Scholar]
- Herrero, M.I.; Murúa, M.G.; Casmuz, A.S.; Gastaminza, G.; Sosa-Gómez, D.R. Microsatellite variation in Helicoverpa gelotopoeon (Lepidoptera: Noctuidae) populations from Argentina. Agric. For. Èntomol. 2021, 23, 536–544. [Google Scholar] [CrossRef]
- Llewellyn, K.S.; Loxdale, H.D.; Harrington, R.; Brookes, C.P.; Clark, S.J.; Sunnucks, P. Migration and genetic structure of the grain aphid (Sitobion avenae) in Britain related to climate and clonal fluctuation as revealed using microsatellites. Mol. Ecol. 2002, 12, 21–34. [Google Scholar] [CrossRef]
- Endersby, N.M.; Mckechnie, S.W.; Ridland, P.M.; Weeks, A.R. Microsatellites reveal a lack of structure in Australian populations of the diamondback moth, Plutella xylostella (L.). Mol. Ecol. 2005, 15, 107–118. [Google Scholar] [CrossRef]
- Scott, L.J.; Lawrence, N.; Lange, C.L.; Corinna, L.; Graham, G.C.; Hardwick, S.; Rossiter, L.; Dillon, M.L.; Scott, K.D. Population dynamics and gene flow of Helicoverpa armigera (Lepidoptera: Noctuidae) on cotton and grain crops in the Murrum-bidgee Valley, Australia. J. Econ. Entomol. 2006, 99, 155–163. [Google Scholar] [CrossRef]
- Domingues, F.A.; Silva-Brandão, K.L.; Abreu, A.G.; Perera, O.P.; Blanco, C.A.; Cônsoli, F.L.; Omoto, C. Genetic structure and gene flow among Brazilian populations of Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Èntomol. 2012, 105, 2136–2146. [Google Scholar] [CrossRef]
- Whitlock, M.C.; McCauley, D.E. Indirect measures of gene flow and migration: FST≠1/ (4Nm+1). Heredity 1999, 82, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.T.; Ritland, C.E.; Myers, J.H. Genetic analysis of cabbage loopers, Trichoplusia ni (Lepidoptera: Noctuidae), a seasonal migrant in western North America. Evol. Appl. 2011, 4, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.I.; Pierce, A.A.; Barribeau, S.M.; Sternberg, E.D.; Mongue, A.J.; De Roode, J.C. Lack of genetic differentiation between monarch butterflies with divergent migration destinations. Mol. Ecol. 2012, 21, 3433–3444. [Google Scholar] [CrossRef]
- Winnie, R.M.; Raffiudin, R.; Widiarta, I.N.; Rauf, A. The Genetic Structure of Nilaparvata lugens (Stal.) in Java Populations. HAYATI J. Biosci. 2020, 27, 333–334. [Google Scholar] [CrossRef]

| City | Population | Longitude | Latitude | Sample Size | Date |
|---|---|---|---|---|---|
| Hezhou | ML | 111.33° E | 25.05° N | 28 | 2022.06 |
| Hezhou | XH | 111.43° E | 24.85° N | 30 | 2022.06 |
| Hezhou | FY | 111.32° E | 24.90° N | 20 | 2022.06 |
| Hezhou | CB | 111.24° E | 24.98° N | 26 | 2022.06 |
| Hezhou | HH | 111.17° E | 24.56° N | 23 | 2022.06 |
| Hezhou | QT | 111.09° E | 24.43° N | 17 | 2022.06 |
| Hezhou | TG | 111.18° E | 24.40° N | 23 | 2022.07 |
| Hezhou | HL | 111.29° E | 24.41° N | 23 | 2022.05 |
| Hezhou | HY | 111.24° E | 24.30° N | 14 | 2022.07 |
| Hechi | BX | 107.66° E | 24.98° N | 24 | 2022.06 |
| Hechi | MC | 107.46° E | 25.21° N | 24 | 2022.06 |
| Hechi | ZB | 107.07° E | 25.48° N | 15 | 2022.06 |
| Baise | BD | 105.86° E | 24.36° N | 24 | 2022.05 |
| Baise | YP | 106.46° E | 24.95° N | 24 | 2022.06 |
| Baise | PH | 105.20° E | 24.44° N | 24 | 2022.05 |
| Baise | CL | 106.54° E | 24.23° N | 24 | 2022.05 |
| Baise | SL | 106.83° E | 24.25° N | 24 | 2022.05 |
| Baise | SC | 105.31° E | 24.52° N | 14 | 2022.07 |
| Baise | JD | 106.32° E | 23.42° N | 24 | 2022.05 |
| Baise | ZR | 106.73° E | 23.43° N | 24 | 2022.05 |
| Baise | LG | 106.88° E | 23.25° N | 24 | 2022.05 |
| Baise | RZ | 106.48° E | 22.98° N | 24 | 2022.05 |
| Baise | WP | 106.52° E | 23.18° N | 24 | 2022.05 |
| Baise | KX | 106.16° E | 23.42° N | 24 | 2022.05 |
| Population | n | NA | HO | HE | AR | PIC | I |
|---|---|---|---|---|---|---|---|
| XH | 30 | 6.86 | 0.4690 | 0.6405 | 5.6761 | 0.6028 | 1.3729 |
| ML | 28 | 6.29 | 0.4261 | 0.6103 | 5.3120 | 0.5681 | 1.2786 |
| CB | 26 | 6.71 | 0.4176 | 0.6002 | 5.6280 | 0.5649 | 1.2999 |
| FY | 20 | 5.14 | 0.4820 | 0.5799 | 4.8323 | 0.5349 | 1.1677 |
| HH | 23 | 5.86 | 0.4848 | 0.6038 | 5.1305 | 0.5606 | 1.2457 |
| QT | 17 | 5.14 | 0.3613 | 0.5635 | 4.8244 | 0.5138 | 1.1130 |
| HL | 23 | 5.71 | 0.5590 | 0.6215 | 5.2608 | 0.5767 | 1.2789 |
| TG | 23 | 6.29 | 0.4596 | 0.5836 | 5.4250 | 0.5445 | 1.2334 |
| HY | 14 | 4.57 | 0.4184 | 0.5601 | 4.5714 | 0.5151 | 1.1226 |
| BD | 24 | 5.57 | 0.4501 | 0.5991 | 4.9598 | 0.5549 | 1.2192 |
| CL | 24 | 5.14 | 0.4881 | 0.5927 | 4.7082 | 0.5499 | 1.1947 |
| SL | 24 | 6.14 | 0.5153 | 0.6138 | 5.4851 | 0.5760 | 1.3013 |
| JD | 24 | 5.86 | 0.5774 | 0.6173 | 5.3008 | 0.5739 | 1.2757 |
| ZR | 24 | 6.00 | 0.5357 | 0.6037 | 5.3328 | 0.5659 | 1.2709 |
| SC | 14 | 4.14 | 0.5204 | 0.6750 | 4.1429 | 0.5892 | 1.2059 |
| RZ | 24 | 5.71 | 0.4702 | 0.5512 | 5.0390 | 0.5127 | 1.1450 |
| WP | 24 | 6.14 | 0.5179 | 0.5966 | 5.5135 | 0.5624 | 1.2889 |
| YP | 24 | 6.43 | 0.4829 | 0.6142 | 5.5779 | 0.5754 | 1.3086 |
| PH | 24 | 5.57 | 0.4622 | 0.5557 | 5.0395 | 0.5216 | 1.1833 |
| MC | 24 | 6.00 | 0.5321 | 0.5910 | 5.3389 | 0.5532 | 1.2486 |
| BX | 24 | 5.00 | 0.5060 | 0.6040 | 4.6105 | 0.5619 | 1.2190 |
| ZB | 15 | 5.86 | 0.4952 | 0.6230 | 5.7783 | 0.5784 | 1.3145 |
| KX | 24 | 5.57 | 0.4428 | 0.5556 | 4.8706 | 0.5133 | 1.1339 |
| LG | 24 | 5.86 | 0.4286 | 0.5716 | 5.0947 | 0.5322 | 1.1974 |
| Mean | 22.6310 | 5.73 | 0.4793 | 0.5970 | 5.1439 | 0.5543 | 1.2342 |
| Source of Variation | d.f. | Sum of Squares | Variance Components | Variation | Fixation Index | p-Value |
|---|---|---|---|---|---|---|
| Among populations | 23 | 101.154 | 0.04114 | 1.92% | FST = 0.0191 | <0.001 |
| Among individuals within populations | 521 | 1319.110 | 0.42924 | 20.02% | FIS = 0.2041 | <0.001 |
| Within individuals | 545 | 912.000 | 1.67339 | 78.06% | FIT = 0.2194 | <0.001 |
| Total | 1098 | 2332.264 | 2.14377 |
| XH | ML | CB | FY | HH | QT | HL | TG | HY | BD | CL | SL | JD | ZR | SC | RZ | WP | YP | PH | MC | BX | ZB | KX | LG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XH | 0.024 | 0.038 | 0.032 | 0.058 | 0.035 | 0.031 | 0.047 | 0.069 | 0.047 | 0.046 | 0.032 | 0.047 | 0.030 | 0.123 | 0.046 | 0.060 | 0.032 | 0.067 | 0.059 | 0.077 | 0.058 | 0.081 | 0.101 | |
| ML | 0.015 | 0.034 | 0.039 | 0.049 | 0.036 | 0.040 | 0.057 | 0.097 | 0.049 | 0.068 | 0.042 | 0.048 | 0.032 | 0.131 | 0.058 | 0.063 | 0.039 | 0.066 | 0.058 | 0.084 | 0.081 | 0.101 | 0.121 | |
| CB | 0.024 | 0.023 | 0.033 | 0.054 | 0.031 | 0.030 | 0.036 | 0.064 | 0.019 | 0.038 | 0.045 | 0.053 | 0.036 | 0.139 | 0.041 | 0.036 | 0.025 | 0.034 | 0.030 | 0.065 | 0.065 | 0.104 | 0.095 | |
| FY | 0.023 | 0.027 | 0.024 | 0.077 | 0.032 | 0.064 | 0.040 | 0.087 | 0.057 | 0.048 | 0.043 | 0.048 | 0.033 | 0.139 | 0.033 | 0.039 | 0.042 | 0.055 | 0.068 | 0.101 | 0.067 | 0.103 | 0.095 | |
| HH | 0.036 | 0.032 | 0.035 | 0.052 | 0.068 | 0.051 | 0.054 | 0.091 | 0.058 | 0.057 | 0.066 | 0.055 | 0.054 | 0.141 | 0.066 | 0.096 | 0.073 | 0.050 | 0.061 | 0.092 | 0.097 | 0.115 | 0.164 | |
| QT | 0.027 | 0.027 | 0.023 | 0.024 | 0.049 | 0.049 | 0.054 | 0.101 | 0.043 | 0.059 | 0.049 | 0.053 | 0.025 | 0.143 | 0.060 | 0.036 | 0.039 | 0.054 | 0.079 | 0.098 | 0.076 | 0.097 | 0.110 | |
| HL | 0.019 | 0.025 | 0.020 | 0.043 | 0.032 | 0.036 | 0.059 | 0.067 | 0.022 | 0.046 | 0.041 | 0.066 | 0.042 | 0.114 | 0.059 | 0.067 | 0.029 | 0.069 | 0.042 | 0.086 | 0.074 | 0.100 | 0.120 | |
| TG | 0.031 | 0.038 | 0.025 | 0.029 | 0.037 | 0.040 | 0.039 | 0.085 | 0.041 | 0.050 | 0.048 | 0.062 | 0.049 | 0.171 | 0.051 | 0.079 | 0.074 | 0.035 | 0.067 | 0.089 | 0.047 | 0.107 | 0.137 | |
| HY | 0.048 | 0.067 | 0.046 | 0.064 | 0.064 | 0.075 | 0.048 | 0.062 | 0.090 | 0.048 | 0.058 | 0.080 | 0.083 | 0.187 | 0.065 | 0.101 | 0.065 | 0.081 | 0.085 | 0.092 | 0.083 | 0.128 | 0.111 | |
| BD | 0.030 | 0.032 | 0.013 | 0.039 | 0.038 | 0.032 | 0.014 | 0.029 | 0.063 | 0.054 | 0.050 | 0.058 | 0.050 | 0.149 | 0.062 | 0.065 | 0.034 | 0.041 | 0.038 | 0.080 | 0.059 | 0.112 | 0.130 | |
| CL | 0.030 | 0.044 | 0.026 | 0.034 | 0.038 | 0.043 | 0.030 | 0.035 | 0.036 | 0.036 | 0.046 | 0.060 | 0.046 | 0.104 | 0.054 | 0.064 | 0.055 | 0.065 | 0.052 | 0.095 | 0.073 | 0.106 | 0.099 | |
| SL | 0.020 | 0.026 | 0.029 | 0.030 | 0.041 | 0.036 | 0.026 | 0.032 | 0.042 | 0.032 | 0.030 | 0.036 | 0.034 | 0.157 | 0.043 | 0.070 | 0.040 | 0.062 | 0.069 | 0.065 | 0.029 | 0.063 | 0.099 | |
| JD | 0.028 | 0.030 | 0.034 | 0.033 | 0.035 | 0.038 | 0.040 | 0.041 | 0.056 | 0.037 | 0.039 | 0.023 | 0.047 | 0.131 | 0.064 | 0.088 | 0.046 | 0.055 | 0.073 | 0.080 | 0.045 | 0.103 | 0.123 | |
| ZR | 0.019 | 0.021 | 0.024 | 0.023 | 0.035 | 0.020 | 0.027 | 0.034 | 0.059 | 0.033 | 0.031 | 0.022 | 0.030 | 0.141 | 0.041 | 0.031 | 0.036 | 0.054 | 0.062 | 0.081 | 0.059 | 0.065 | 0.085 | |
| SC | 0.061 | 0.070 | 0.075 | 0.080 | 0.076 | 0.086 | 0.060 | 0.093 | 0.108 | 0.080 | 0.060 | 0.081 | 0.069 | 0.076 | 0.166 | 0.166 | 0.131 | 0.192 | 0.146 | 0.207 | 0.202 | 0.238 | 0.237 | |
| RZ | 0.034 | 0.042 | 0.031 | 0.026 | 0.048 | 0.046 | 0.043 | 0.039 | 0.051 | 0.045 | 0.040 | 0.032 | 0.046 | 0.031 | 0.099 | 0.065 | 0.042 | 0.067 | 0.070 | 0.084 | 0.069 | 0.076 | 0.100 | |
| WP | 0.037 | 0.041 | 0.024 | 0.028 | 0.061 | 0.027 | 0.042 | 0.053 | 0.070 | 0.043 | 0.042 | 0.045 | 0.054 | 0.021 | 0.088 | 0.048 | 0.050 | 0.071 | 0.072 | 0.103 | 0.099 | 0.105 | 0.085 | |
| YP | 0.019 | 0.025 | 0.017 | 0.029 | 0.046 | 0.029 | 0.018 | 0.048 | 0.047 | 0.023 | 0.036 | 0.025 | 0.029 | 0.023 | 0.070 | 0.032 | 0.033 | 0.058 | 0.048 | 0.060 | 0.066 | 0.083 | 0.075 | |
| PH | 0.047 | 0.047 | 0.026 | 0.042 | 0.037 | 0.042 | 0.049 | 0.027 | 0.062 | 0.031 | 0.047 | 0.044 | 0.040 | 0.039 | 0.109 | 0.052 | 0.051 | 0.042 | 0.053 | 0.063 | 0.062 | 0.104 | 0.109 | |
| MC | 0.038 | 0.038 | 0.021 | 0.047 | 0.040 | 0.056 | 0.028 | 0.045 | 0.061 | 0.026 | 0.035 | 0.045 | 0.047 | 0.041 | 0.081 | 0.051 | 0.048 | 0.032 | 0.039 | 0.084 | 0.098 | 0.135 | 0.127 | |
| BX | 0.047 | 0.053 | 0.043 | 0.067 | 0.058 | 0.068 | 0.054 | 0.059 | 0.065 | 0.052 | 0.062 | 0.042 | 0.050 | 0.052 | 0.106 | 0.060 | 0.066 | 0.039 | 0.046 | 0.055 | 0.058 | 0.070 | 0.085 | |
| ZB | 0.034 | 0.049 | 0.041 | 0.045 | 0.059 | 0.053 | 0.045 | 0.032 | 0.058 | 0.038 | 0.046 | 0.019 | 0.028 | 0.038 | 0.099 | 0.050 | 0.061 | 0.041 | 0.044 | 0.061 | 0.038 | 0.069 | 0.090 | |
| KX | 0.056 | 0.069 | 0.072 | 0.074 | 0.078 | 0.072 | 0.068 | 0.075 | 0.093 | 0.077 | 0.074 | 0.046 | 0.070 | 0.048 | 0.130 | 0.059 | 0.073 | 0.059 | 0.077 | 0.091 | 0.051 | 0.050 | 0.066 | |
| LG | 0.063 | 0.077 | 0.063 | 0.066 | 0.101 | 0.078 | 0.076 | 0.090 | 0.080 | 0.084 | 0.067 | 0.065 | 0.078 | 0.057 | 0.124 | 0.072 | 0.058 | 0.050 | 0.078 | 0.083 | 0.058 | 0.059 | 0.050 |
| Distance Class (Km) | N | r |
|---|---|---|
| 44 | 56 | −0.0380 |
| 89 | 68 | 0.0261 |
| 133 | 40 | −0.0241 |
| 178 | 44 | −0.1003 |
| 222 | 38 | −0.0584 |
| 267 | 34 | −0.0184 |
| 311 | 2 | −0.1166 |
| 356 | 2 | −0.3507 |
| 400 | 32 | −0.0619 |
| 448 | 24 | −0.1292 |
| 489 | 60 | −0.0679 |
| 534 | 78 | −0.0621 |
| 578 | 38 | −0.3657 |
| 622 | 32 | 0.0232 |
| XH | ML | CB | FY | HH | QT | HL | TG | HY | BD | CL | SL | JD | ZR | SC | RZ | WP | YP | PH | MC | BX | ZB | KX | LG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XH | 0.6929 | 0.0066 | 0.1098 | 0.0061 | 0.0066 | 0.0066 | 0.0063 | 0.0062 | 0.0065 | 0.0064 | 0.0065 | 0.0066 | 0.0065 | 0.0073 | 0.0063 | 0.0202 | 0.0081 | 0.0068 | 0.0129 | 0.0076 | 0.0074 | 0.0064 | 0.0370 | 0.0065 |
| ML | 0.0620 | 0.6731 | 0.1080 | 0.0064 | 0.0064 | 0.0066 | 0.0064 | 0.0062 | 0.0065 | 0.0064 | 0.0066 | 0.0066 | 0.0063 | 0.0066 | 0.0065 | 0.0126 | 0.0066 | 0.0064 | 0.0155 | 0.0066 | 0.0068 | 0.0065 | 0.0118 | 0.0065 |
| CB | 0.0306 | 0.0079 | 0.7806 | 0.0069 | 0.0078 | 0.0067 | 0.0076 | 0.0072 | 0.0073 | 0.0070 | 0.0070 | 0.0076 | 0.0076 | 0.0073 | 0.0069 | 0.0160 | 0.0081 | 0.0074 | 0.0134 | 0.0085 | 0.0082 | 0.0070 | 0.0180 | 0.0075 |
| FY | 0.0242 | 0.0078 | 0.1245 | 0.6744 | 0.0075 | 0.0078 | 0.0075 | 0.0076 | 0.0078 | 0.0076 | 0.0076 | 0.0078 | 0.0080 | 0.0076 | 0.0076 | 0.0115 | 0.0082 | 0.0077 | 0.0116 | 0.0076 | 0.0078 | 0.0078 | 0.0147 | 0.0077 |
| HH | 0.0508 | 0.0070 | 0.1024 | 0.0072 | 0.6739 | 0.0072 | 0.0072 | 0.0071 | 0.0072 | 0.0073 | 0.0072 | 0.0070 | 0.0073 | 0.0073 | 0.0072 | 0.0105 | 0.0076 | 0.0073 | 0.0170 | 0.0073 | 0.0071 | 0.0069 | 0.0161 | 0.0071 |
| QT | 0.0110 | 0.0086 | 0.1216 | 0.0083 | 0.0084 | 0.6750 | 0.0081 | 0.0080 | 0.0082 | 0.0082 | 0.0083 | 0.0084 | 0.0082 | 0.0085 | 0.0084 | 0.0125 | 0.0092 | 0.0086 | 0.0158 | 0.0086 | 0.0084 | 0.0083 | 0.0132 | 0.0084 |
| HL | 0.0301 | 0.0074 | 0.1335 | 0.0074 | 0.0070 | 0.0073 | 0.6740 | 0.0072 | 0.0070 | 0.0072 | 0.0075 | 0.0072 | 0.0073 | 0.0071 | 0.0073 | 0.0094 | 0.0073 | 0.0071 | 0.0109 | 0.0074 | 0.0072 | 0.0072 | 0.0118 | 0.0072 |
| TG | 0.0235 | 0.0072 | 0.1309 | 0.0067 | 0.0070 | 0.0069 | 0.0072 | 0.6737 | 0.0070 | 0.0073 | 0.0071 | 0.0072 | 0.0070 | 0.0072 | 0.0069 | 0.0115 | 0.0074 | 0.0070 | 0.0175 | 0.0069 | 0.0075 | 0.0073 | 0.0152 | 0.0070 |
| HY | 0.0100 | 0.0088 | 0.0988 | 0.0088 | 0.0088 | 0.0088 | 0.0087 | 0.0086 | 0.6754 | 0.0088 | 0.0086 | 0.0090 | 0.0088 | 0.0086 | 0.0086 | 0.0170 | 0.0090 | 0.0089 | 0.0144 | 0.0090 | 0.0088 | 0.0088 | 0.0262 | 0.0089 |
| BD | 0.0233 | 0.0072 | 0.1410 | 0.0073 | 0.0070 | 0.0072 | 0.0071 | 0.0069 | 0.0070 | 0.6736 | 0.0070 | 0.0070 | 0.0070 | 0.0070 | 0.0074 | 0.0087 | 0.0072 | 0.0071 | 0.0144 | 0.0073 | 0.0074 | 0.0073 | 0.0105 | 0.0072 |
| CL | 0.0336 | 0.0070 | 0.1329 | 0.0069 | 0.0069 | 0.0068 | 0.0070 | 0.0070 | 0.0069 | 0.0068 | 0.6737 | 0.0068 | 0.0071 | 0.0069 | 0.0069 | 0.0098 | 0.0070 | 0.0069 | 0.0084 | 0.0071 | 0.0068 | 0.0069 | 0.0171 | 0.0067 |
| SL | 0.0256 | 0.0068 | 0.1200 | 0.0068 | 0.0070 | 0.0069 | 0.0071 | 0.0069 | 0.0069 | 0.0069 | 0.0070 | 0.6737 | 0.0071 | 0.0067 | 0.0069 | 0.0098 | 0.0074 | 0.0071 | 0.0096 | 0.0072 | 0.0074 | 0.0070 | 0.0350 | 0.0070 |
| JD | 0.0445 | 0.0069 | 0.1190 | 0.0072 | 0.0071 | 0.0070 | 0.0071 | 0.0069 | 0.0069 | 0.0071 | 0.0068 | 0.0069 | 0.6737 | 0.0071 | 0.0069 | 0.0080 | 0.0072 | 0.0071 | 0.0099 | 0.0073 | 0.0072 | 0.0069 | 0.0181 | 0.0071 |
| ZR | 0.0277 | 0.0072 | 0.1267 | 0.0068 | 0.0072 | 0.0072 | 0.0073 | 0.0070 | 0.0072 | 0.0071 | 0.0070 | 0.0071 | 0.0071 | 0.6738 | 0.0069 | 0.0101 | 0.0075 | 0.0072 | 0.0093 | 0.0072 | 0.0071 | 0.0070 | 0.0241 | 0.0071 |
| SC | 0.0828 | 0.0090 | 0.0484 | 0.0088 | 0.0088 | 0.0092 | 0.0091 | 0.0086 | 0.0089 | 0.0088 | 0.0090 | 0.0088 | 0.0087 | 0.0088 | 0.6755 | 0.0113 | 0.0087 | 0.0086 | 0.0103 | 0.0088 | 0.0088 | 0.0087 | 0.0125 | 0.0089 |
| RZ | 0.0220 | 0.0071 | 0.1280 | 0.0069 | 0.0070 | 0.0068 | 0.0068 | 0.0070 | 0.0071 | 0.0070 | 0.0069 | 0.0071 | 0.0070 | 0.0068 | 0.0069 | 0.6816 | 0.0075 | 0.0071 | 0.0209 | 0.0072 | 0.0073 | 0.0071 | 0.0139 | 0.0070 |
| WP | 0.0177 | 0.0069 | 0.1379 | 0.0070 | 0.0069 | 0.0070 | 0.0074 | 0.0072 | 0.0070 | 0.0071 | 0.0070 | 0.0071 | 0.0069 | 0.0071 | 0.0068 | 0.0150 | 0.6745 | 0.0073 | 0.0094 | 0.0070 | 0.0074 | 0.0070 | 0.0186 | 0.0068 |
| YP | 0.0294 | 0.0072 | 0.1233 | 0.0070 | 0.0072 | 0.0072 | 0.0072 | 0.0072 | 0.0070 | 0.0073 | 0.0069 | 0.0072 | 0.0072 | 0.0072 | 0.0071 | 0.0109 | 0.0077 | 0.6740 | 0.0129 | 0.0075 | 0.0080 | 0.0071 | 0.0197 | 0.0070 |
| PH | 0.0084 | 0.0070 | 0.1388 | 0.0068 | 0.0070 | 0.0070 | 0.0070 | 0.0071 | 0.0071 | 0.0069 | 0.0069 | 0.0069 | 0.0067 | 0.0070 | 0.0070 | 0.0091 | 0.0073 | 0.0067 | 0.6854 | 0.0072 | 0.0079 | 0.0068 | 0.0249 | 0.0071 |
| MC | 0.0274 | 0.0066 | 0.1462 | 0.0071 | 0.0070 | 0.0068 | 0.0067 | 0.0072 | 0.0069 | 0.0069 | 0.0068 | 0.0069 | 0.0068 | 0.0070 | 0.0070 | 0.0102 | 0.0073 | 0.0070 | 0.0082 | 0.6739 | 0.0073 | 0.0067 | 0.0092 | 0.0070 |
| BX | 0.0301 | 0.0068 | 0.0467 | 0.0071 | 0.0071 | 0.0072 | 0.0071 | 0.0072 | 0.0069 | 0.0070 | 0.0069 | 0.0070 | 0.0069 | 0.0071 | 0.0071 | 0.0105 | 0.0072 | 0.0071 | 0.0085 | 0.0071 | 0.6746 | 0.0071 | 0.1026 | 0.0069 |
| ZB | 0.0179 | 0.0086 | 0.0761 | 0.0087 | 0.0089 | 0.0090 | 0.0087 | 0.0088 | 0.0086 | 0.0089 | 0.0086 | 0.0088 | 0.0090 | 0.0091 | 0.0090 | 0.0125 | 0.0090 | 0.0089 | 0.0123 | 0.0091 | 0.0097 | 0.6755 | 0.0448 | 0.0089 |
| KX | 0.0142 | 0.0071 | 0.0239 | 0.0078 | 0.0073 | 0.0073 | 0.0105 | 0.0085 | 0.0070 | 0.0092 | 0.0082 | 0.0080 | 0.0081 | 0.0072 | 0.0078 | 0.0109 | 0.0077 | 0.0082 | 0.0110 | 0.0073 | 0.0084 | 0.0096 | 0.7868 | 0.0076 |
| LG | 0.0137 | 0.0068 | 0.0165 | 0.0069 | 0.0068 | 0.0069 | 0.0069 | 0.0069 | 0.0069 | 0.0070 | 0.0068 | 0.0069 | 0.0069 | 0.0068 | 0.0070 | 0.0102 | 0.0070 | 0.0069 | 0.0080 | 0.0071 | 0.0070 | 0.0068 | 0.1536 | 0.6737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Yang, F.; Zhang, D.; Zhang, S.; Yu, X.; Yang, M. Genetic Diversity and Fine-Scale Genetic Structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China Based on Microsatellite Markers. Animals 2023, 13, 560. https://doi.org/10.3390/ani13040560
Hu Z, Yang F, Zhang D, Zhang S, Yu X, Yang M. Genetic Diversity and Fine-Scale Genetic Structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China Based on Microsatellite Markers. Animals. 2023; 13(4):560. https://doi.org/10.3390/ani13040560
Chicago/Turabian StyleHu, Zhongwen, Fangyuan Yang, Deping Zhang, Shimeng Zhang, Xiaofei Yu, and Maofa Yang. 2023. "Genetic Diversity and Fine-Scale Genetic Structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China Based on Microsatellite Markers" Animals 13, no. 4: 560. https://doi.org/10.3390/ani13040560
APA StyleHu, Z., Yang, F., Zhang, D., Zhang, S., Yu, X., & Yang, M. (2023). Genetic Diversity and Fine-Scale Genetic Structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China Based on Microsatellite Markers. Animals, 13(4), 560. https://doi.org/10.3390/ani13040560







