Comparative Analyses Reveal the Genetic Mechanism of Ambergris Production in the Sperm Whale Based on the Chromosome-Level Genome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome Data Collection
2.2. Gene Family and Phylogenetic Tree Construction
2.3. Gene Family Expansion and Contraction
2.4. Positive Selection Analysis
2.5. Functional Enrichment Analysis
2.6. Identification and Chromosomal Distribution of CYPs and HSDs
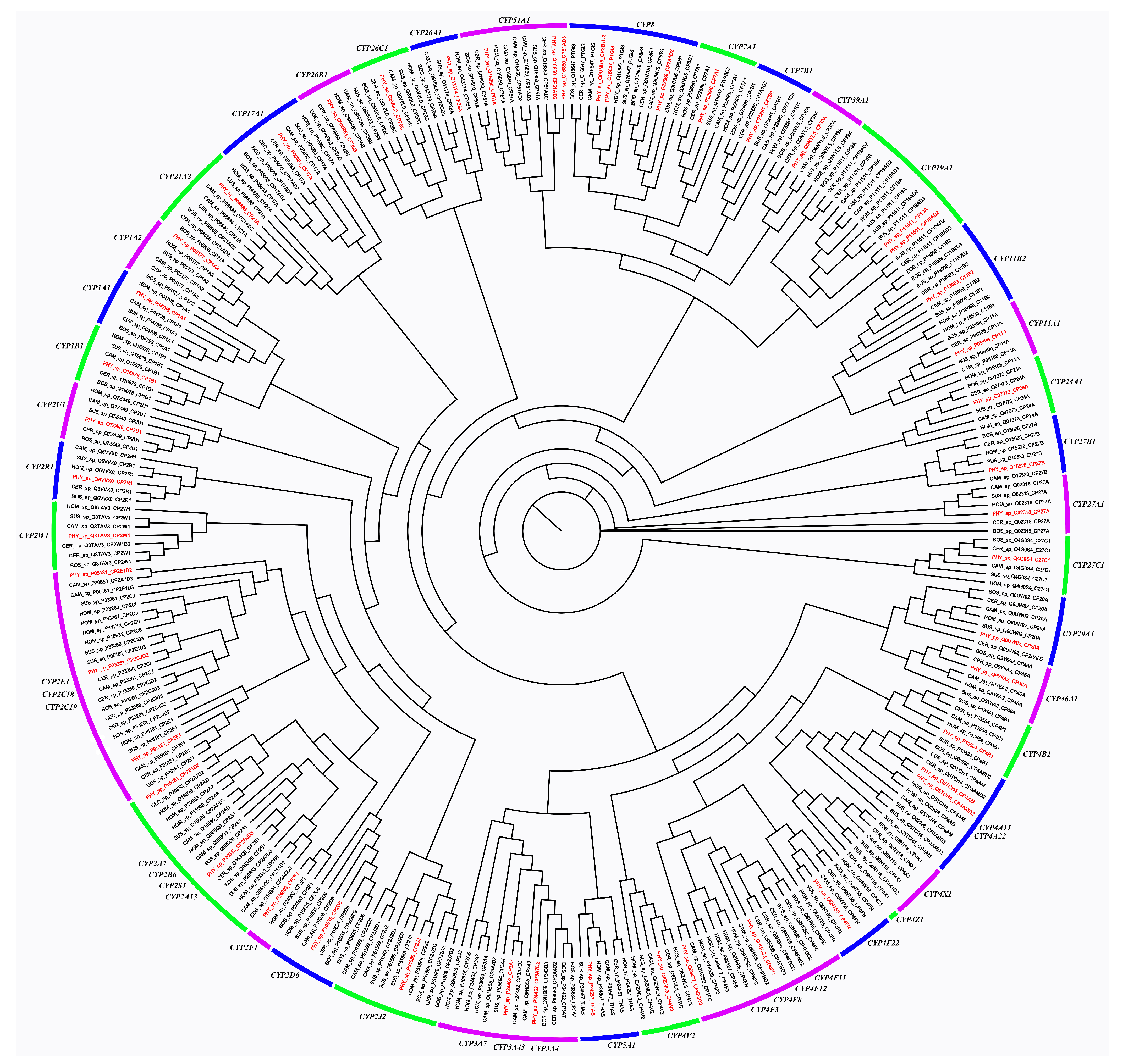
2.7. Phylogenetic Analysis of CYPs
3. Results
3.1. Phylogenetic Analysis and Divergence Time Estimation
3.2. Gene Family Expansion Related to Ambergris Production
3.3. Positive Selection and Functional Enrichment
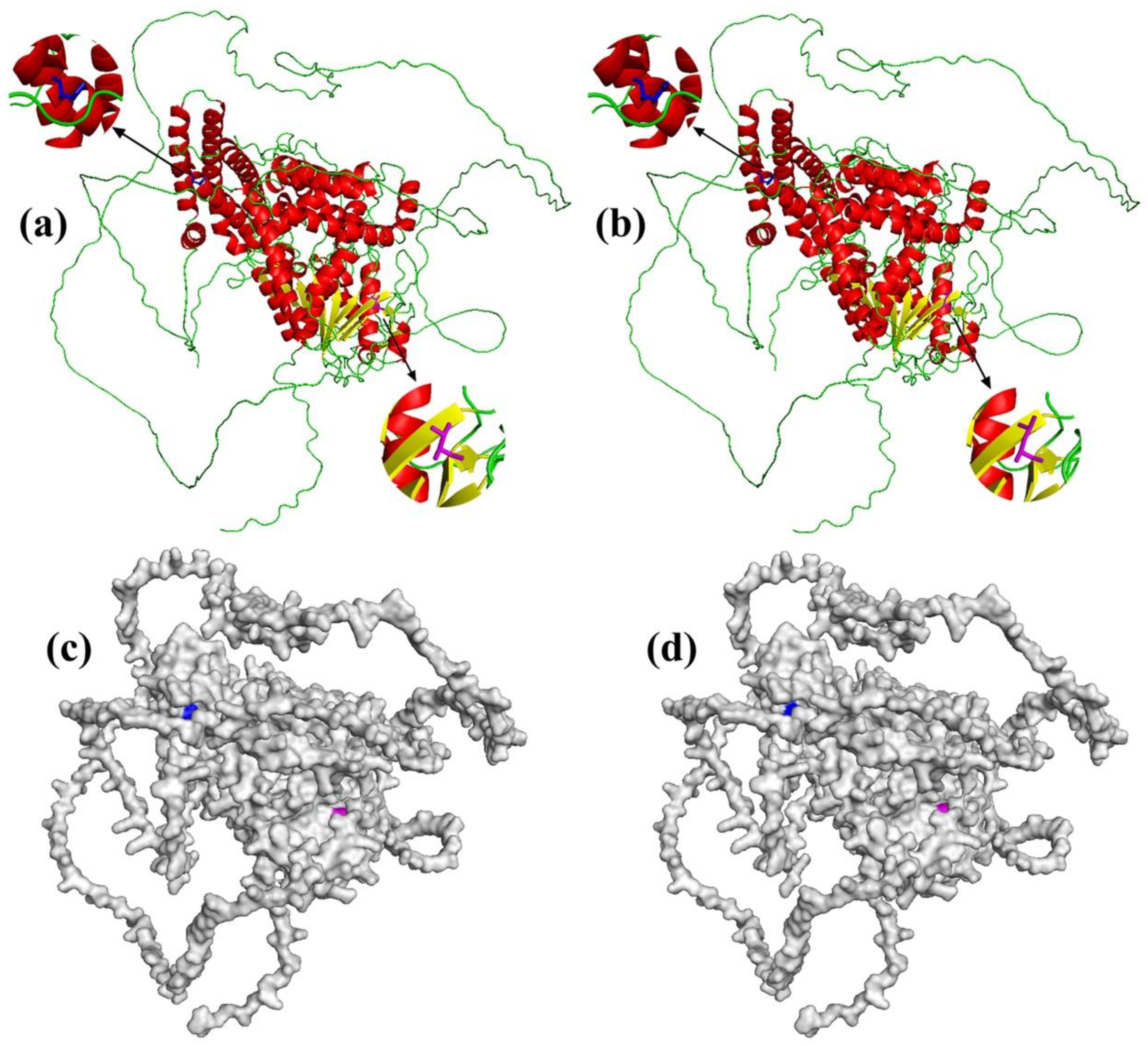
3.4. Sperm Whale-Specific Missense Mutations
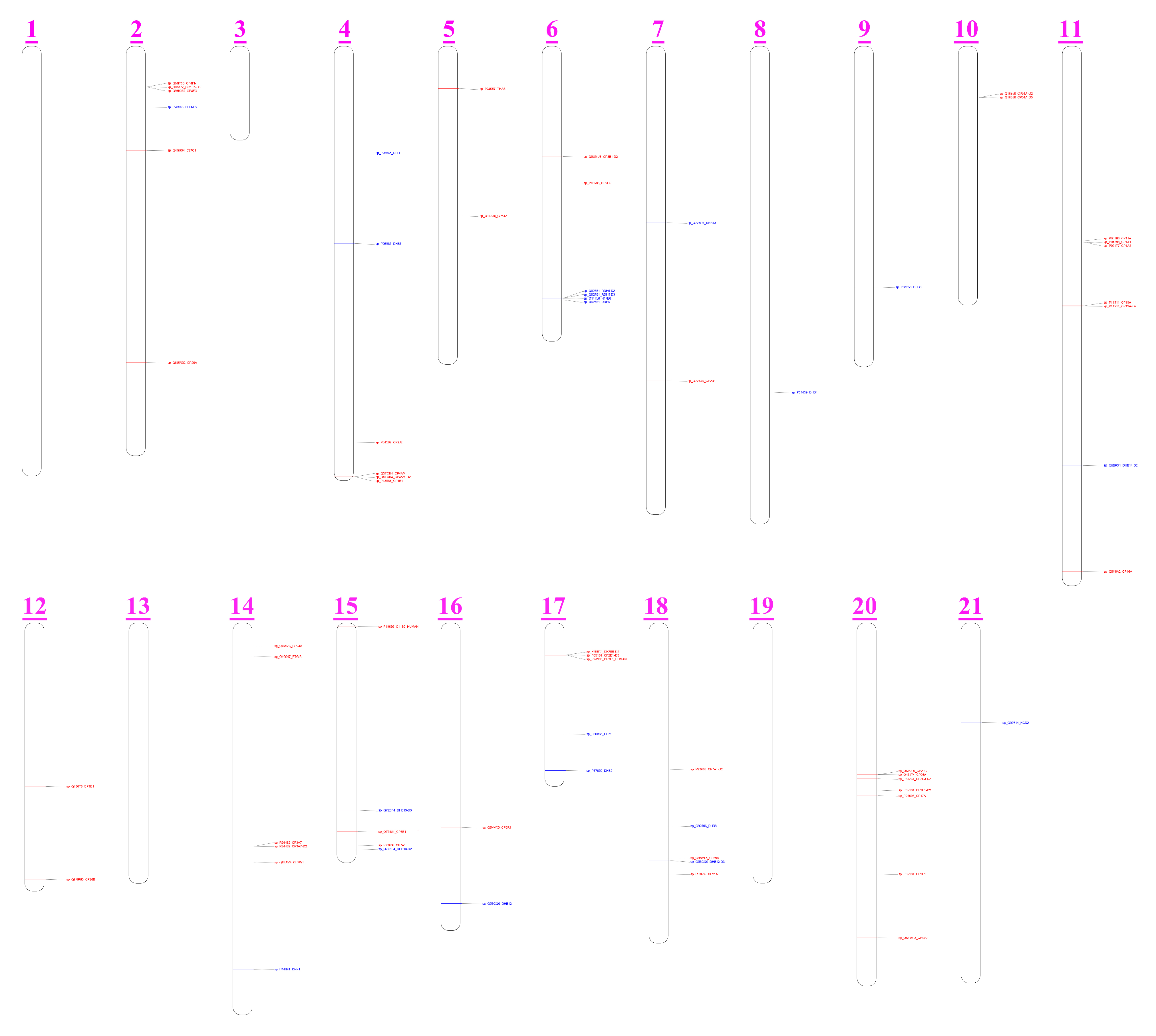
3.5. CYPs and HSDs
4. Discussion
4.1. Steroid and Terpenoid Metabolism in Sperm Whale
4.2. Aldosterone Synthesis and Secretion in Sperm Whale
4.3. Adaptation of Commensal Gut Microbiota and Intestinal Immune Network
4.4. Evolution and Adaptation of CYP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitehead, H. Sperm Whale: Physeter macrocephalus. In Encyclopedia of Marine Mammals; Academic Press: Cambridge, MA, USA, 2018; pp. 919–925. [Google Scholar]
- Jaquet, N.; Whitehead, H. Scale-Dependent Correlation of Sperm Whale Distribution with Environmental Features and Productivity in the South Pacific. Mar. Ecol. Prog. Ser. 1996, 135, 1–9. [Google Scholar] [CrossRef]
- Kawakami, T. A Review of Sperm Whale Food. Sci. Rep. Whales Res. Inst. 1980, 32, 199–218. [Google Scholar]
- Clarke, M.R.; Martins, H.R.; Pascoe, P. The Diet of Sperm Whales (Physeter macrocephalus Linnaeus 1758) off the Azores. Philos. Trans. R. Soc. B Biol. Sci. 1993, 339, 67–82. [Google Scholar] [CrossRef]
- Taylor, B.L.; Baird, R.; Barlow, J.; Dawson, S.M.; Ford, J.; Mead, J.G.; Notarbartolo di Sciara, G.; Wade, P.; Pitman, R.L. Physeter macrocephalus, Sperm Whale (Amended Version). The IUCN Red List of Threatened Species. 2019. Available online: https://www.iucnredlist.org/species/41755/10554884 (accessed on 1 April 2022).
- Brito, C.; Jordão, V.L.; Pierce, G.J. Ambergris as an Overlooked Historical Marine Resource: Its Biology and Role as a Global Economic Commodity. J. Mar. Biol. Assoc. U. K. 2016, 96, 585–596. [Google Scholar] [CrossRef]
- Britannica, T.E. Ambergris. Available online: https://www.britannica.com/science/ambergris (accessed on 1 April 2022).
- Clarke, R. The Origin of Ambergris. Lat. Am. J. Aquat. Mamm. 2006, 5, 7–21. [Google Scholar] [CrossRef]
- Macleod, R.; Sinding, M.H.S.; Olsen, M.T.; Collins, M.J.; Rowland, S.J. DNA Preserved in Jetsam Whale Ambergris. Biol. Lett. 2020, 16, 20190819. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A. Chromatographic and Spectral Studies of Jetsam and Archived Ambergris. Nat. Prod. Res. 2017, 31, 1752–1757. [Google Scholar] [CrossRef]
- Ueda, D.; Hoshino, T.; Sato, T. Cyclization of Squalene from Both Termini: Identification of an Onoceroid Synthase and Enzymatic Synthesis of Ambrein. J. Am. Chem. Soc. 2013, 135, 18335–18338. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A.; Wolff, G.A. Biosynthesis of Ambrein in Ambergris: Evidence from Isotopic Data and Identification of Possible Intermediates. Nat. Prod. Res. 2019, 35, 1235–1241. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A.; von der Lühe, B.; Volkman, J.K.; Vane, C.H.; Ingram, S.N.; Dunn, C.; Claridge, D. Ambrein: A Minor, but Common Constituent of Mammalian Faeces? Nat. Prod. Res. 2021, 35, 4843–4848. [Google Scholar] [CrossRef]
- Rowland, S.J.; Sutton, P.A.; Belt, S.T.; Fitzsimmons-Thoss, V.; Scarlett, A.G. Further Spectral and Chromatographic Studies of Ambergris. Nat. Prod. Res. 2018, 32, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.W. Ambergris. In Encyclopedia of Marine Mammals, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 28–29. ISBN 9780123735539. [Google Scholar]
- Lambertsen, R.H.; Kohn, B.A. Unusual Multisystemic Pathology in a Sperm Whale Bull. J. Wildl. Dis. 1987, 23, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, T.M. Historical Note: Ambergris in Perfumery in the Past and Present Indian Context and the Western World. Indian J. Hist. Sci. 2015, 50, 306–323. [Google Scholar] [CrossRef]
- Li, C.; Tan, X.; Bai, J.; Xu, Q.; Liu, S.; Guo, W.; Yu, C.; Fan, G.; Lu, Y.; Zhang, H.; et al. A Survey of the Sperm Whale (Physeter Catodon) Commensal Microbiome. PeerJ 2019, 7, e7257. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.C.; Kuderna, L.; Alexander, A.; Catchen, J.; Pérez-Silva, J.G.; López-Otín, C.; Quesada, V.; Minx, P.; Tomlinson, C.; Montague, M.J.; et al. The Novel Evolution of the Sperm Whale Genome. Genome Biol. Evol. 2017, 9, 3260–3264. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, Y.; Liu, X.; Wang, J.; Sun, Z.; Sun, S.; Zhang, H.; Chen, J.; Lv, M.; Han, K.; et al. The First Chromosome-Level Genome for a Marine Mammal as a Resource to Study Ecology and Evolution. Mol. Ecol. Resour. 2019, 19, 944–956. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust Conversion of Protein Sequence Alignments into the Corresponding Codon Alignments. Nucleic Acids Res. 2006, 34, 609–612. [Google Scholar] [CrossRef]
- Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- De Bie, T.; Cristianini, N.; Demuth, J.P.; Hahn, M.W. CAFE: A Computational Tool for the Study of Gene Family Evolution. Bioinformatics 2006, 22, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A Web Server for Annotation and Identification of Enriched Pathways and Diseases. Nucleic Acids Res. 2011, 39, 316–322. [Google Scholar] [CrossRef]
- Ip, E.; Molenberghs, G. Empirical Bayes Methods. In International Encyclopedia of Education; Elsevier: Oxford, UK, 2010; pp. 142–149. [Google Scholar]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Tripathi, S.; Pohl, M.O.; Zhou, Y.; Rodriguez-Frandsen, A.; Wang, G.; Stein, D.A.; Moulton, H.M.; DeJesus, P.; Che, J.; Mulder, L.C.; et al. Meta- and Orthogonal Integration of Influenza “OMICs” Data Defines a Role for UBR4 in Virus Budding. Cell Host Microbe 2015, 18, 723–735. [Google Scholar] [CrossRef]
- Kent, W.J. BLAT—The BLAST-Like Alignment Tool. Genome Res. 2002, 12, 656–664. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. TrimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-Hit: A Fast Program for Clustering and Comparing Large Sets of Protein or Nucleotide Sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.W.; De Bie, T.; Stajich, J.E.; Nguyen, C.; Cristianini, N. Estimating the Tempo and Mode of Gene Family Evolution from Comparative Genomic Data. Genome Res. 2005, 15, 1153–1160. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Shen, W.J. Hormone-Sensitive Lipase: Control of Intracellular Tri-(Di-)Acylglycerol and Cholesteryl Ester Hydrolysis. J. Lipid Res. 2002, 43, 1585–1594. [Google Scholar] [CrossRef]
- Holm, C.; Kirchgessner, T.; Svenson, K.; Fredrikson, G.; Nilsson, S.; Miller, C.; Shively, J.; Heinzmann, C.; Sparkes, R.; Mohandas, T.; et al. Hormone-Sensitive Lipase: Sequence, Expression, and Localizaton. Science 1988, 241, 1503–1506. [Google Scholar] [CrossRef]
- Grober, J.; Lucas, S.; Sörhede-Winzell, M.; Zaghini, I.; Mairal, A.; Contreras, J.A.; Besnard, P.; Holm, C.; Langin, D. Hormone-Sensitive Lipase Is a Cholesterol Esterase of the Intestinal Mucosa. J. Biol. Chem. 2003, 278, 6510–6515. [Google Scholar] [CrossRef]
- Obrowsky, S.; Chandak, P.G.; Patankar, J.V.; Pfeifer, T.; Povoden, S.; Schreiber, R.; Haemmerle, G.; Levak-Frank, S.; Kratky, D. Cholesteryl Ester Accumulation and Accelerated Cholesterol Absorption in Intestine-Specific Hormone Sensitive Lipase-Null Mice. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2012, 1821, 1406–1414. [Google Scholar] [CrossRef]
- Baker, M.E. Origin and Diversification of Steroids: Co-Evolution of Enzymes and Nuclear Receptors. Mol. Cell. Endocrinol. 2011, 334, 14–20. [Google Scholar] [CrossRef]
- Nelson, D.R.; Koymans, L.; Kamataki, T.; Stegeman, J.J.; Feyereisen, R.; Waxman, D.J.; Waterman, M.R.; Gotoh, O.; Coon, M.J.; Estabrook, R.W.; et al. P450 Superfamily: Update on New Sequences, Gene Mapping, Accession Numbers and Nomenclature. Pharmacogenetics 1996, 6, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A. Cytochrome P450s and Cholesterol Homeostasis. Pharmacol. Ther. 2006, 112, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Molecular Endocrinology of Hydroxysteroid Dehydrogenases. Endocr. Rev. 1997, 18, 281–305. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef]
- Machalz, D.; Pach, S.; Bermudez, M.; Bureik, M.; Wolber, G. Structural Insights into Understudied Human Cytochrome P450 Enzymes. Drug Discov. Today 2021, 26, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Read, S. Ambergris and Early Modern Languages of Scent. Seventeenth Century 2013, 28, 221–237. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Zhang, L.; Gaur, U.; Ma, T.; Jie, H.; Zhao, G.; Wu, N.; Xu, Z.; Xu, H.; et al. The Musk Chemical Composition and Microbiota of Chinese Forest Musk Deer Males. Sci. Rep. 2016, 6, 18975. [Google Scholar] [CrossRef]
- Huff, M.W.; Telford, D.E. Lord of the Rings—The Mechanism for Oxidosqualene:Lanosterol Cyclase Becomes Crystal Clear. Trends Pharmacol. Sci. 2005, 26, 333–335. [Google Scholar] [CrossRef]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K.; et al. Lanosterol Synthase Mutations Cause Cholesterol Deficiency-Associated Cataracts in the Shumiya Cataract Rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef]
- Iatrino, R.; Lanzani, C.; Bignami, E.; Casamassima, N.; Citterio, L.; Meroni, R.; Zagato, L.; Zangrillo, A.; Alfieri, O.; Fontana, S.; et al. Lanosterol Synthase Genetic Variants, Endogenous Ouabain, and Both Acute and Chronic Kidney Injury. Am. J. Kidney Dis. 2019, 73, 504–512. [Google Scholar] [CrossRef]
- Romano, M.T.; Tafazzoli, A.; Mattern, M.; Sivalingam, S.; Wolf, S.; Rupp, A.; Thiele, H.; Altmüller, J.; Nürnberg, P.; Ellwanger, J.; et al. Bi-Allelic Mutations in LSS, Encoding Lanosterol Synthase, Cause Autosomal-Recessive Hypotrichosis Simplex. Am. J. Hum. Genet. 2018, 103, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-Sensitive Lipase: Sixty Years Later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, N.; Dong, L.; Zheng, X.; Zhang, Y.; Ding, C.; Zhao, S.; Ma, Z.; Wang, Y. A Novel Lipid Prognostic Signature of ADCY2, LIPE, and OLR1 in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 4916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, W.; Wen, Q.; Bu, P.; Gao, J.; Wang, G.; Jin, J.; Song, Y.; Sun, X.; Zhang, Y.; et al. Comparative Genomics Reveals the Genetic Mechanisms of Musk Secretion and Adaptive Immunity in Chinese Forest Musk Deer. Genome Biol. Evol. 2019, 11, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Dalai, M.; Su, R.; Lin, W.; Erdenedalai, M.; Luvsantseren, B.; Chimedtseren, C.; Wang, Z.; Hasi, S. Whole-Genome Sequencing of Wild Siberian Musk Deer (Moschus Moschiferus) Provides Insights into Its Genetic Features. BMC Genom. 2020, 21, 108. [Google Scholar] [CrossRef]
- Boon, W.C.; Coghlan, J.P.; Curnow, K.M.; McDougall, J.G. Aldosterone Secretion: A Molecular Perspective. Trends Endocrinol. Metab. TEM 1997, 8, 346–354. [Google Scholar] [CrossRef]
- Bollag, W.B. Regulation of Aldosterone Synthesis and Secretion. Compr. Physiol. 2014, 4, 1017–1055. [Google Scholar] [CrossRef]
- Qi, C.; Sorrentino, S.; Medalia, O.; Korkhov, V.M. The Structure of a Membrane Adenylyl Cyclase Bound to an Activated Stimulatory G Protein. Science 2019, 364, 389–394. [Google Scholar] [CrossRef]
- Qi, C.; Lavriha, P.; Mehta, V.; Khanppnavar, B.; Mohammed, I.; Li, Y.; Lazaratos, M.; Schaefer, J.V.; Dreier, B.; Plückthun, A.; et al. Structural Basis of Adenylyl Cyclase 9 Activation. Nat. Commun. 2022, 13, 1045. [Google Scholar] [CrossRef]
- Morth, J.P.; Poulsen, H.; Toustrup-Jensen, M.S.; Schack, V.R.; Egebjerg, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. The Structure of the Na+, K+-ATPase and Mapping of Isoform Differences and Disease-Related Mutations. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 217–227. [Google Scholar] [CrossRef]
- Brandt, P.; Ibrahim, E.; Bruns, G.A.P.; Neve, R.L. Determination of the Nucleotide Sequence and Chromosomal Localization of the ATP2B2 Gene Encoding Human Ca2+-Pumping ATPase Isoform PMCA2. Genomics 1992, 14, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Sarker, A.H.; Akiyama, K.; Ikeda, S.; Yao, M.; Tsutsui, K.; Shohmori, T.; Seki, S. Genomic Structure and Sequence of a Human Homologue (NTHL1/NTH1) of Escherichia coli Endonuclease III with Those of the Adjacent Parts of TSC2 and SLC9A3R2 Genes. Gene 1998, 222, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yeruva, S.; He, P.; Singh, A.K.; Zhang, H.; Chen, M.; Lamprecht, G.; de Jonge, H.R.; Tse, M.; Donowitz, M.; et al. Lysophosphatidic Acid Stimulates the Intestinal Brush Border Na+/H+ Exchanger 3 and Fluid Absorption via LPA5 and NHERF2. Gastroenterology 2010, 138, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Horna, P.; Shi, M.; Olteanu, H.; Johansson, U. Emerging Role of T-Cell Receptor Constant β Chain-1 (TRBC1) Expression in the Flow Cytometric Diagnosis of T-Cell Malignancies. Int. J. Mol. Sci. 2021, 22, 1817. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.F.; Styrishave, B.; Løbner-Olesen, A.; Andersen, O. Marine Invertebrate Cytochrome P450: Emerging Insights from Vertebrate and Insect Analogies. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2006, 143, 363–381. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 2E1 and Its Roles in Disease. Chem.-Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef]
- Hsu, M.H.; Savas, Ü.; Griffin, K.J.; Johnson, E.F. Human Cytochrome P450 Family 4 Enzymes: Function, Genetic Variation and Regulation. Drug Metab. Rev. 2007, 39, 515–538. [Google Scholar] [CrossRef]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14α-Demethylase Cytochrome P450 (CYP51), a P450 in All Biological Kingdoms. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 467–477. [Google Scholar] [CrossRef]
- Yoshida, Y.; Aoyama, Y.; Noshiro, M.; Gotoh, O. Sterol 14-Demethylase P450 (CYP51) Provides a Breakthrough for the Discussion on the Evolution of Cytochrome P450 Gene Superfamily. Biochem. Biophys. Res. Commun. 2000, 273, 799–804. [Google Scholar] [CrossRef]
- Waterman, M.R.; Lepesheva, G.I. Sterol 14α-Demethylase, an Abundant and Essential Mixed-Function Oxidase. Biochem. Biophys. Res. Commun. 2005, 338, 418–422. [Google Scholar] [CrossRef]
- Ishida, H.; Kuruta, Y.; Gotoh, O.; Yamashita, C.; Yoshida, Y.; Noshiro, M. Structure, Evolution, and Liver-Specific Expression of Sterol 12α-Hydroxylase P450 (CYP8B). J. Biochem. 1999, 126, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, C.; Cheng, J.; Krausz, K.W.; Li, T.; Ferrell, J.M.; Gonzalez, F.J.; Chiang, J.Y.L. Bile Acid Signaling in Lipid Metabolism: Metabolomic and Lipidomic Analysis of Lipid and Bile Acid Markers Linked to Anti-Obesity and Anti-Diabetes in Mice. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acids: Regulation of Synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Eggertsen, G.; Gåfvels, M.; Andersson, U.; Einarsson, C.; Björkhem, I.; Chiang, J.Y.L. Mechanisms of Cholesterol and Sterol Regulatory Element Binding Protein Regulation of the Sterol 12α-Hydroxylase Gene (CYP8B1). Biochem. Biophys. Res. Commun. 2004, 320, 1204–1210. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Kim, D.H.; Iwasaki, M. Role of Human Cytochrome P-450 IIE1 in the Oxidation of Many Low Molecular Weight Cancer Suspects. Chem. Res. Toxicol. 1991, 4, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Bulus, H.; Oguztuzun, S.; Güler Simsek, G.; Kilic, M.; Ada, A.O.; Göl, S.; Kocdogan, A.K.; Kaygın, P.; Bozer, B.; Iscan, M. Expression of CYP and GST in Human Normal and Colon Tumor Tissues. Biotech. Histochem. 2019, 94, 1–9. [Google Scholar] [CrossRef]
- Güler Şimşek, G.; Oguztuzun, S.; Bozer, B.; Kilic, M.; Kocdogan, A.K.; Kaygin, P.; Gurbuz, N.; Bulus, H. Expressions of CYP and GST Isoenzymes in Human Gastric Tumor and Non-Tumor Tissues. UHOD-Uluslar. Hematol.-Onkol. Derg. 2018, 28, 36–44. [Google Scholar] [CrossRef]
- Moses, S.K.; Harley, J.R.; Lieske, C.L.; Muir, D.C.G.; Whiting, A.V.; O’Hara, T.M. Variation in Bioaccumulation of Persistent Organic Pollutants Based on Octanol-Air Partitioning: Influence of Respiratory Elimination in Marine Species. Mar. Pollut. Bull. 2015, 100, 122–127. [Google Scholar] [CrossRef]
- Wolkers, J.; Burkow, I.C.; Monshouwer, M.; Lydersen, C.; Dahle, S.; Witkamp, R.F. Cytochrome P450-Mediated Enzyme Activities and Polychlorinated Biphenyl Accumulation in Harp Seal (Phoca Groenlandica). Mar. Environ. Res. 1999, 48, 59–72. [Google Scholar] [CrossRef]
- Hooker, S.K.; Metcalfe, T.L.; Metcalfe, C.D.; Angell, C.M.; Wilson, J.Y.; Moore, M.J.; Whitehead, H. Changes in Persistent Contaminant Concentration and CYP1A1 Protein Expression in Biopsy Samples from Northern Bottlenose Whales, Hyperoodon Ampullatus, Following the Onset of Nearby Oil and Gas Development. Environ. Pollut. 2008, 152, 205–216. [Google Scholar] [CrossRef]
- Bandiera, S.M.; Torok, S.M.; Lin, S.; Ramsay, M.A.; Norstrom, R.J. Catalytic and Immunologic Characterization of Hepatic and Lung Cytochromes P450 in the Polar Bear. Biochem. Pharmacol. 1995, 49, 1135–1146. [Google Scholar] [CrossRef] [PubMed]

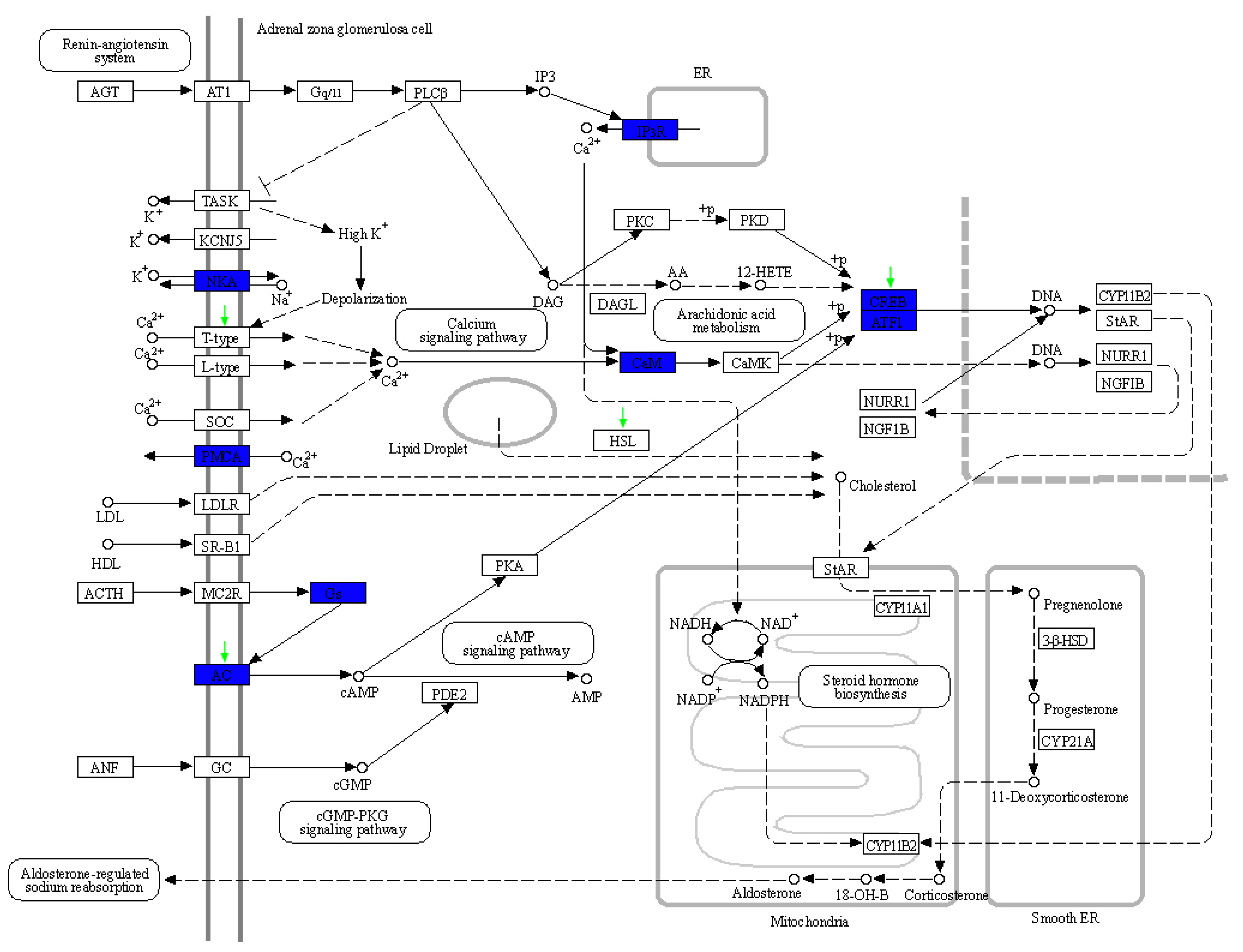
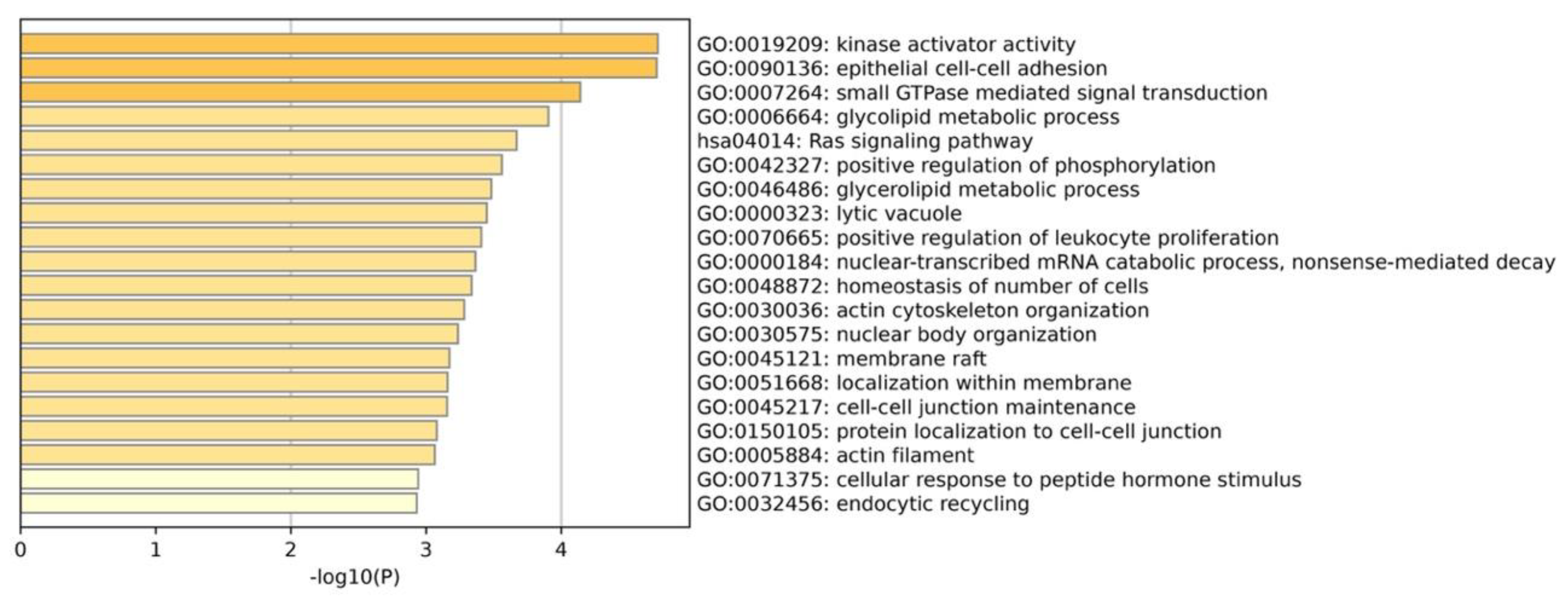


| KEGG Pathway | Map ID | Number of PSGs | Positively Selected Genes |
|---|---|---|---|
| Steroid biosynthesis | map00100 | 1 | LSS |
| Aldosterone synthesis and secretion | map04925 | 4 | LIPE |
| ADCY2 | |||
| ATF6B | |||
| CACNA1I | |||
| Aldosterone-regulated sodium reabsorption | map04960 | 1 | SLC9A3R2 |
| Gene | Mutation Sites (Human) | Amino Acids (Human/Sperm Whale) | Polarity | PolyPhen-2 | |
|---|---|---|---|---|---|
| HumDiv | HumVar | ||||
| LIPE | 393 | Y(Tyr)/H(His) | polar/polar | 0.014 (benign) | 0.028 (benign) |
| 567 | L(Leu)/V(Val) | unpolar/unpolar | 0.999 (probably damaging) | 0.994 (probably damaging) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Peng, K.; Liu, Y.; Zhang, R.; Zheng, X.; Yue, B.; Du, C.; Wu, Y. Comparative Analyses Reveal the Genetic Mechanism of Ambergris Production in the Sperm Whale Based on the Chromosome-Level Genome. Animals 2023, 13, 361. https://doi.org/10.3390/ani13030361
Zhou C, Peng K, Liu Y, Zhang R, Zheng X, Yue B, Du C, Wu Y. Comparative Analyses Reveal the Genetic Mechanism of Ambergris Production in the Sperm Whale Based on the Chromosome-Level Genome. Animals. 2023; 13(3):361. https://doi.org/10.3390/ani13030361
Chicago/Turabian StyleZhou, Chuang, Kexin Peng, Yi Liu, Rusong Zhang, Xiaofeng Zheng, Bisong Yue, Chao Du, and Yongjie Wu. 2023. "Comparative Analyses Reveal the Genetic Mechanism of Ambergris Production in the Sperm Whale Based on the Chromosome-Level Genome" Animals 13, no. 3: 361. https://doi.org/10.3390/ani13030361
APA StyleZhou, C., Peng, K., Liu, Y., Zhang, R., Zheng, X., Yue, B., Du, C., & Wu, Y. (2023). Comparative Analyses Reveal the Genetic Mechanism of Ambergris Production in the Sperm Whale Based on the Chromosome-Level Genome. Animals, 13(3), 361. https://doi.org/10.3390/ani13030361






