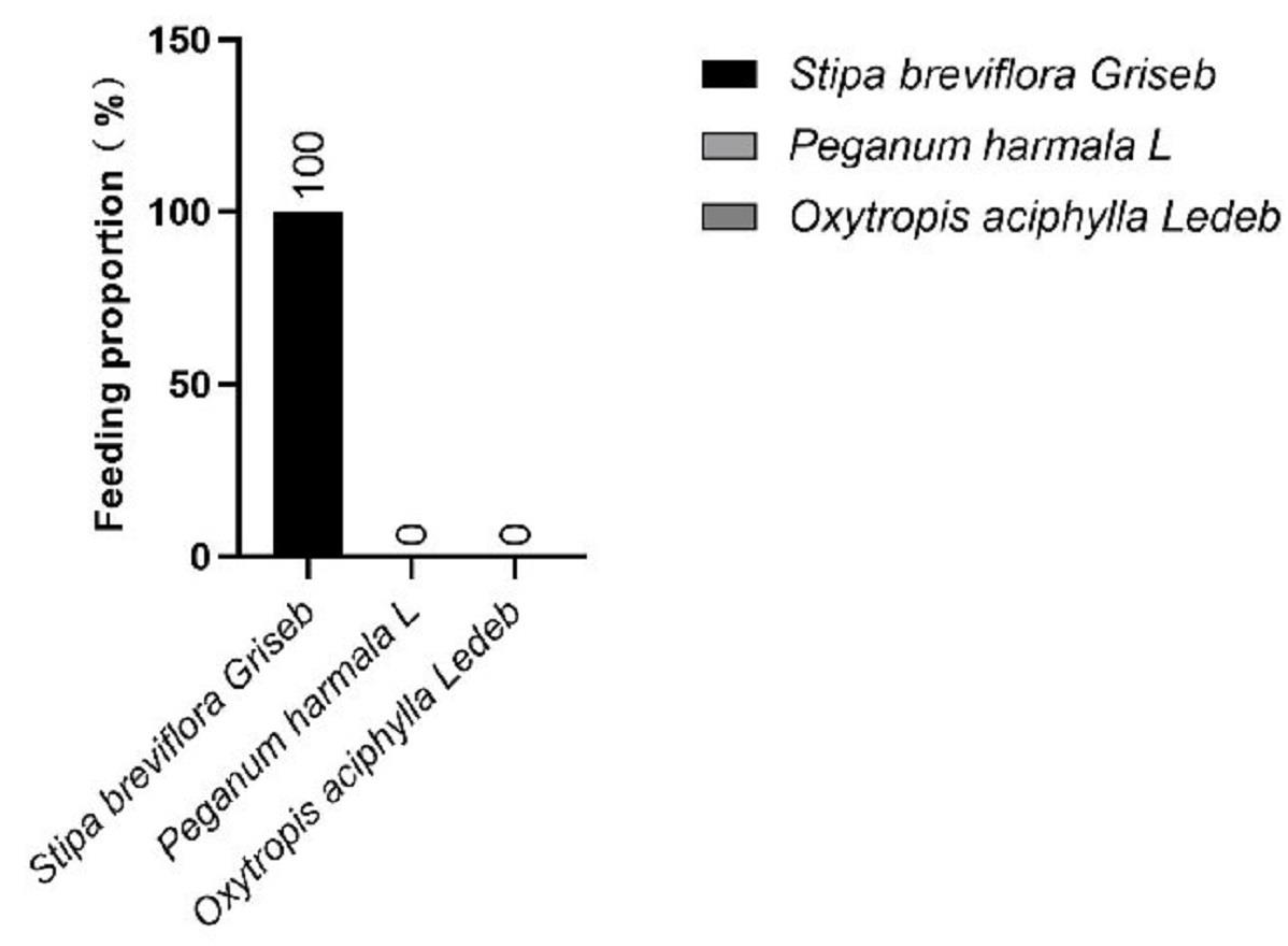
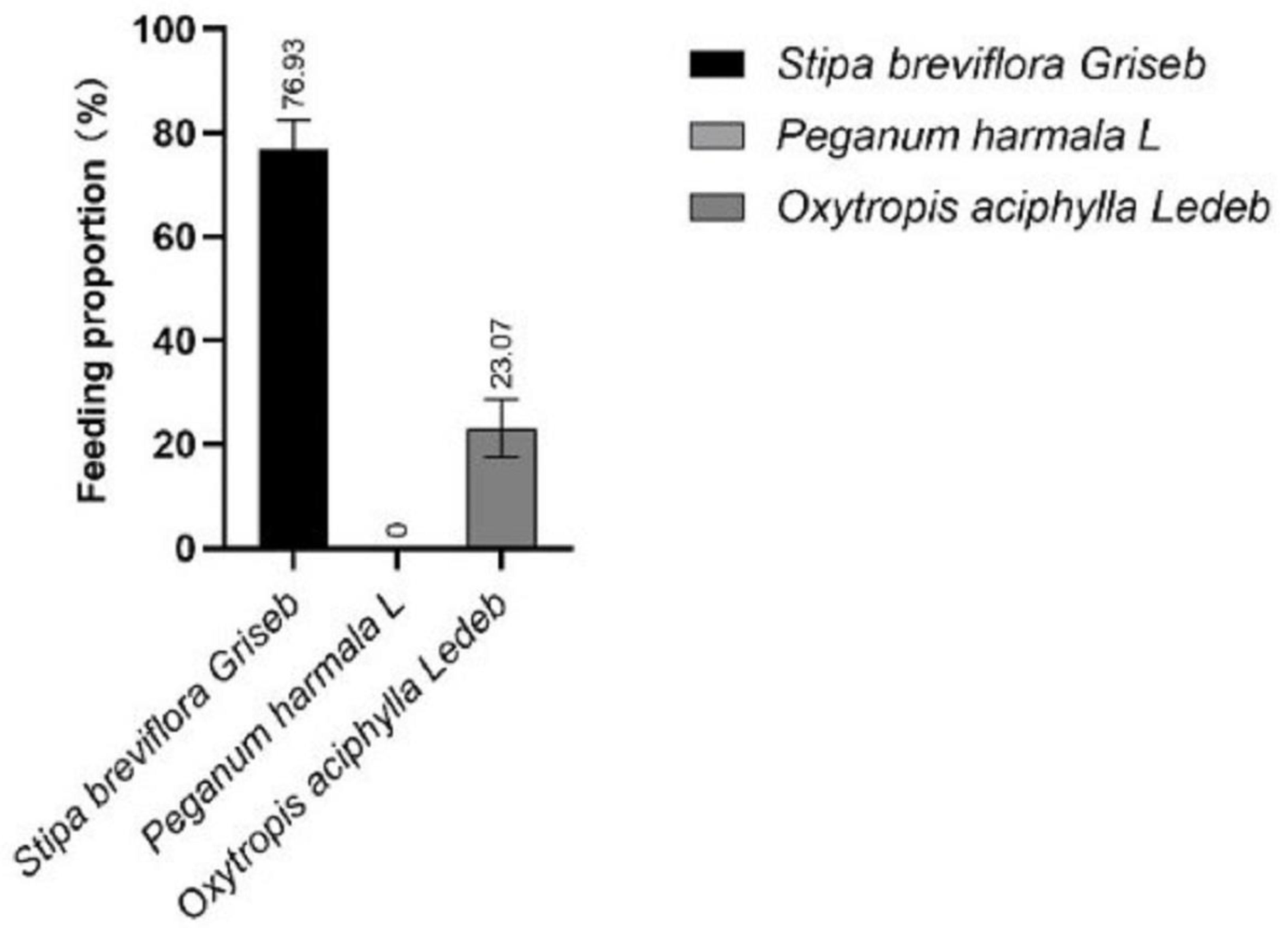
1. Introduction
Cashmere goats are mainly distributed in Central Asia and Mongolia, especially in Western China [
1]. Inner Mongolia cashmere goats are known worldwide for their high-quality cashmere fiber; moreover, they are also raised locally for meat and milk products [
2]. In traditional management, Inner Mongolia cashmere goats usually graze on a full grazing system with only forage as feed. It has been reported that the crude protein (CP) content of nature grasses decreases from 9.57%–21.26% to 2.58%–10.03% and neutral washing fiber (NDF) and acid detergent fiber (ADF) contents increase gradually from summer to the winter in the YiWei White Cashmere Goat Farm located in the Inner Mongolia Autonomous Region [
3]. Moreover, the mineral element level in the soil–grazing–livestock ecosystem of the
Stipa breviflora steppe located in the Inner Mongolia Siziwangqi Region is high in Fe, Mn and Ca, low in P, Na, K and Se, and seasonally deficient in Cu, Zn and S [
4,
5]. The nutritional value of natural grasslands varies seasonally, and the pasture nutritional value may not meet the needs of pregnant ruminants in the dry and autumn winter season [
6,
7,
8]. It has been shown that the energy or protein deficiency of pregnant sheep or goats can inhibit the development of fetal renal vasculature and renal units, resulting in an imbalance in superoxide dismutase, hydrogen peroxide inactivation systems in the thymus, injury of the jejunum, antioxidant capacity, and immune responses of newborn lambs or kids [
9,
10,
11]. A study has shown that in an extensive farming system, electrolyte levels of female goats display different concentrations in the bloodstream according to physiological stage and kid numbers, and it is necessary to consider these differences in actual feed and management [
12]. The mineral element deficiency decreases production performance, resulting in associated illnesses, even death, especially Cu, Zn and S, which are particularly important for wool-bearing animals; their deficiency can affect fur color and traits; wool yield, elasticity and strength; and animal reproductive performance, etc. [
13,
14].
Not only the Inner Mongolia region, but also many other parts of the world are faced with low pasture nutritional value and limits for grazing animal growth. In the natural grassland of Dangxiong, Tibet, the nutritional value of forage grasses during the spring re-greening and summer vigorous periods is significantly higher than that during the autumn and winter dry periods, and the intake of Ca and P by grazing pregnant ewes is insufficient in alpine meadows during the winter dry period and in river meadows during the summer peak period [
15]. In Ethiopia and the semi-arid regions of northeastern Brazil, the quantity and quality of natural pasture resources limit the production of grazing goats, and supplemental feeding is often used as an effective measure to improve the growth performance and production performance of goats [
16,
17]. Hence, supplemental feeding is necessary for grazing pregnant goats in the dry and autumn winter season [
18].
However, before the promulgation of “Nutrient Requirements of Cashmere Goats”, supplemental feeding in actual production was mainly based on NRC (1981), AFRC (1998) and experience, which were not suitable for the actual production of cashmere goats [
19]. It has been reported that 300 g/day of corn is the only feedstuff used as supplementary feeding for castrated goat kids and female kids in the YiWei White Cashmere Goat Farm located in the main producing area of Inner Mongolia cashmere goats [
3]. Similarly, McGregor et al. provided 300 g/day grain supplements for cashmere goats, which significantly increased the total cashmere diameter (2.12 µm) and made cashmere lose its economic value [
20]. It remains unclear how to supplement feeding pregnant cashmere goats scientifically and precisely under pasture with a poor nutrient content. “Nutrient Requirements of Cashmere Goats (NY/T4048–2021)”, China’s and the world’s first cashmere goat feeding standards, was published in 2021 [
21], which provides a nutrition reference for the accurate supplementary feeding of pregnant cashmere goats. Hence, in this study, “Nutrient Requirements of Cashmere Goats” was used as a guide to provide supplement diets for goats during early and late gestation. Comparing relevant indicators with on-farm supplementation, the aim of this study was to investigate the effectiveness of supplementary feeding based on the feeding standard for Inner Mongolian Cashmere Goats during pregnancy and provide a theoretical and data basis for the precise feeding of cashmere goats in other physiological stages. This will give full play to the advantages of germplasm resources of cashmere goats and guide and promote the high-quality and sustainable development of the cashmere goat industry.
4. Discussion
This study indicated that, compared with that in the “Nutrient Requirements of Cashmere Goats”, the daily intake of DMI, DE, CP, P, Cu, Zn, K, Mg, Se and S of goats in early gestation under grazing conditions was not enough, and the goats in late gestation lacked Ca on this basis. The forage DMI at early gestation, 0.85 kg, in this study was similar to 0.83 kg for Inner Mongolia cashmere goats during the fattening period determined by Wenqi; both studies were carried out using the saturated alkane method [
3]. In late gestation, the decrease in forage DMI may be caused by the decrease in forage palatability and increase in the NDF content of the pasture [
33]. Similarly, Xinjiang fine-wool sheep has been reported in which intakes of ME and CP were 5.15 MJ/day·ewe and 85.8 g/day·ewe lower than the nutrient requirements of sheep under winter grazing conditions, respectively [
34]. As mentioned above, the mineral element level in the soil–grazing–livestock ecosystem of the
Stipa breviflora steppe located in the Inner Mongolia Siziwangqi Region was high in Fe, Mn and Ca, low in P, Na, K and Se, and seasonally deficient in Cu, Zn and S. Moreover, the result of the mineral element surplus and deficit in this study was similar with that in [
4,
5], and the results in our study are similar, except that cashmere goats were deficient in Ca intake in late pregnancy. On the one hand, the experimental animals in the two studies were different, and on the other hand, the Ca requirement of cashmere goats became greater into late gestation, and the Ca content in the pasture decreased. In addition, the goats’ intake ratio of Ca and P in this study approached to 7:1, which reduced P absorption, further reducing the absorption of P and Ca [
35]. The above results also show that it is necessary to supplement the cashmere goats in this study.
Cashmere or wool yield can be enhanced by supplementing with energy and protein [
36,
37,
38]. Mineral elements are related to the cashmere yield, Zn showed a significant positive correlation with the cashmere percentage [
39] and supplementation with 25 mg/kg DM Cu from copper sulfate or copper methionine can enhance cashmere production [
40]. Cashmere length and cashmere fineness are relevant to energy, CP and mineral elements, while too much energy makes the cashmere get thicker and lose economic value [
41]. Protein has no effect on cashmere fineness when protein levels meet the maintenance needs of goats [
42]. Usually, cashmere fibers reached their finest at medium protein and low energy levels [
43]. It was reported that supplementation with 20 mg/kg DM (total dietary Cu level of 25.6 mg/kg DM) can promote cashmere growth [
44]. However, in this study, the cashmere length increased and the lower cashmere fineness were not statistically significant. A similar result has been reported; under grazing conditions, pregnant Inner Mongolia cashmere goats were supplemented with 9.73 MJ/kg DE + 9.9% CP in the early stage (1st December–1st February) and with 9.46 MJ/kg DE + 9.52% CP in the later period (1st February–31st March), which significantly increased the cashmere yield of goats, but had no statistical effect on cashmere length and cashmere fineness [
45]. This is perhaps because the supplementary feeding experiment was performed in a non-increasing period and the secondary hair follicles of Inner Mongolian cashmere goats were in a state of relative ‘rest’ (telogen) during this period (December to March), when the hair shaft gradually stopped growing and shed [
46]. These results indicate that cashmere production performance can be enhanced by the supplementary feeding of pregnant cashmere goats according to the feeding standard, and compared with on-farm supplementary, a supplementary feeding scheme is reasonable.
In Liaoning cashmere goats, studies showed that growth traits are closely related to the cashmere yield, and body weight had the greatest direct effect on the cashmere yield [
47]. Similarly, Rayeni cashmere goat total fleece weight increased by 45 g and post-weight after shearing was enhanced by 1.5 g after supplementation with a 10.12 MJ/kg diet during pregnancy and lactation periods [
48]. It was reported that there is a significant effect of ewe weight on lamb weight [
49], and the single and twin-birth kid weight was also increased due to supplementation in this study. Similarly, Ewes’ liveweight gain significantly increased by 134 g/day and lamb birth weights were significantly enhanced by 1 kg, with a supplement of 150 g cottonseed meal + 50 g molasses daily for medium-wooled Peppin Merino ewes during late gestation (last 4 weeks) [
50]. Palm kernel cake (PKC) provides a protein source in a supplement (comprising 35% crushed maize, 30% rice bran, 32% PKC, 2% vitamin mineral premix and 1% salt), and supplementation at 0.5% of the live weight significantly enhanced the weight by 0.93 kg after 82 days of the feeding trial for Boer × local female goats (12.4 ± 2.6 kg, 7–9 months) [
51]. In addition, supplementation of ewes with Zn, Se and Co in late gestation improved the mineral status of ewes and their kids before weaning and increased lamb weights [
52,
53]. These results suggested that supplementation based on the standard can enhance pregnant goats’ growth performance and fertility. Studies showed that there is a positive relationship between the lamb birth weight and lamb growth rate [
54], and we speculate that supplementation may have a sustained impact on the growth performance of offspring kids, which requires continuous follow-up to verify.
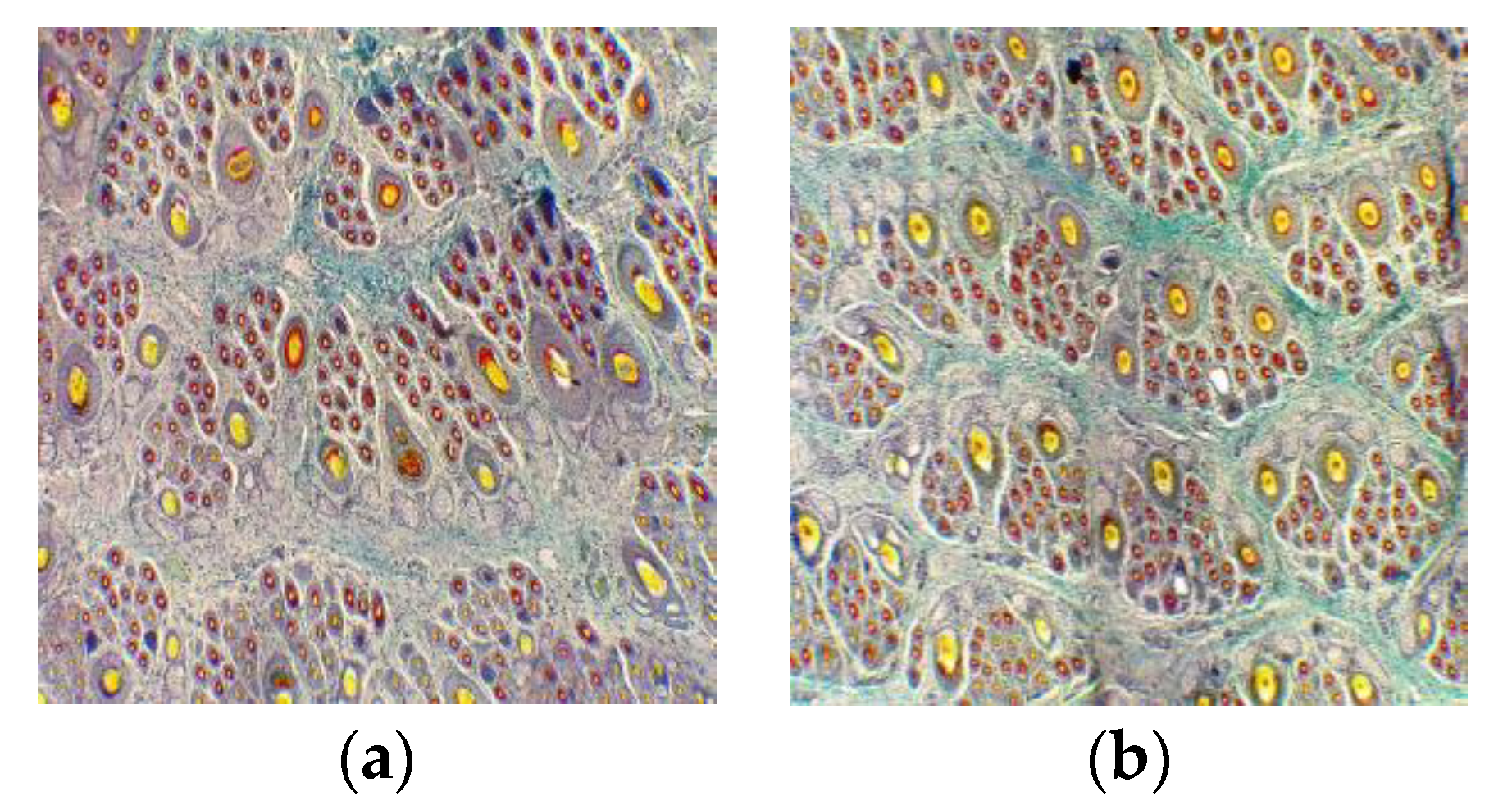
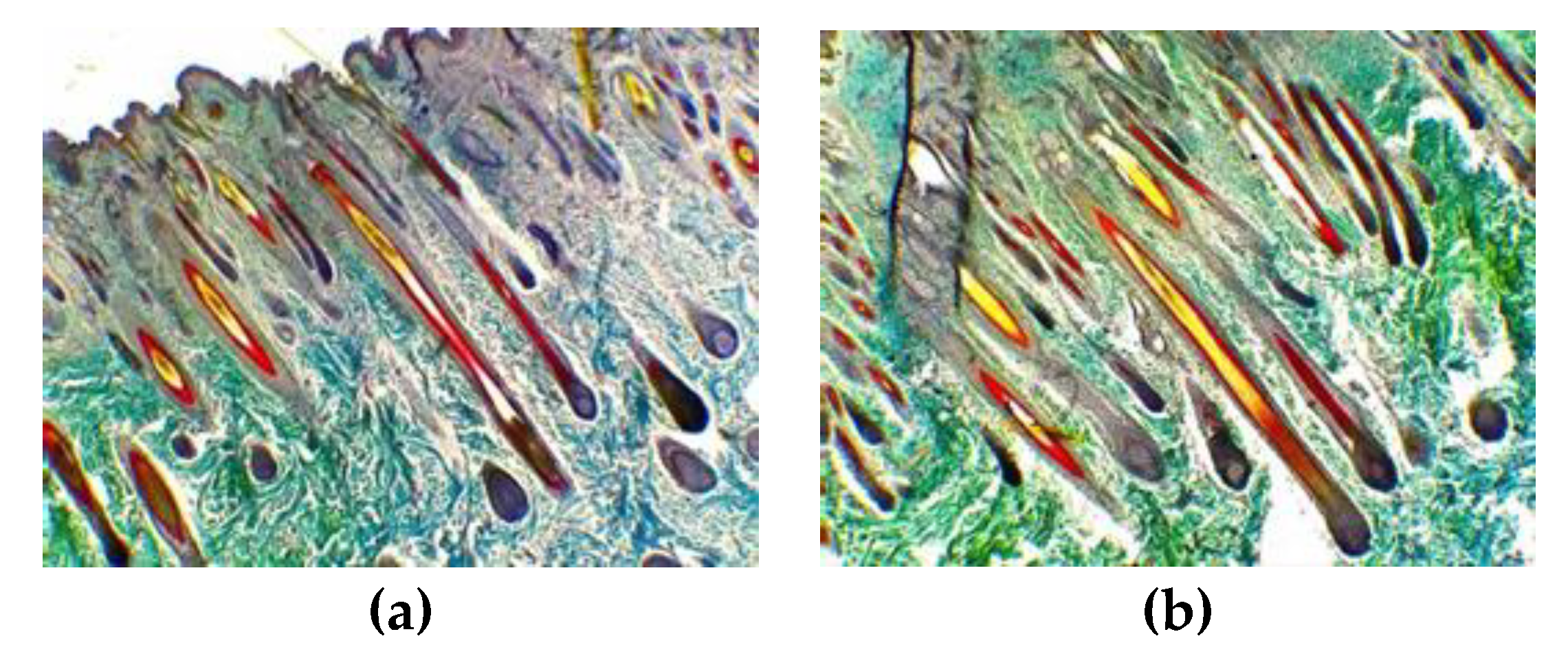
Supplementation based on the standard for pregnant cashmere goats enhanced the mature SF density of kids in this study. Similarly, kids’ SF density can be increased significantly at 12.30 and 28.09 n/mm
2 with supplements of 5 g urea + 7.5 g Na
2SO
4 and 250 g corn in cashmere goats during mid and late gestation, respectively [
55]. It was reported that supplementation with Nano-Selenium (declared Se content 0.5 mg/kg DM daily) for cashmere goats during pregnancy can to promote the development and growth of fetal hair follicles [
56]. In Angora goats, supplements provided to goats in the middle gestation and lactation stage had a positive effect on the SF density and SF number in kids at all ages after birth, increasing the S:P at 4, 6 and 15 months of age [
57]. We speculate that supplementation may improve the hair follicle development of offspring lambs for a long time in this study, which requires continuous follow-up to verify.