The Occurrence of Cattle Tick Fever in a Region of the Atlantic Forest on the Border with the Caatinga in Brazil
Abstract
Simple Summary
Abstract
1. Introduction
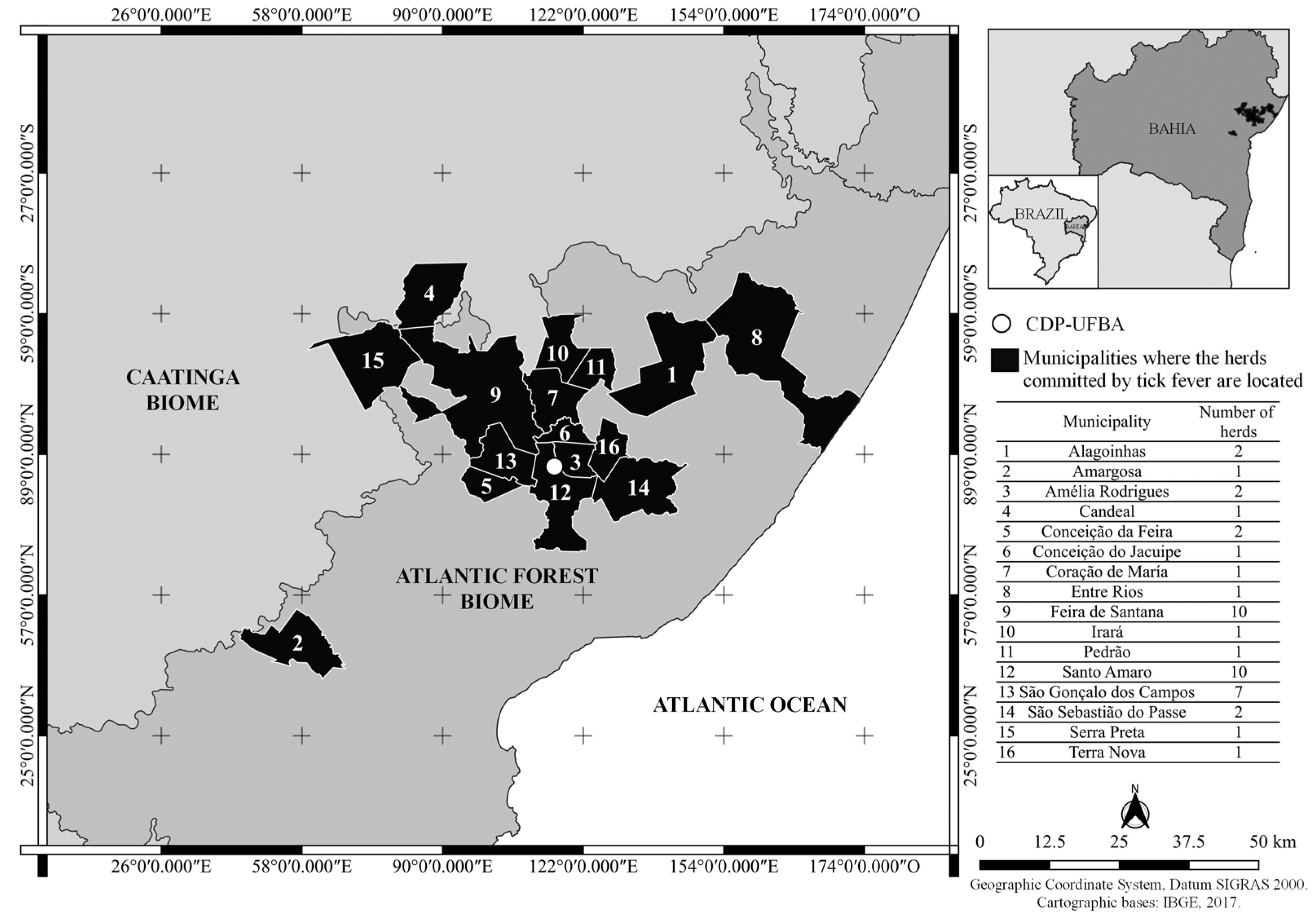
2. Materials and Methods
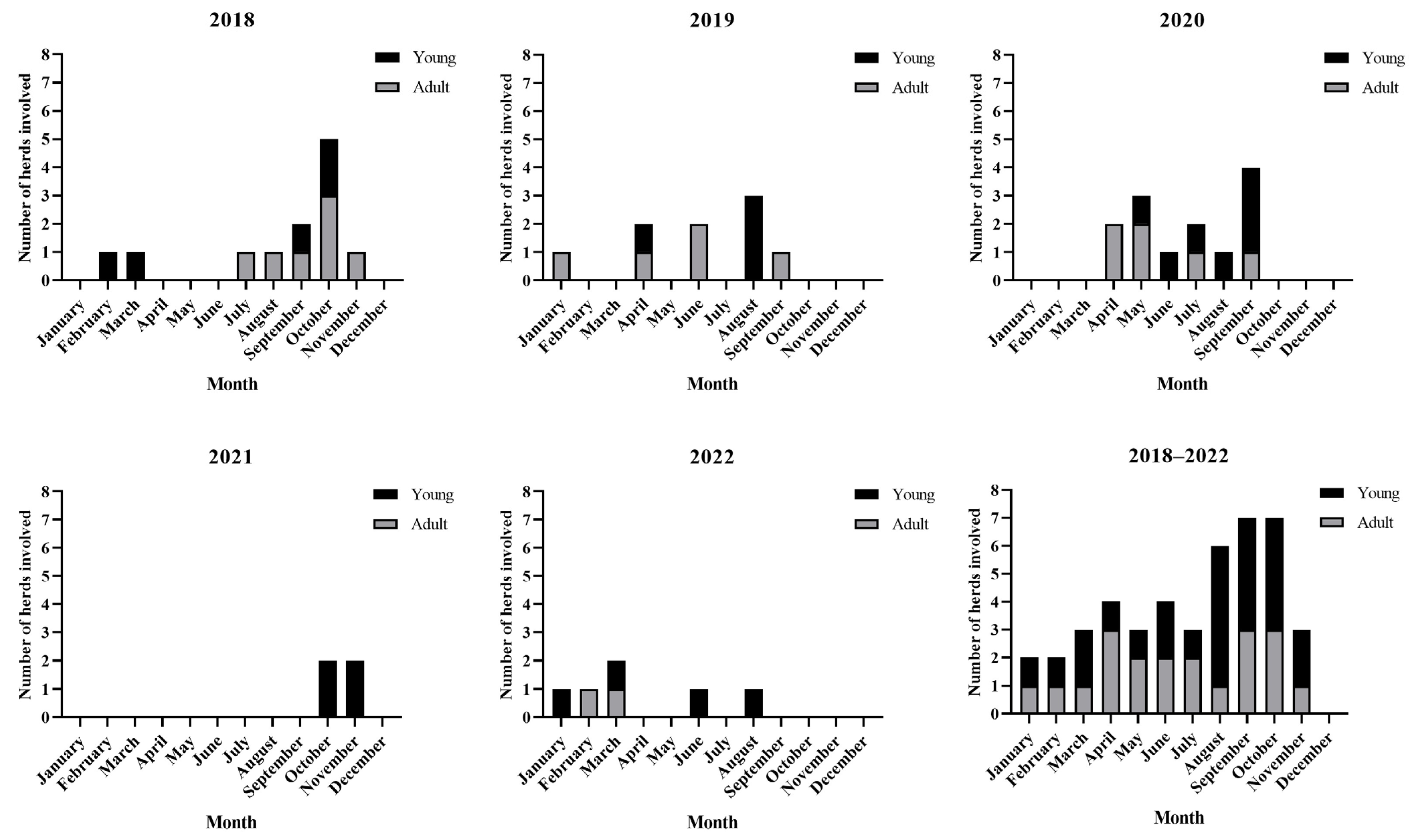
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of Genera in the Families Rickettsiaceae and Anaplasmataceae in the Order Rickettsiales: Unification of Some Species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, Descriptions of Six New Species Combinations and Designation of Ehrlichia Equi and “HGE Agent” as Subjective Synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [CrossRef] [PubMed]
- Levine, N.D. Taxonomy of the Piroplasms. Trans. Am. Microsc. Soc. 1971, 90, 2. [Google Scholar] [CrossRef]
- Gray, J.S.; Estrada-Peña, A.; Peña, P.; Zintl, A. Vectors of Babesiosis. Annu. Rev. Entomol. 2018, 64, 149–165. [Google Scholar] [CrossRef]
- Connell, M.; Hall, W.T.K. Transmission of Anaplasma marginale the Cattle Tick Boophilus microplus. Aust. Vet. J. 1972, 48, 477. [Google Scholar] [CrossRef]
- Costa, S.C.L.; de Magalhães, V.C.S.; de Oliveira, U.V.; Carvalho, F.S.; de Almeida, C.P.; Machado, R.Z.; Munhoz, A.D. Transplacental Transmission of Bovine Tick-Borne Pathogens: Frequency, Co-Infections and Fatal Neonatal Anaplasmosis in a Region of Enzootic Stability in the Northeast of Brazil. Ticks Tick. Borne Dis. 2016, 7, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The Natural History of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of Cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef]
- Richey, E.J. Bovine Anaplasmosis. In American Association of Bovine Practitioners Conference Proceedings; U.S. Department of Agriculture: Washington, DC, USA, 1992; pp. 3–11. [Google Scholar] [CrossRef]
- Madruga, C.R.; Aycardi, E.; Kessler, R.H.; Aparecida, M.; Schenk, M.; De Figueredo, G.R.; Curvo, J. Níveis de Anticorpos Anti-Babesia Bigemina e Babesia Bovis, Em Bezerros Da Raça Nelore, Ibage e Cruzamentos de Nelore. Pesqui. Agropecu. Bras. 1984, 19, 1163–1168. [Google Scholar]
- Mahoney, D.F.; Ross, D.R. Epizootiological Factors in the Control of Bovine Babesiosis. Aust. Vet. J. 1972, 48, 292–298. [Google Scholar] [CrossRef]
- Puentes, J.D.; Riet-Correa, F. Epidemiological Aspects of Cattle Tick Fever in Brazil. Braz. J. Vet. Parasitol. 2023, 32, 1–11. [Google Scholar] [CrossRef]
- Coleman, P.; Perry, B.; Woolhouse, M. Endemic Stability—A Veterinary Idea Applied to Human Public Health. Lancet 2001, 357, 1284–1286. [Google Scholar] [CrossRef]
- de Almeida, M.B.; Tortelli, F.P.; Riet-Correa, B.; Ferreira, J.L.M.; Soares, M.P.; Farias, N.A.R.; Riet-Correa, F.; Schild, A.L. Tristeza Parasitária Bovina Na Região Sul Do Rio Grande Do Sul: Estudo Retrospectivo de 1978–2005. Pesq. Vet. Bras. 2006, 26, 237–242. [Google Scholar] [CrossRef]
- Costa, V.M.M.; Rodrigues, A.L.; Medeiros, J.M.A.; Labruna, M.B.; Simões, S.V.D.; Riet-Correa, F. Tristeza Parasitária Bovina No Sertão Da Paraíba. Pesq. Vet. Bras. 2011, 31, 239–243. [Google Scholar] [CrossRef]
- Gonçalves, R.; Silva, A.; Ferreira, D.; Chiacchio, S.; Lopes, R.; Borges, A.; Amorim, R. Tristeza Parasitária Em Bovinos Na Região de Botucatu–SP: Estudo Retrospectivo de 1986–2007. Semin. Cienc. Agrar. 2011, 32, 307–312. [Google Scholar] [CrossRef][Green Version]
- Instituto Brasileiro de Geografia e Estatística—IBGE Censo Agropecuário. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/21814-2017-censo-agropecuario.html?=&t=resultados (accessed on 14 April 2022).
- Instituto do Meio Ambiente e Recursos Hídricos—INEMA Sistema Estadual de Informações Ambientais e de Recursos Hídricos. Available online: http://monitoramento.seia.ba.gov.br/ (accessed on 14 June 2023).
- George, J.W.; Snipes, J.; Lane, V.M. Comparison of Bovine Hematology Reference Intervals from 1957 to 2006. Vet. Clin. Pathol. 2010, 39, 138–148. [Google Scholar] [CrossRef]
- Miraballes, C.; Taño, M.; Riet-Correa, F. Evaluation of the One-Side Tick Counting Technique and of the Level of Infestation of Bovines with Rhipicephalus Microplus. Exp. Appl. Acarol. 2022, 86, 443–453. [Google Scholar] [CrossRef]
- Costa, V.M.d.M.; Simões, S.V.D.; Riet-Correa, F. Doenças Parasitárias Em Ruminantes No Semi-Árido Brasileiro. Pesq. Vet. Bras. 2009, 29, 563–568. [Google Scholar] [CrossRef]
- Perosa, F.; Gris, A.; Piva, M.; Schwertz, C.; Henker, L.; Lorenzett, M.; Santiani, F.; Baldi, K.; Gomes, T.; Casagrande, R.; et al. Doenças Diagnosticadas Pelo Laboratório de Patologia Veterinária No Octênio 2013–2020. In Boletim de Diagnóstico do Laboratório de Patologia Veterinária 2013–2020; Mendes, R., Perosa, F., Gomes, T., Eds.; IFC: Concordia, Brazil, 2022; Volume 3, pp. 43–65. [Google Scholar]
- de Oliveira, P.A.; Ruas, J.L.; Riet-Correa, F.; Coelho, A.C.B.; Santos, B.L.; Marcolongo-Pereira, C.; Sallis, E.S.V.; Schild, A.L. Doenças Parasitárias Em Bovinos e Ovinos No Sul Do Brasil: Frequência e Estimativa de Perdas Econômicas. Pesq. Vet. Bras. 2017, 37, 797–801. [Google Scholar] [CrossRef]
- Pupin, R.C.; Guizelini, C.d.C.; de Lemos, R.A.A.; Martins, T.B.; Borges, F.d.A.; Borges, D.G.L.; Gomes, D.C. Retrospective Study of Epidemiological, Clinical and Pathological Findings of Bovine Babesiosis in Mato Grosso Do Sul, Brazil (1995–2017). Ticks Tick. Borne Dis. 2019, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, L.A.S.; Silva, G.S.; Bezerra, K.S.; Rondelli, A.L.H.; Lima, S.R.; Furlan, F.H.; Pescador, C.A.; Colodel, E.M. Doenças de Bovinos Em Mato Grosso Diagnosticadas No Laboratório de Patologia Veterinária Da UFMT (2005–2014). Pesq. Vet. Bras. 2017, 37, 432–440. [Google Scholar] [CrossRef]
- Amorim, L.S.; Wenceslau, A.A.; Carvalho, F.S.; Carneiro, P.L.S.; Albuquerque, G.R. Complexo Tristeza Parasitária Bovina: Diagnóstico e Avaliação Dos Fatores de Risco Na Bahia, Brasil. Braz. J. Vet. Parasitol. 2014, 23, 328–336. [Google Scholar] [CrossRef]
- Araujo, F.R.; Madruga, C.; Almeida, M.; Leal, C.; Miguita, M. Serological Survey of Babesia Bovis and Babesia Bigemina in the State of Bahia by Indirect Immunofluorescence and Rapid Conglutination Test. Braz. J. Vet. Parasitol. 1997, 6, 111–115. [Google Scholar]
- Suarez, C.E.; Noh, S. Emerging Perspectives in the Research of Bovine Babesiosis and Anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Goff, W.L.; Johnson, W.C.; Parish, S.M.; Barrington, G.M.; Tuo, W.; Valdez, R.A. The Age-Related Immunity in Cattle to Babesia Bovis Infection Involves the Rapid Induction of Interleukin-12, Interferon-γ and Inducible Nitric Oxide Synthase MRNA Expression in the Spleen. Parasite Immunol. 2001, 23, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Aubry, P.; Geale, D.W. A Review of Bovine Anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.Z.; da Silva, J.B.; André, M.R.; Gonçalves, L.R.; Matos, C.A.; Obregón, D. Surto de Anaplasmose Associado Com a Presença de Diferentes Cepas de Anaplasma Marginale Em Gado Leiteiro Dos Estados de São Paulo e Goiás, Brasil. Braz. J. Vet. Parasitol. 2015, 24, 438–446. [Google Scholar] [CrossRef]
- Jonsson, N.N.; Bock, R.E.; Jorgensen, W.K. Productivity and Health Effects of Anaplasmosis and Babesiosis on Bos Indicus Cattle and Their Crosses, and the Effects of Differing Intensity of Tick Control in Australia. Vet. Parasitol. 2008, 155, 1–9. [Google Scholar] [CrossRef]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a Bovine Blood Parasite of Veterinary and Zoonotic Importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, N.; Liu, C.; Han, M.; Wang, H.; Chen, X.; Kang, J.; Li, X.; Liu, Y. Residue Depletion of Imidocarb in Bovine Tissues by UPLC-MS/MS. Animals 2022, 13, 104. [Google Scholar] [CrossRef]
- Brayton, K.A.; Kappmeyer, L.S.; Herndon, D.R.; Dark, M.J.; Tibbals, D.L.; Palmer, G.H.; McGuire, T.C.; Knowles, D.P. Complete Genome Sequencing of Anaplasma marginale Reveals That the Surface Is Skewed to Two Superfamilies of Outer Membrane Proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 844–849. [Google Scholar] [CrossRef]
- Raynal, J.T.; da Silva, A.A.B.; Sousa, T.d.J.; Bahiense, T.C.; Meyer, R.; Portela, R.W. Acaricides Efficiency on Rhipicephalus(Boophilus) microplus from Bahia State North-Central Region. Braz. J. Vet. Parasitol. 2013, 22, 71–77. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puentes, J.D.; Carvalho, V.S.d.; Caymmi, L.G.; Mendonça, M.F.F.d.; Riet-Correa, F. The Occurrence of Cattle Tick Fever in a Region of the Atlantic Forest on the Border with the Caatinga in Brazil. Animals 2023, 13, 3636. https://doi.org/10.3390/ani13233636
Puentes JD, Carvalho VSd, Caymmi LG, Mendonça MFFd, Riet-Correa F. The Occurrence of Cattle Tick Fever in a Region of the Atlantic Forest on the Border with the Caatinga in Brazil. Animals. 2023; 13(23):3636. https://doi.org/10.3390/ani13233636
Chicago/Turabian StylePuentes, Juan Dario, Vitor Santiago de Carvalho, Lais Gouveia Caymmi, Múcio Fernando Ferraro de Mendonça, and Franklin Riet-Correa. 2023. "The Occurrence of Cattle Tick Fever in a Region of the Atlantic Forest on the Border with the Caatinga in Brazil" Animals 13, no. 23: 3636. https://doi.org/10.3390/ani13233636
APA StylePuentes, J. D., Carvalho, V. S. d., Caymmi, L. G., Mendonça, M. F. F. d., & Riet-Correa, F. (2023). The Occurrence of Cattle Tick Fever in a Region of the Atlantic Forest on the Border with the Caatinga in Brazil. Animals, 13(23), 3636. https://doi.org/10.3390/ani13233636




