Consumption of Rodenticide Baits by Invertebrates as a Potential Route into the Diet of Insectivores
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Pilot Study
2.2. Field Exposure Study
2.3. Statistical Analysis
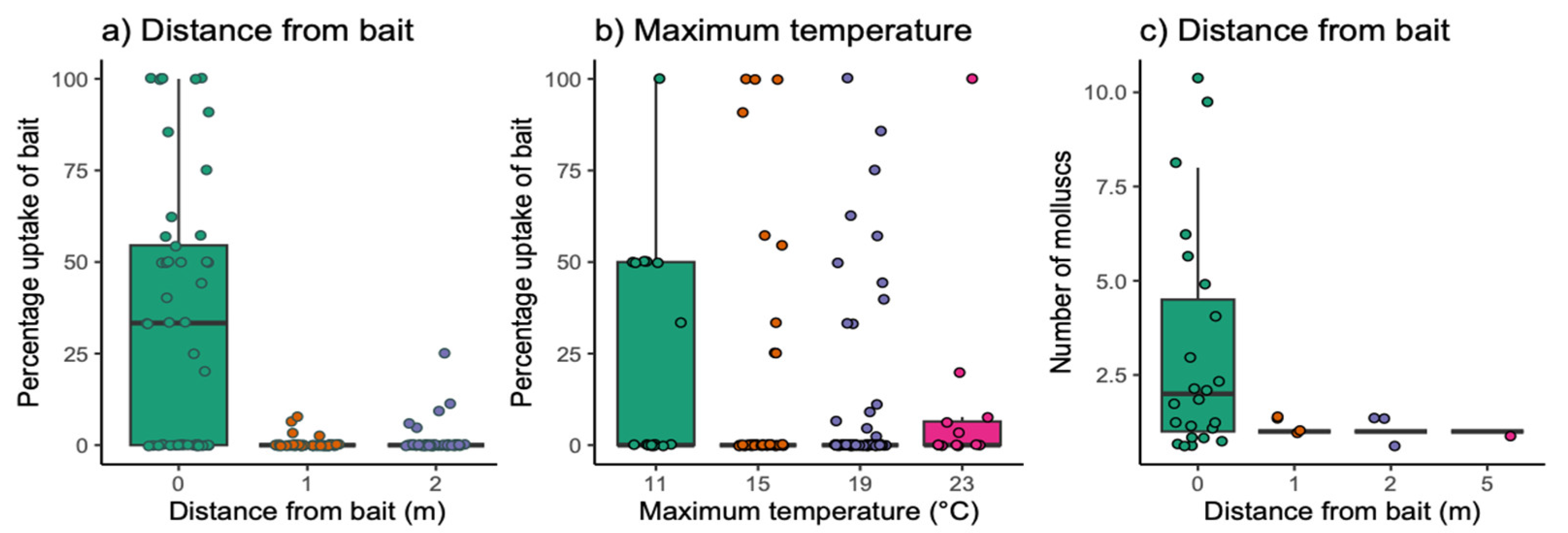
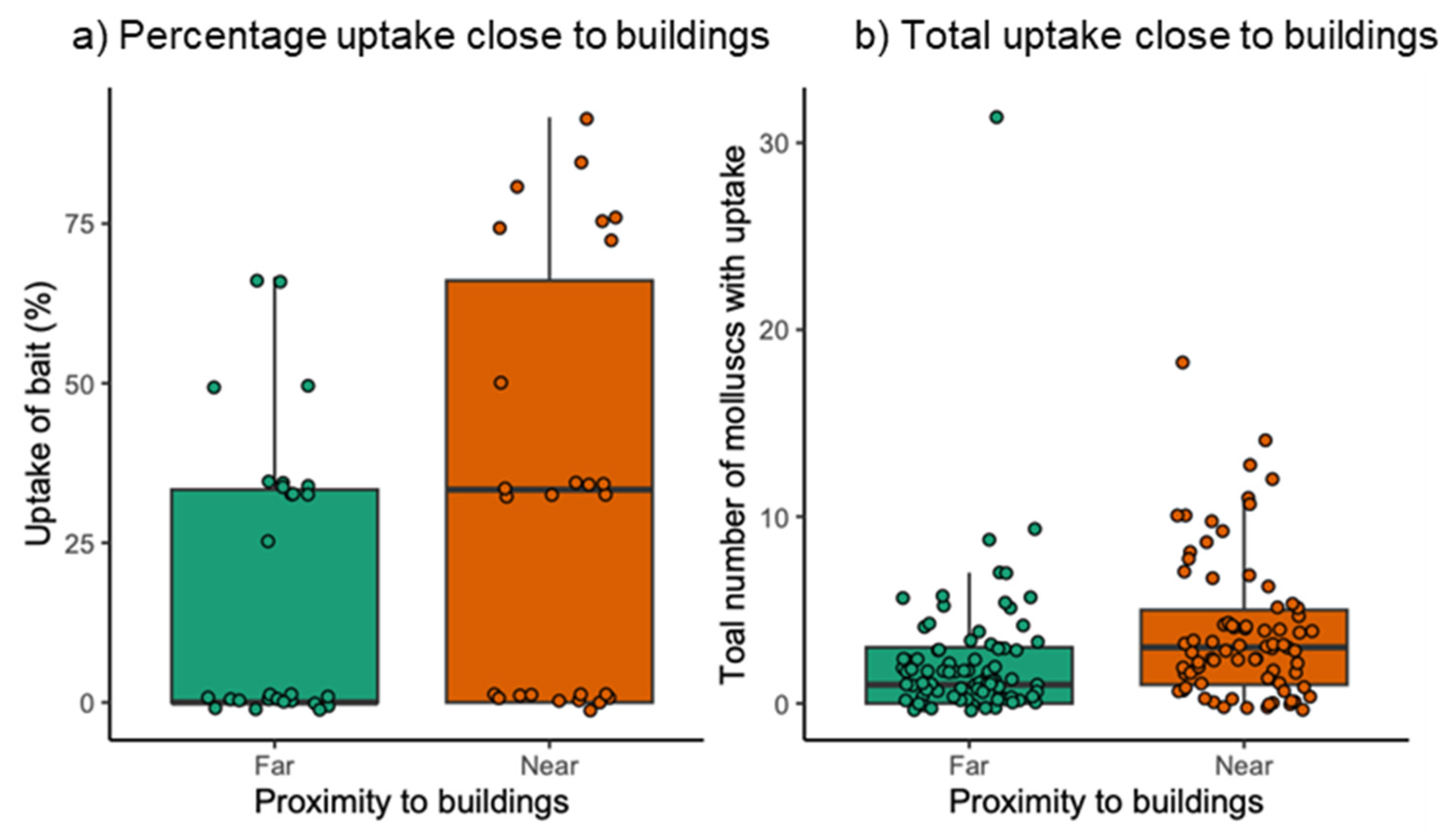
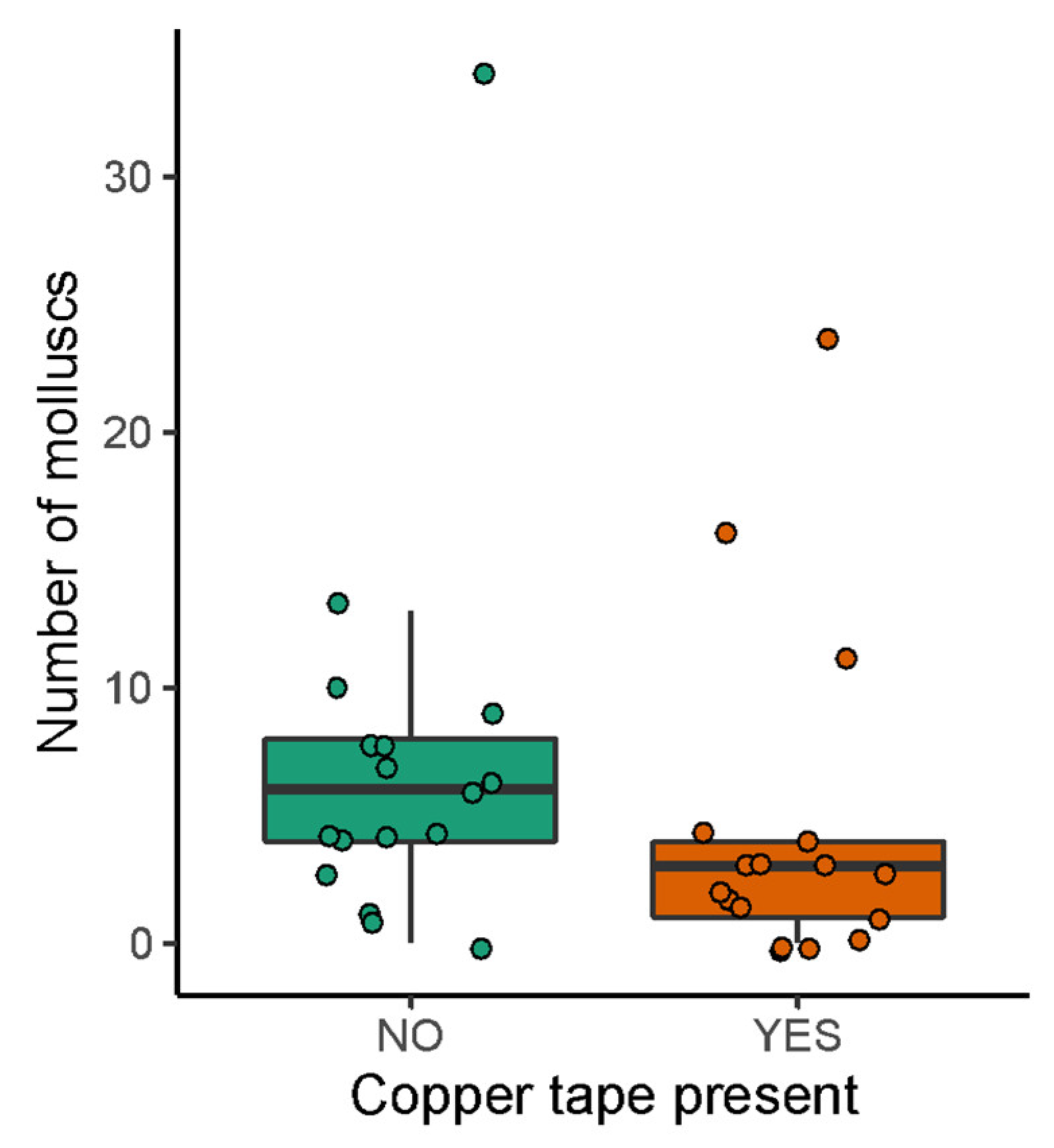
3. Results
3.1. Laboratory Pilot Study
3.2. Field Exposure Study
4. Discussion
Bait Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sainsbury, K.A.; Shore, R.F.; Schofield, H.; Croose, E.; Pereira, M.G.; Sleep, D.; Kitchener, A.C.; Hantke, G.; McDonald, R.A. Long-term increase in secondary exposure to anticoagulant rodenticides in European polecats Mustela putorius in Great Britain. Environ. Pollut. 2018, 236, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Witmer, G.W. The Changing Role of Rodenticides and Their Alternatives in the Management of Commensal Rodents. Hum. Wildl. Interact. 2019, 13, 186–199. [Google Scholar]
- Geduhn, A.; Esther, A.; Schenke, D.; Mattes, H.; Jacob, J. Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci. Total Environ. 2014, 496, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.F.; Walker, L.A.; Potter, E.D.; Chaplow, J.S.; Pereira, M.G.; Sleep, D.; Hunt, A.G. Second Generation Anticoagulant Rodenticide Residues in Barn Owls 2018; NERC/Centre for Ecology & Hydrolog: Lancaster, UK, 2019. [Google Scholar]
- Dowding, C.V.; Shore, R.F.; Worgan, A.; Baker, P.J.; Harris, S. Accumulation of anticoagulant rodenticides in a non-target insectivore, the European hedgehog (Erinaceus europaeus). Environ. Pollut. 2010, 158, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Brakes, C.R.; Smith, R.H. Exposure of non-target small mammals to rodenticides: Short-term effects, recovery and implications for secondary poisoning. J. Appl. Ecol. 2005, 42, 118–128. [Google Scholar] [CrossRef]
- Smith, R.; Shore, R. Environmental Impacts of Rodenticides; CABI: Wallingford, UK, 2015; pp. 330–345. [Google Scholar]
- Walker, L.A.; Turk, A.; Long, S.M.; Wienburg, C.L.; Best, J.; Shore, R.F. Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Sci. Total Environ. 2008, 392, 93–98. [Google Scholar] [CrossRef]
- Masuda, B.M.; Fisher, P.; Jamieson, I.G. Anticoagulant rodenticide brodifacoum detected in dead nestlings of an insectivorous passerine. N. Z. J. Ecol. 2014, 38, 110–115. [Google Scholar]
- Alomar, H.; Chabert, A.; Coeurdassier, M.; Vey, D.; Berny, P. Accumulation of anticoagulant rodenticides (chlorophacinone, bromadiolone and brodifacoum) in a non-target invertebrate, the slug, Deroceras reticulatum. Sci. Total Environ. 2018, 610–611, 576–582. [Google Scholar] [CrossRef]
- Quinn, N. Assessing Individual and Population-Level Effects of Anticoagulant Rodenticides on Wildlife. Hum. Wildl. Interact. 2019, 13, 7. [Google Scholar]
- Lohr, M.T.; Davis, R.A. Anticoagulant rodenticide use, non-target impacts and regulation: A case study from Australia. Sci. Total Environ. 2018, 634, 1372–1384. [Google Scholar] [CrossRef]
- Tosh, D.G.; McDonald, R.A.; Bearhop, S.; Lllewellyn, N.R.; Fee, S.; Sharp, E.A.; Barnett, E.A.; Shore, R.F. Does small mammal prey guild affect the exposure of predators to anticoagulant rodenticides? Environ. Pollut. 2011, 159, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, E.; Santangeli, A.; Koivisto, P.; Korkolainen, T.; Vuorisalo, T.; Hanski, I.K.; Loivamaa, I.; Koivisto, S. The prevalence and correlates of anticoagulant rodenticide exposure in non-target predators and scavengers in Finland. Sci. Total Environ. 2018, 642, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Lettoof, D.C.; Lohr, M.T.; Busetti, F.; Bateman, P.W.; Davis, R.A. Toxic time bombs: Frequent detection of anticoagulant rodenticides in urban reptiles at multiple trophic levels. Sci Total Env. 2020, 724, 138218. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.H.; Fisher, P.; Heppelthwaite, V.; Eason, C.T. Toxicity and Residues of Brodifacoum in Snails and Earthworms; Department of Conservation: Wellington, New Zealand, 2003; p. 14. [Google Scholar]
- Barker, G.M. Natural Enemies of Terrestrial Molluscs; CABI: Wallingford, UK, 2004. [Google Scholar]
- Elmeros, M.; Bossi, R.; Christensen, T.K.; Kjær, L.J.; Lassen, P.; Topping, C.J. Exposure of non-target small mammals to anticoagulant rodenticide during chemical rodent control operations. Environ. Sci. Pollut. Res. 2019, 26, 6133–6140. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Geduhn, A.; Schenke, D.; Jacob, J. Exposure of passerine birds to brodifacoum during management of Norway rats on farms. Sci. Total Environ. 2021, 762, 144160. [Google Scholar] [CrossRef] [PubMed]
- Dowding, J.E.; Lovegrove, T.i.G.; Ritchie, J.; Kast, S.N.; Puckett, M. Mortality of northern New Zealand dotterels (Charadrius obscurus aquilonius) following an aerial poisoning operation. Notornis 2006, 53, 235. [Google Scholar]
- Spurr, E.; Maitland, M.; Taylor, G.; Wright, G.; Radford, C.; Brown, L. Residues of brodifacoum and other anticoagulant pesticides in target and non-target species, Nelson Lakes National Park, New Zealand. N. Z. J. Zool. 2005, 32, 237–249. [Google Scholar] [CrossRef]
- López-Perea, J.J.; Camarero, P.R.; Molina-López, R.A.; Parpal, L.; Obón, E.; Solá, J.; Mateo, R. Interspecific and geographical differences in anticoagulant rodenticide residues of predatory wildlife from the Mediterranean region of Spain. Sci. Total Environ. 2015, 511, 259–267. [Google Scholar] [CrossRef]
- The Royal Parks. Appendix 8 Post-Mortem Analysis of Hedgehog Liver Tissue Samples for Second-Generation Rodenticides; The Royal Parks Foundation: London, UK, 2016. [Google Scholar]
- Wilson, E.; Wembridge, D. The State of Britain’s Hedgehogs 2018; People’s Trust for Endangered Species: London, UK, 2018. [Google Scholar]
- BTO. Spotted Flycatcher. Available online: https://www.bto.org/understanding-birds/birdfacts/spotted-flycatcher (accessed on 24 November 2023).
- Finch, T.; Bell, J.R.; Robinson, R.A.; Peach, W.J. Demography of Common Swifts (Apus apus) breeding in the UK associated with local weather but not aphid biomass. Ibis 2023, 165, 420–435. [Google Scholar] [CrossRef]
- Fuller, R.J.; Noble, D.G.; Smith, K.W.; Vanhinsbergh, D. Recent declines in populations of woodland birds in Britain. Br. Birds 2005, 98, 116–143. [Google Scholar]
- Blanco, C.A.; Perera, O.; Ray, J.D.; Taliercio, E.; Williams, L., III. Incorporation of rhodamine B into male tobacco budworm moths Heliothis virescens to use as a marker for mating studies. J. Insect Sci. 2006, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Mascari, T.M.; Foil, L.D. Evaluation of Rhodamine B as an Orally Delivered Biomarker for Rodents and a Feed-Through Transtadial Biomarker for Phlebotomine Sand Flies (Diptera: Psychodidae). J. Med. Entomol. 2009, 46, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- ProRep. Bug Gel. Available online: https://www.pro-rep.co.uk/portfolio/bug-gel/ (accessed on 25 November 2023).
- Tosh, D.G.; Shore, R.F.; Jess, S.; Withers, A.; Bearhop, S.; Ian Montgomery, W.; McDonald, R.A. User behaviour, best practice and the risks of non-target exposure associated with anticoagulant rodenticide use. J. Environ. Manag. 2011, 92, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Cranshaw, W. Attractiveness of Beer and Fermentation Products to the Gray Garden Slug, Agriolimax Reticulatum (Muller) (Mollusca: Limacidae); Colorado State University: Colorado State University Agricultural Experiment Station: Fort Collins, CO, USA, 1997. [Google Scholar]
- World Weather Online. World Weather API and Weather Forecast. Available online: https://www.worldweatheronline.com/ (accessed on 1 September 2021).
- Schüder, I.; Port, G.; Bennison, J. Barriers, repellents and antifeedants for slug and snail control. Crop Prot. 2003, 22, 1033–1038. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- RStudioTeam. RStudio: Integrated Development Environment for R, RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Elliott, J.E.; Hindmarch, S.; Albert, C.A.; Emery, J.; Mineau, P.; Maisonneuve, F. Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ. Monit. Assess. 2014, 186, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Dunlevy, P.A.; Campbell, E.W.; Lindsey, G.D. Broadcast application of a placebo rodenticide bait in a native Hawaiian forest. Int. Biodeterior. Biodegrad. 2000, 45, 199–208. [Google Scholar] [CrossRef]
- Széles, G.L.; Purger, J.J.; Molnár, T.; Lanszki, J. Comparative analysis of the diet of feral and house cats and wildcat in Europe. Mammal Res. 2018, 63, 43–53. [Google Scholar] [CrossRef]
- Spurr, E.B.; Drew, K.W. Invertebrates feeding on baits used for vertebrate pest control in new zealand. N. Z. J. Ecol. 1999, 23, 167–173. [Google Scholar]
- Morgan, D.R.; Wright, G.; Ogilvie, S.C.; Pierce, R.; Thomson, P. Assessment of the Environmental Impact of Brodifacoum during Rodent Eradication Operations in New Zealand. 1996. Available online: https://digitalcommons.unl.edu/vpc17/38/ (accessed on 17 October 2021).
- Evans, M.J.; Wallman, J.F.; Barton, P.S. Traits reveal ecological strategies driving carrion insect community assembly. Ecol. Entomol. 2020, 45, 966–977. [Google Scholar] [CrossRef]
- Morii, Y.; Ohkubo, Y.; Watanabe, S. Activity of invasive slug Limax maximus in relation to climate conditions based on citizen’s observations and novel regularization based statistical approaches. Sci. Total Environ. 2018, 637–638, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Rosin, Z.M.; Kwieciński, Z.; Lesicki, A.; Skórka, P.; Kobak, J.; Szymańska, A.; Osiejuk, T.S.; Kałuski, T.; Jaskulska, M.; Tryjanowski, P. Shell colour, temperature, (micro)habitat structure and predator pressure affect the behaviour of Cepaea nemoralis. Sci. Nat. 2018, 105, 35. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.; Triebskorn, R.; Köhler, H.-R. Snails in the sun: Strategies of terrestrial gastropods to cope with hot and dry conditions. Ecol. Evol. 2019, 9, 12940–12960. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, A.; Ansart, A. Conservation at a slow pace: Terrestrial gastropods facing fast-changing climate. Conserv. Physiol. 2017, 5, cox007. [Google Scholar] [CrossRef] [PubMed]
- Rial-Berriel, C.; Acosta-Dacal, A.; Cabrera Pérez, M.Á.; Suárez-Pérez, A.; Melián Melián, A.; Zumbado, M.; Henríquez Hernández, L.A.; Ruiz-Suárez, N.; Rodriguez Hernández, Á.; Boada, L.D.; et al. Intensive livestock farming as a major determinant of the exposure to anticoagulant rodenticides in raptors of the Canary Islands (Spain). Sci. Total Environ. 2021, 768, 144386. [Google Scholar] [CrossRef]
- van den Brink, N.W.; Elliott, J.E.; Shore, R.F.; Rattner, B.A. Anticoagulant Rodenticides and Wildlife; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Hindmarch, S.; Elliott, J.E.; McCann, S.; Levesque, P. Habitat use by barn owls across a rural to urban gradient and an assessment of stressors including, habitat loss, rodenticide exposure and road mortality. Landsc. Urban Plan. 2017, 164, 132–143. [Google Scholar] [CrossRef]
- Schüder, I.; Port, G.; Bennison, J. The behavioural response of slugs and snails to novel molluscicides, irritants and repellents. Pest Manag. Sci. 2004, 60, 1171–1177. [Google Scholar] [CrossRef]
- Rautio, A.; Isomursu, M.; Valtonen, A.; Hirvelä-Koski, V.; Kunnasranta, M. Mortality, diseases and diet of European hedgehogs (Erinaceus europaeus) in an urban environment in Finland. Mammal Res. 2016, 61, 161–169. [Google Scholar] [CrossRef]
- Grosshans, W. The food of the hedgehog erinaceus europaeus studies of stomach contents of hedgehogs from schleswig holstein west germany. Zool. Anz. 1983, 211, 364–384. [Google Scholar]
- Yalden, D. The food of the hedgehog in England. Acta Theriol. 1976, 21, 401–424. [Google Scholar] [CrossRef]
- Wroot, A.J. Feeding ecology of the European hedgehog Erinaceus europaeus. Ph.D. Thesis, Royal Holloway, University of London, London, UK, 1984. [Google Scholar]
- Howes, C. Field Notes: Notes on Suburban Hedgehogs. Naturalis 1976, 101, 147–148. [Google Scholar]
- Kruuk, H. Predators and anti-predator behaviour of the black-headed gull (Larus ridibundus L.). Behaviour. Suppl. 1964, 11, III-129. [Google Scholar]
- South, A. Predators, parasites and disease. In Terrestrial Slugs: Biology, Ecology and Control; Springer: Dordrecht, The Netherlands, 1992; pp. 220–241. [Google Scholar]
- Churchfield, S.; Rychlik, L.; Taylor, J.R.E. Food resources and foraging habits of the common shrew, Sorex araneus: Does winter food shortage explain Dehnel’s phenomenon? Oikos 2012, 121, 1593–1602. [Google Scholar] [CrossRef]
- Damin-Pernik, M.; Espana, B.; Lefebvre, S.; Fourel, I.; Caruel, H.; Benoit, E.; Lattard, V. Management of Rodent Populations by Anticoagulant Rodenticides: Toward Third-Generation Anticoagulant Rodenticides. Drug Metab. Dispos. 2017, 45, 160–165. [Google Scholar] [CrossRef]


| Category | Total Caught | % Bait Uptake |
|---|---|---|
| Ant | 32 | 0.0 |
| Aphid | 2 | 0.0 |
| Beetle | 589 | 0.2 |
| Caterpillar | 8 | 0.0 |
| Earthworm | 6 | 0.0 |
| Earwig | 63 | 6.8 |
| Lepidoptera | 1 | 0.0 |
| Millipede | 23 | 0.0 |
| Slug | 239 | 20.7 |
| Snail | 251 | 18.4 |
| Springtail | 178 | 1.1 |
| Woodlouse | 196 | 1.0 |
| Model | Parameter | X2 | df | p |
|---|---|---|---|---|
| (a) Percentage uptake | Distance | 37.51 | 1 | <0.001 |
| Max. temperature | 4.50 | 1 | 0.034 | |
| Precipitation | 0.55 | 1 | 0.460 | |
| (b) Total number showing uptake | Distance | 4.98 | 1 | 0.026 |
| Max. temperature | 1.63 | 1 | 0.202 | |
| Precipitation | 3.73 | 1 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.J.; Cotter, S.C.; Soulsbury, C.D. Consumption of Rodenticide Baits by Invertebrates as a Potential Route into the Diet of Insectivores. Animals 2023, 13, 3873. https://doi.org/10.3390/ani13243873
Williams EJ, Cotter SC, Soulsbury CD. Consumption of Rodenticide Baits by Invertebrates as a Potential Route into the Diet of Insectivores. Animals. 2023; 13(24):3873. https://doi.org/10.3390/ani13243873
Chicago/Turabian StyleWilliams, Emily J., Sheena C. Cotter, and Carl D. Soulsbury. 2023. "Consumption of Rodenticide Baits by Invertebrates as a Potential Route into the Diet of Insectivores" Animals 13, no. 24: 3873. https://doi.org/10.3390/ani13243873
APA StyleWilliams, E. J., Cotter, S. C., & Soulsbury, C. D. (2023). Consumption of Rodenticide Baits by Invertebrates as a Potential Route into the Diet of Insectivores. Animals, 13(24), 3873. https://doi.org/10.3390/ani13243873







