1. Introduction
The season is an important factor affecting the growth and development of many animals, especially fur animals. Hair length and density features could affect the transfer of heat from the domestic animal’s skin to the environment [
1]. The environmental temperature can affect the activities of livestock and poultry and, as a result, impact their production performance [
2]. Temperature and humidity factors could affect intestinal water metabolism and cause changes in the composition of the intestinal flora [
3]. Rabbit sweat glands are not well developed; this, coupled with their whole-body hair, means that they are highly sensitive to changes in the external environment’s temperature, more so than most farm animal species [
4]. Rex rabbits are important fur rabbits, and the environmental temperature in different seasons seriously affects the quality of their fur. The main indicators used to evaluate the fur quality of Rex rabbits are hair density, skin area, skin weight, skin thickness, hair length, hair color, skin tensile strength, etc., [
5]. Hair follicles are the tissues that produce fur in mammals, and they are one of the few organs with a periodic regeneration function after birth. According to the morphological characteristics, the hair follicle cycle is divided into anagen, catagen, and telogen [
6,
7,
8]. Hair follicles rely on periodic cyclic growth to achieve hair growth and renewal, and their periodic changes determine the periodic growth and loss of hair, while the periodic development of hair follicles is affected by a variety of factors and is regulated by a series of signal molecules [
9,
10,
11]. As the “material basis” of fur growth, hair follicles are not only regulated by genetic and nutritional factors but are also affected by environmental temperature and humidity [
12,
13,
14,
15]. Many studies have been conducted on animals under heat and cold stresses [
16,
17,
18], while the effect of the seasons on fur rabbits has rarely been reported, and the mechanism of seasonal influence on hair follicle development remains unclear. In this study, Rex rabbits reared outdoors for five months were skinned in spring, summer, autumn, or winter, and their skin was removed to determine the most suitable slaughter season for Rex rabbits by assessing their fur quality, the relative expression of genes, and protein expression related to hair follicle development.
4. Discussion
Global warming and abnormally high ambient temperatures have adversely affected the livestock sector in recent decades [
22]. In China, where the temperature changes in different seasons, rabbits in particular are sensitive to extreme environmental temperature and humidity. This is not conducive to the performance of rabbit production and the improvement of product quality. Compared with the summer, the shoulder fat weight, perirenal fat weight, and perigastric fat increased in winter by 28.81%, 62.90%, and 41.54%, respectively (
Table 5). The observed differences are consistent with those in previous research reports [
23,
24,
25,
26]. Ambient temperatures affect the lipid metabolism of broilers, and high cholesterol and triglyceride contents in the blood reflect enhanced lipid catabolism [
27]. Leptin is a hormone secreted by adipose tissue; it is involved in promoting fat breakdown and utilization, and its content in serum is proportional to the amount of adipose tissue in the animal [
28,
29,
30]. Leptin acts on receptors located in the central nervous system to regulate the behavior and metabolism of organisms [
31], and it regulates the energy balance and weight of organisms via a negative feedback mechanism [
32]. The HDL receptor mediates the selective uptake of cholesterol [
33], and heat stress may reduce the activity of glucose-6-phosphate dehydrogenase, thus reducing the activity of nicotinamide adenine dinucleotide dehydrogenase and inhibiting energy metabolism [
34]. In this study, cholesterol, HDL, and LDL were higher in summer than in winter in the Rex rabbits (
Table 6), suggesting that the season can affect lipid metabolism and energy balance.
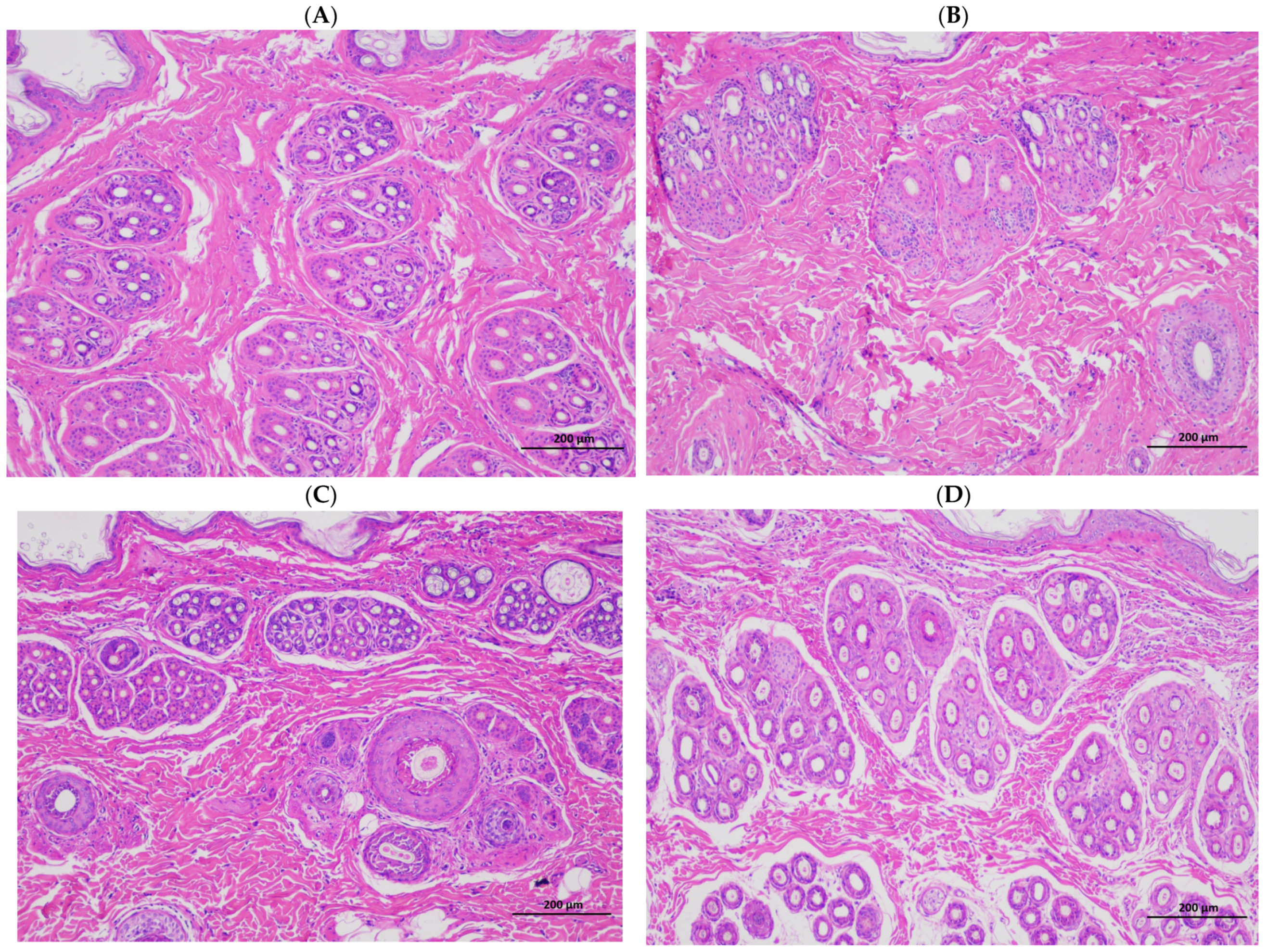
Rabbits with fur have poor heat dissipation mechanisms and are more easily affected by heat stress, and this reduces their performance and the economic efficiency of farms. The quality of fur is mainly based on the hair density, hair length, skin area, and skin thickness, and the density of the hair follicle is a particularly important indicator for evaluating the quality of Rex rabbits’ skin. The skin in summer is generally thin and breaks easily, and the wool can also fall off easily, which affects the rabbit’s skin quality. Compared with winter, the present results show that skinning in summer decreased the skin weight, skin area, skin thickness (
Table 3), and hair follicle density of the rabbits (
Table 4;
Figure 1). This finding is consistent with previous experimental results, which showed that heat stress can reduce the hair length and hair follicle density of Rex rabbits [
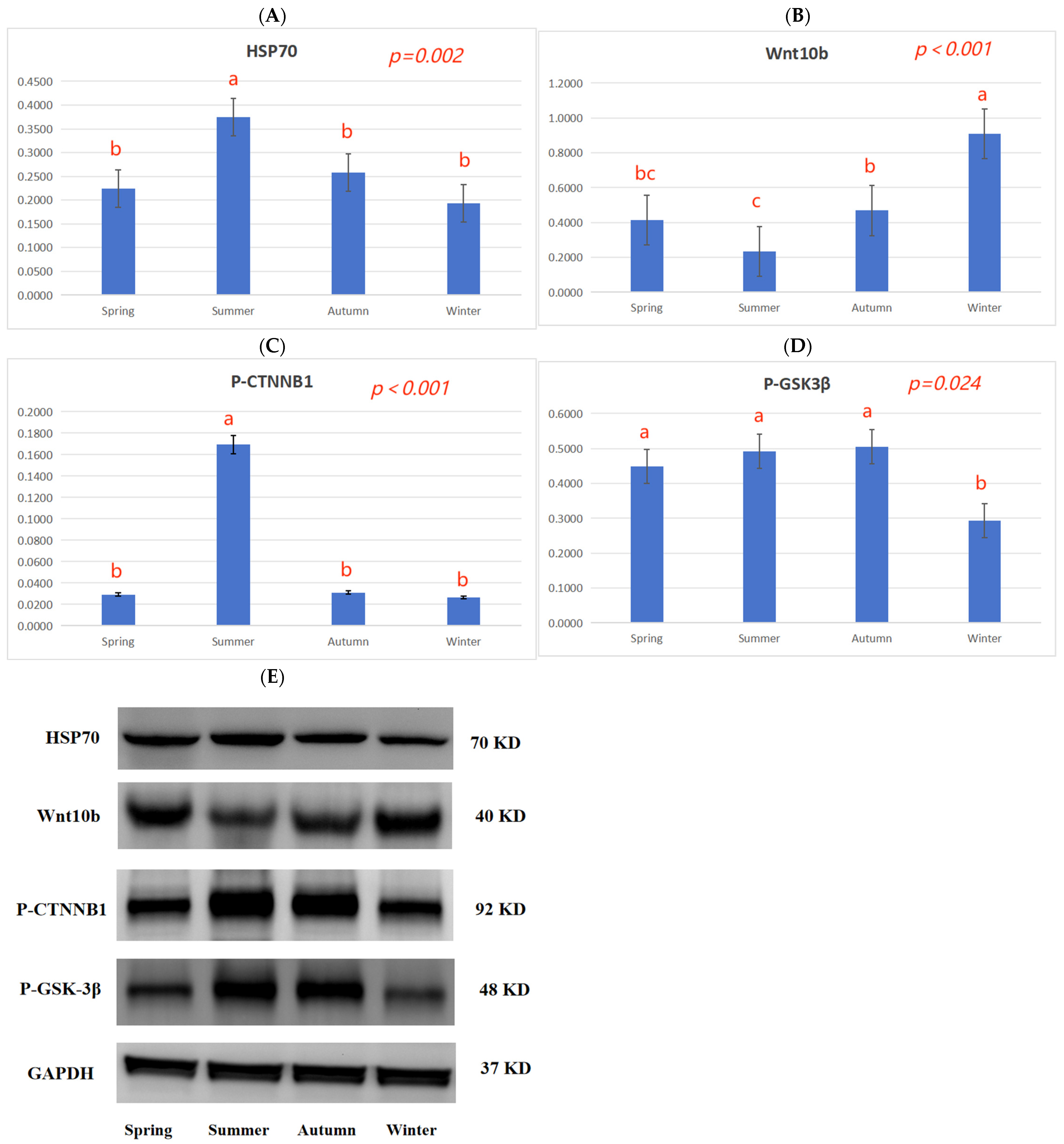
35]. The expression of several heat shock proteins is increased during summer; this helps maintain cellular redox homeostasis to ensure cell survival. In particular, HSP70 is highly expressed and is a key marker of heat stress in hair follicle tissue [
36,
37,
38]. In summer, the HSP70 protein expression in skin tissue was higher than in spring, autumn, and winter (
Figure 2A), and the fur quality was poor (
Table 3); this is closely related to the high temperatures experienced in summer.
The Wnt family is a class of secreted lipid-modified glycoproteins, which play an important regulatory role in the development of animal hair follicles [
39]. The classical Wnt signaling pathway, composed of the Wnt protein, β-catenin, frizzled-4, and the adenomatous polyposis coli complex, is involved in skin biological processes [
40]. Wnt10b promotes hair follicle growth and dermal papilla cell proliferation via the Wnt/β-catenin signaling pathway in Rex rabbits [
41]. Wnt signaling requires the sequestration of glycogen synthase kinase 3 (GSK3β) inside multivesicular endosomes [
42]. Dickkopf-associated protein 1 (DKK1) is an important antagonist of the Wnt signaling pathway. The continuous overexpression of DKK1 during the embryonic development stage prevents the formation of hair follicles in animals, and the overexpression of DKK1 after birth inhibits the growth of hair follicles. DKK1 can inhibit the Wnt signaling pathway by inhibiting β-catenin activity, leading to hair follicle degeneration [
43]. In winter,
Wnt10b,
CTNNB1, and
GSK3β gene expression was higher than in summer, and the
DDK1 gene expression was lower than in summer (
Table 7), indicating that high temperatures could inhibit hair follicle development by inhibiting the Wnt10b/β-catenin signal.
The bone morphogenetic protein (BMP) signaling pathway plays an important role in controlling the growth and development of animal skin hair follicles [
44]. BMP signals are conducive to the maintenance of dermal papilla activity; BMP2 and BMP4 participate in the formation of the hair follicle matrix, and the expression levels of BMP2 and BMP4 during the growth phase are lower than during the resting phase, inhibiting the transformation of hair follicles from the resting phase to the growth phase [
45]. After the overexpression of BMP2, cell proliferation and differentiation between hair follicle stem cells and dermal papilla cease [
46]. BMP signaling inhibits Wnt signaling by regulating β-catenin phosphorylation. Transforming growth factor-β1 (TGF-β1) is expressed in the inner root sheath during the transition from the growth stage to the degeneration stage, which can cause hair follicles to transition from the growth stage to the degeneration stage and inhibit cell proliferation [
47]. In winter, the expression of the
TGFβ-1,
BMP2, and
BMP4 genes was lower than that in summer (
Table 7), and the CTNNB1 protein phosphorylation levels in the skin tissue in summer were higher than in spring, autumn, and winter (
Figure 2C), indicating that the seasons may regulate hair follicle development via the TGF-β/BMP signaling pathway.
IGF1 has a positive effect on hair follicle development; it is expressed in both the hair follicles and dermal fibroblasts of rats, participates in apoptosis, and promotes the proliferation of dermal cells [
48]. In addition, EGF stimulates hair follicle elongation and maintains hair follicle growth [
49], and a subcutaneous injection of EGF in Angora rabbits can increase the growth of hair follicles and induce hair follicle regeneration [
50]. Knockdown of the EGF receptor gene leads to a decrease in hair follicle formation and facilitates the transition of hair follicles from anagen to catagen, which is in line with previous findings [
51]. The expression of
IGF-I,
IGF-IR, and
EGF in the rabbits skinned in summer was lower than that in the rabbits skinned in winter (
Table 7), which suggests that IGF1 and EGF signaling may be the key target signals in temperature-regulated hair follicle development in Rex rabbits.