Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Sample Collection
2.2.1. Sample Collection for Antimicrobial Residue Analysis
2.2.2. Feces Samples for Antimicrobial Susceptibility Testing (AST)
2.3. Identification of Commensal E. coli
2.4. Isolation and Identification of Presumptive ESBL/AmpC and Carbapenemase-Producing Commensal E. coli
2.5. Antimicrobial Susceptibility Test (AST) Determination by Broth Microdilution
2.6. Analytical Standards
2.7. Isotopically Labelled Internal Standards
2.8. Preparation of Stock, Intermediate and Working Standard Solutions
2.9. Chemicals and Reagents
2.10. Sample Preparation
2.11. LC-MS/MS Analysis
2.12. Method Validation
3. Results
3.1. Detection and Quantification of Antimicrobial Residues in Milk and Feces Samples
3.1.1. LC–MS/MS Optimization
3.1.2. Linearity of the Method
3.1.3. Selectivity
3.1.4. Limit of Detection (LOD), Limit of Quantification (LOQ), Decion Limit (CCα) and Detection Capability (CCβ)
3.1.5. Accuracy and Precision of the Method
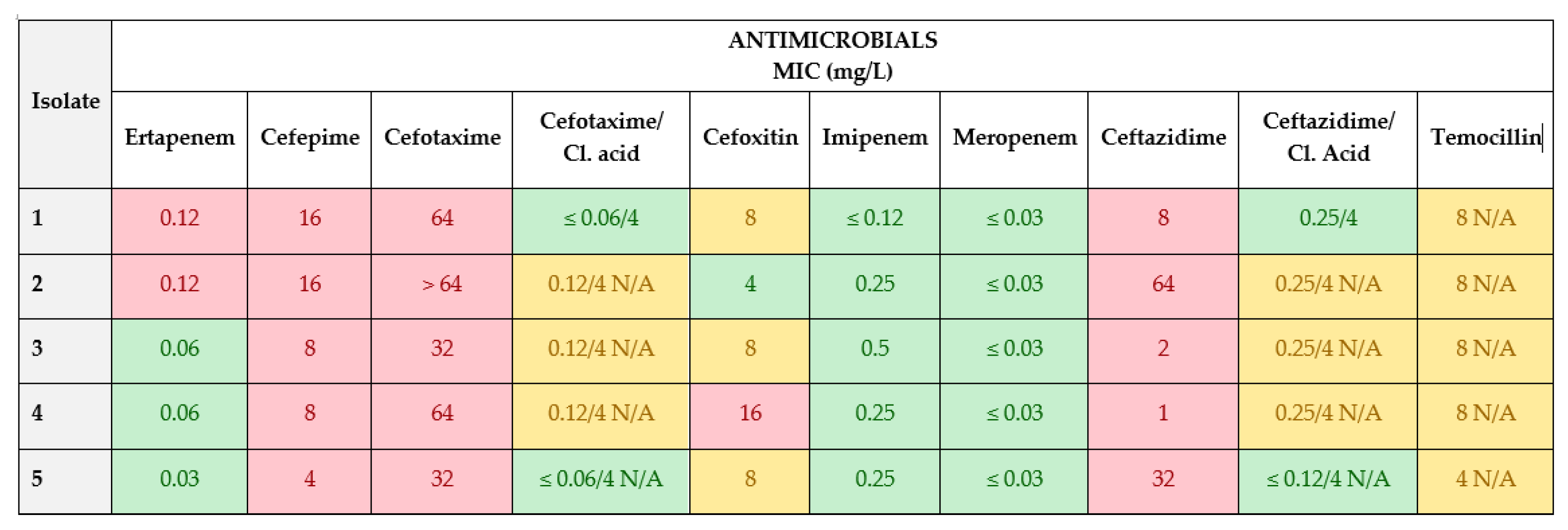
3.2. Isolation of Presumptive ESBL/AmpC- and Carbapenemase-Producing E. coli from Feces Samples
3.3. Antimicrobial Susceptibility Test (AST) Determination by Broth Microdilution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Desta, H.; Wieland, B. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef]
- Ribeiro da Cunha, B.; Fonseca, L.P.; Calado, C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Knapp, W.C.; Dolfing, J.; Ehlert, A.I.P.; Graham, W.D. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (OJ L 15, 20.1.2010, pp. 1–72). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0037 (accessed on 15 October 2023).
- Rice, B.L. The clinical consequences of antimicrobial resistance. Curr. Opin. Microbiol. 2009, 12, 476–481. [Google Scholar] [CrossRef]
- Tenover, F.C.; McGowan, J.E. Reasons for the emergence of antibiotic resistance. Am. J. Med. Sci. 1996, 311, 9–16. [Google Scholar] [CrossRef]
- Blake, D.P.; Humphry, R.W.; Scott, K.P.; Hillman, K.; Fenlon, D.R.; Low, J.C. Influence of tetracycline exposure on tetracycline resistance and the carriage of tetracycline resistance genes within commensal Escherichia coli populations. J. Appl. Microbiol. 2003, 94, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Modi, R.S.; Collins, J.J.; Relman, A.D. Antibiotics and the gut microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef]
- Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 2018, 9, 76–86. [Google Scholar] [CrossRef]
- Filippitzi, M.E.; Devreese, M.; Broekaert, K.; Rasschaert, G.; Daeseleire, E.; Meirlaen, J.; Dewulf, J. Quantitative risk model to estimate the level of antimicrobial residues that can be transferred to soil via manure, due to oral treatments of pigs. Prev. Vet. Med. 2019, 167, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef]
- Winckler, C.; Engels, H.; Hund-Rinke, K.; Luckow, T.; Simon, M.; Steffens, G. Verhalten von Tetracyclinen und anderen Veterina rantibiotika in Wirtschaftsdunger und Boden. Ufoplan 2003, 200, 248. [Google Scholar]
- Pawelzick, H.T.; Per, H.O.H.; Nau, H.; Hamscher, G. A survey of the occurrence of various tetracyclines and sulfamethazine in sandy soils in northwestern Germany fertilized with liquid manure. In Proceedings of the SETAC Euro 14th Annual Meeting, Prague, Czech Republic, 18–22 April 2004. [Google Scholar]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.A.; Soares, A.M.V.M.; Domingues, I.; Van Den Brink, P.J. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lapara, T.M.; Burch, T.R.; McNamara, P.J.; Tan, D.T.; Yan, M.; Eichmiller, J.J. Tertiary-Treated municipal wastewater is a significant point-source of antibiotic resistance genes into Duluth-superior Harbor. Environ. Sci. Technol. 2011, 45, 9543–9549. [Google Scholar] [CrossRef]
- Krapac, I.G.; Koike, S.; Meyer, M.T.; Snow, D.D.; Chou, S.J.; Mackie, R.I.; Roy, W.; Chee, J.C. Long-Term Monitoring of the Occurrence of Antibiotic Residues and Antibiotic Resistance Genes in Groundwater near Swine Confinement Facilities; U.S. Department of Agriculture: Washington, DC, USA, 2004; pp. 158–174. [Google Scholar]
- Kivits, T.; Broers, P.H.; Beeltje, H.; Van Vliet, M.; Griffioen, J. Presence and fate of veterinary antibiotics in age-dated groundwater in areas with intensive livestock farming. Environ. Pollut. 2018, 241, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and Transport of Antibiotic Residues and Antibiotic Resistance Genes following Land Application of Manure Waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [PubMed]
- Boerlin, P.; Travis, R.; Gyles, C.L.; Reid-Smith, R.; Janecko, N.; Lim, H.; Nicholson, V.; Mcewen, S.A.; Friendship, R.; Archambault, M. Antimicrobial Resistance and Virulence Genes of Escherichia coli Isolates from Swine in Ontario. Appl. Environ. Microbiol. 2005, 71, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS 7218; Microbiology of the Food Chain—General Requirements and Guidance for Microbiological Examinations. ISO: Geneva, Switzerland, 2007.
- Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products, Amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and Repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/ EC and Council Decision 92/438/EEC (Official Controls Regulation). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0625&from=EN (accessed on 15 October 2023).
- Laboratory Protocol Isolation of ESBL-, AmpC- and Carbapenemase-Producing, E. coli from Caecal Samples December 2019 Version 7, Version 7 Was Reviewed and Updated by: Rene, S. Hendriksen and Valeria Bortolaia, Authors of the Document: Henrik Hasman, Yvonne Agersø, Rene Hendriksen, Lina, M. Cavaco (DTU Food) and Beatriz Guerra-Roman. Available online: https://www.eurl-ar.eu/CustomerData/Files/Folders/21-protocols/530_esbl-ampc-cpeprotocol-version-caecal-v7-09-12-19.pdf (accessed on 5 June 2023).
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. ISO: Geneva, Switzerland, 2019.
- ISO 20776-2:2021; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 2: Evaluation of Performance of Antimicrobial Susceptibility Test Devices against Reference Broth Micro-Dilution. ISO: Geneva, Switzerland, 2021.
- Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria and repealing Implementing Decision 2013/652/EU (notified under document C (2020) 7894). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020D1729&rid=4 (accessed on 15 October 2023).
- Patyra, E.; Kwiatek, K.; Nebot, C.; Gavilán, R.E. Quantification of Veterinary Antibiotics in Pig and Poultry Feces and Liquid Manure as a Non-Invasive Method to Monitor Antibiotic Usage in Livestock by Liquid Chromatography Mass-Spectrometry. Molecules 2022, 25, 3265. [Google Scholar] [CrossRef] [PubMed]
- Pokrant, E.; Trincado, L.; Yévenes, K.; Terraza, G.; Maddaleno, A.; San Martín, B.; Zavala, S.; Hidalgo, H.; Lapierre, L.; Cornejo, J. Determination of five antimicrobial families in droppings of therapeutically treated broiler chicken by high-performance liquid chromatography-tandem mass spectrometry. Poultry Sci. 2021, 100, 101313. [Google Scholar] [CrossRef]
- 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044) (OJ L 221 17.08.2002, p. 8). Available online: http://data.europa.eu/eli/dec/2002/657/oj (accessed on 5 June 2023).
- Hajrulai-Musliu, Z.; Uzunov, R.; Jovanov, S.; Jankuloski, D.; Stojkovski, V.; Pendovski, L.; Sasanya, J.J. A new LC–MS/MS method for multiple residues/contaminants in bovine meat. BMC Chem. 2021, 15, 62. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Validation of analytical procedures: Text and methodology Q2(R1). Current Step 4 version Parent Guideline dated 27 October 1994. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 15 October 2023).
- EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance, Version 2.0.1 2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 5 June 2023).
- Kimera, Z.I.; Mshana, S.E.; Rweyemamu, M.M.; Mboera, L.E.G.; Matee, M.I.N. Antimicrobial use and resistance in food producing animals and the environment: An African perspective. Antimicrob. Resist. Infect. Control 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Nisyriou, S. Multi-residue methods for confirmatory determination of antibiotics in milk. J. Sep. Sci. 2008, 31, 2068–2090. [Google Scholar] [CrossRef] [PubMed]
- Alija, G.; Hajrulai-Musliu, Z.; Uzunov, R. Development and validation of confirmatory LC–MS/MS method for multi-residue analysis of antibiotic drugs in bovine milk. SN Appl. Sci. 2020, 2, 1563. [Google Scholar] [CrossRef]
- Chowdhury, S.; Hassan, M.M.; Alam, M.; Sattar, S.; Bari, M.S.; Saifuddin, A.K.M.; Hoque, M.A. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Vet. World. 2015, 8, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Mehran, M.A.; Hossein, B.; Masoud, A.; Ashraf-O-Sadat, N.; Mahboob, N. Simultaneous determination of tetracyclines residues in bovine milk samples by solid phase extraction and HPLC-FL method. Adv. Pharm. Bull. 2011, 1, 34–39. [Google Scholar] [CrossRef]
- Hassan, B.A.R. HPLC uses and importance in the pharmaceutical analysis and industrial field. Pharm. Anal. Acta 2012, 3, 9. [Google Scholar] [CrossRef]
- Martínez-Cortés, I.; Rosiles, R.; Gutierrez, L.; Díaz, D.; Sumano, H. Amoxicillin resides in milk of Holstein cows with double or triple intramammary administration of amoxicillin-potassium clavulanate. Int. J. Appl. Res. Vet. Med. 2014, 12, 186–192. [Google Scholar]
- Burmańczuk, A.; Tomasz, G.; Gbylik-Sikorska, M.; Gajda, A.; Kowalski, C. Withdrawal of Amoxicillin and Penicillin G Procaine from Milk after Intramammary Administration in Dairy Cows with Mastitis. J. Vet. Res. 2017, 61, 37–43. [Google Scholar] [CrossRef]
- Mahmood, T.; Abbas, M.; Ilyas, S.; Afzal, N.; Nawaz, R. Quantification of fluoroquinolone (enrofloxacin, norfloxacin and ciprofloxacin) residues in cow milk. Int. J. Chem. Biochem. Sci. 2016, 10, 10–15. [Google Scholar]
- Azabo, R.R.; Mshana, S.E.; Matee, M.I.; Kimera, S.I. Antimicrobial Resistance Pattern of Escherichia coli Isolates from Small Scale Dairy Cattle in Dar es Salaam, Tanzania. Animals 2022, 12, 1853. [Google Scholar] [CrossRef]
- Kijima-Tanaka, M.; Ishihara, K.; Morioka, A.; Kojima, A.; Ohzono, T.; Ogikubo, K.; Takahashi, T.; Tamura, Y. A national surveillance of antimicrobial resistance in Escherichia coli isolated from food-producing animals in Japan. J. Antimicrob. Chemother. 2003, 51, 447–451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bywater, R.; Deluyker, H.; Deroover, E.; De Jong, A.; Marion, H.; McConville, M.; Rowan, T.; Shryock, T.; Shuster, D.; Thomas, V.; et al. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food-producing animals. J. Antimicrob. Chemother. 2004, 54, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Kim, S.J.; Moon, D.C.; Mechesso, A.F.; Choi, J.H.; Kang, H.Y.; Boby, N.; Yoon, S.S.; Lim, S.K. Antimicrobial Resistance in Escherichia coli Isolates from Healthy Food Animals in South Korea. Microorganisms 2022, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Jeckel, S.; Snow, L.; Stubbs, R.; Teale, C.; Wearing, H.; Horton, R.; Toszeghy, M.; Tearne, O.; Ellis-Iversen, J.; et al. Epidemiology of extended spectrum beta-lactamase E. coli (CTX-M-15) on a commercial dairy farm. Vet Microbiol. 2012, 154, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kerluku, M.; Jankuloski, D.; Manovska Ratkova, M.; Prodanov, M.; Dimzoska Stojanovska, B.; Dodovski, A.; Blagoevska, K. β-Lactamase genes (blaCTX-M, blaSHV, blaTEM, blaOXA1 AND blaOXA2) and phylogenetic groups in ESBL producing commensal Escherichia coli isolated from faecal samples from dairy farm in the Municipality of Debar. Mac. Vet. Rev. 2023, 46, 89–97. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Huang, F.Y.; Gan, L.L.; Yu, X.; Cai, D.J.; Fang, J.; Zhong, Z.J.; Guo, H.R.; Xie, Y.; Wang, Z.S.; et al. High prevalence of blaCTX-M and blaSHV among ESBL producing E. coli isolates from beef cattle in China’s Sichuan-Chongqing circle. Sci. Rep. 2021, 11, 13725. [Google Scholar] [CrossRef]
- Braun, S.D.; Ahmed, M.F.E.; El-Adawy, H.; Hotzel, H.; Engelmann, I.; Weiß, D.; Monecke, S.; Ehricht, R. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile delta. Egypt. Front. Microbiol. 2016, 7, 1020. [Google Scholar] [CrossRef] [PubMed]

| Standard | Ionisation Mode (ESI) | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy | Cone Voltage | Retention Time |
|---|---|---|---|---|---|---|
| Amoxicillin | + | 367.07 | 159.96 | 16 | 28 | 5.55 |
| 90.89 | 40 | |||||
| Ampicillin | + | 349.97 | 159.94 | 14 | 34 | 3.93 |
| 105.95 | 20 | |||||
| Procaine benzylpenicillin | + | 334.99 | 90.96 | 42 | 44 | 5.52 |
| 80.94 | 52 | |||||
| Lincomycin | + | 407.06 | 126.02 | 34 | 22 | 2.80 |
| 41.75 | 72 | |||||
| Tylosin | + | 916.30 | 100.88 | 52 | 74 | 6.31 |
| 173.99 | 46 | |||||
| Trimethoprim | + | 290.97 | 229.94 | 24 | 26 | 2.90 |
| 122.94 | 28 | |||||
| Cephapirin | + | 423.93 | 291.93 | 14 | 42 | 2.04 |
| 151.89 | 28 | |||||
| Tetracycline | + | 445.03 | 410.01 | 20 | 40 | 5.33 |
| 153.90 | 34 | |||||
| Cloxacillin | + | 435.94 | 159.97 | 18 | 26 | 6.15 |
| 276.96 | 14 | |||||
| Oxacillin | + | 402.05 | 159.96 | 10 | 24 | 5.95 |
| 243.03 | 12 | |||||
| Cefalexin | + | 347.97 | 173.93 | 14 | 30 | 2.75 |
| 157.86 | 8 | |||||
| Ceftiofur | + | 523.96 | 125.17 | 58 | 34 | 4.90 |
| 241.00 | 16 | |||||
| Enrofloxacin | + | 360.05 | 72.02 | 36 | 36 | 3.68 |
| 245.09 | 30 | |||||
| Ciprofloxacin | + | 332.01 | 230.94 | 28 | 38 | 3.56 |
| 245.05 | 40 | |||||
| Oxytetracycline | + | 462.01 | 426.02 | 38 | 36 | 3.17 |
| 200.93 | 30 | |||||
| Sulfachlorpyridazin | + | 284.9 | 155.93 | 16 | 28 | 2.93 |
| 91.93 | 34 | |||||
| Sulfadiazine | + | 250.97 | 155.93 | 14 | 28 | 1.92 |
| 91.93 | 30 | |||||
| Sulfadimethoxine | + | 310.97 | 91.93 | 32 | 36 | 4.36 |
| 155.93 | 20 | |||||
| Sulfadimidine | + | 278.95 | 91.93 | 36 | 34 | 2.71 |
| 185.93 | 18 | |||||
| Sulfamethoxazole | + | 253.91 | 155.94 | 16 | 28 | 3.01 |
| 92.00 | 30 | |||||
| Flunixin–D3 | + | 300.03 | 263.98 | 36 | 25 | 6.80 |
| Penicillin G–d7 | + | 374.03 | 159.94 | 16 | 32 | 5.51 |
| Standard | Calibration Range | R2 | ||
|---|---|---|---|---|
| Matrix | Matrix | |||
| Feces | Milk | Feces | Milk | |
| (μg/kg) | (μg/L) | |||
| Amoxicillin | 20.0–1000 | 1.0–50 | 0.9998 | 0.9993 |
| Ampicillin | 20.0–1000 | 1.0–50 | 0.9989 | 0.9963 |
| Procaine benzylpenicillin | 20.0–1000 | 1.0–50 | 0.9997 | 0.9999 |
| Lincomycin | 20.0–1000 | 50.0–300 | 0.9984 | 0.9978 |
| Tylosin | 20.0–1000 | 10.0–100 | 0.9978 | 0.9991 |
| Trimethoprim | 20.0–1000 | 10.0–100 | 0.9997 | 0.9994 |
| Cephapirin | 20.0–1000 | 10.0–100 | 0.9999 | 0.9994 |
| Tetracycline | 20.0–1000 | 10.0–200 | 0.9944 | 0.9974 |
| Cloxacillin | 20.0–1000 | 10.0–100 | 0.9961 | 0.9954 |
| Oxacillin | 20.0–1000 | 10.0–100 | 0.9984 | 0.9951 |
| Cefalexin | 20.0–1000 | 10.0–200 | 0.9929 | 0.9976 |
| Ceftiofur | 20.0–1000 | 10.0–200 | 0.9937 | 0.9979 |
| Enrofloxacin | 20.0–1000 | 10.0–200 | 0.9999 | 0.9981 |
| Ciprofloxacin | 20.0–1000 | 10.0–200 | 0.9949 | 0.9997 |
| Oxytetracycline | 20.0–1000 | 10.0–200 | 0.9994 | 0.9963 |
| Sulfachlorpyridazin | 20.0–1000 | 10.0–200 | 0.9974 | 0.9967 |
| Sulfadiazine | 20.0–1000 | 10.0–200 | 0.9979 | 0.9958 |
| Sulfadimethoxine | 20.0–1000 | 10.0–200 | 0.9979 | 0.9970 |
| Sulfadimidine | 20.0–1000 | 10.0–200 | 0.9981 | 0.9988 |
| Sulfamethoxazole | 20.0–1000 | 10.0–200 | 0.9978 | 0.9994 |
| Standard | LOD | LOQ | CCα | CCβ | ||||
|---|---|---|---|---|---|---|---|---|
| Matrix | Matrix | Matrix | Matrix | |||||
| Feces | Milk | Feces | Milk | Feces | Milk | Feces | Milk | |
| (μg/kg) | (μg/L) | (μg/kg) | (μg/L) | (μg/kg) | (μg/L) | (μg/kg) | (μg/L) | |
| Amoxicillin | 24.10 | 1.59 | 32.11 | 2.59 | 42.36 | 5.34 | 52.11 | 6.43 |
| Ampicillin | 20.18 | 1.28 | 57.17 | 1.76 | 35.14 | 4.66 | 49.78 | 5.23 |
| Procaine benzylpenicillin | 18.33 | 1.36 | 28.65 | 2.16 | 43.18 | 4.80 | 56.71 | 5.66 |
| Lincomycin | 14.10 | 47.94 | 35.17 | 55.78 | 27.45 | 179.19 | 37.52 | 199.07 |
| Tylosin | 12.80 | 10.71 | 34.12 | 13.62 | 20.12 | 55.31 | 31.40 | 66.09 |
| Trimethoprim | 13.36 | 8.68 | 21.17 | 11.01 | 38.29 | 54.74 | 49.40 | 65.31 |
| Cephapirin | 8.26 | 12.33 | 23.01 | 15.72 | 21.35 | 71.91 | 32.14 | 82.65 |
| Tetracycline | 21.35 | 8.65 | 55.60 | 11.05 | 41.78 | 105.91 | 52.40 | 116.86 |
| Cloxacillin | 22.18 | 10.24 | 59.60 | 12.97 | 37.54 | 31.74 | 54.25 | 35.93 |
| Oxacillin | 17.15 | 9.26 | 41.10 | 14.01 | 30.12 | 35.12 | 41.35 | 41.32 |
| Cefalexin | 16.35 | 12.38 | 35.50 | 15.02 | 21.35 | 105.14 | 34.17 | 118.36 |
| Ceftiofur | 9.12 | 11.22 | 26.40 | 14.67 | 15.35 | 101.66 | 27.14 | 108.76 |
| Enrofloxacin | 20.40 | 10.53 | 35.42 | 14.12 | 47.80 | 112.33 | 59.71 | 132.14 |
| Ciprofloxacin | 23.14 | 12.80 | 55.35 | 16.60 | 39.14 | 102.12 | 57.80 | 121.14 |
| Oxytetracycline | 27.60 | 10.76 | 41.60 | 12.12 | 51.48 | 113.65 | 66.41 | 131.29 |
| Sulfachlorpyridazin | 14.35 | 10.47 | 32.18 | 13.88 | 22.15 | 116.82 | 40.78 | 142.59 |
| Sulfadiazine | 21.30 | 10.89 | 55.60 | 13.39 | 27.15 | 121.95 | 58.35 | 152.05 |
| Sulfadimethoxine | 12.25 | 10.35 | 31.48 | 12.69 | 17.14 | 104.70 | 25.10 | 122.17 |
| Sulfadimidine | 12.70 | 9.06 | 34.15 | 11.42 | 19.48 | 121.30 | 31.40 | 146.93 |
| Sulfamethoxazole | 15.30 | 10.13 | 48.12 | 13.03 | 23.54 | 103.81 | 37.80 | 119.54 |
| Standards | Added | Average Concentration in the Samples (n = 6) | Standard Deviation | Recovery (%) | Repeatability | Reproducibility | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | (μg/kg) | (CVr, %) | (CVR, %) | |||||||||
| Matrix | Matrix | Matrix | Matrix | Matrix | Matrix | |||||||
| Feces | Milk | Feces | Milk (μg/L) | Feces | Milk (μg/L) | Feces | Milk | Feces | Milk | Feces | Milk | |
| (μg/kg) | (μg/L) | (μg/kg) | (μg/kg) | |||||||||
| Amoxicillin | 100 | 2 | 89.00 | 2.18 | 3.81 | 0.24 | 89.00 | 109.00 | 4.28 | 10.99 | 10.69 | 19.37 |
| 250 | 4 | 235.71 | 4.26 | 10.38 | 0.66 | 94.28 | 106.38 | 4.40 | 15.59 | 9.02 | 18.90 | |
| 500 | 6 | 464.26 | 5.22 | 17.66 | 0.61 | 92.85 | 86.97 | 3.80 | 11.61 | 14.37 | 14.57 | |
| Ampicillin | 100 | 2 | 80.07 | 1.74 | 3.13 | 0.21 | 80.07 | 87.51 | 3.90 | 12.07 | 11.35 | 16.64 |
| 250 | 4 | 238.59 | 3.56 | 11.08 | 0.65 | 95.44 | 89.36 | 4.64 | 18.26 | 14.88 | 20.18 | |
| 500 | 6 | 412.29 | 5.71 | 12.18 | 1.03 | 82.46 | 95.17 | 2.96 | 18.04 | 10.43 | 21.35 | |
| Procaine benzylpenicillin | 100 | 2 | 83.48 | 1.42 | 6.37 | 0.14 | 83.48 | 70.83 | 7.62 | 9.53 | 10.48 | 15.99 |
| 250 | 4 | 243.11 | 3.92 | 13.61 | 0.53 | 97.24 | 97.92 | 5.60 | 13.59 | 6.17 | 18.18 | |
| 500 | 6 | 473.10 | 4.93 | 17.87 | 0.35 | 94.62 | 82.08 | 3.78 | 7.10 | 6.60 | 14.23 | |
| Lincomycin | 100 | 75 | 85.56 | 61.25 | 6.00 | 3.14 | 85.56 | 81.67 | 7.01 | 5.13 | 14.22 | 6.82 |
| 250 | 150 | 230.61 | 159.31 | 12.28 | 12.12 | 92.24 | 106.20 | 5.32 | 7.61 | 12.76 | 8.87 | |
| 500 | 225 | 428.11 | 218.62 | 10.78 | 27.88 | 85.62 | 97.16 | 2.52 | 12.76 | 6.16 | 15.78 | |
| Tylosin | 100 | 25 | 86.39 | 22.25 | 4.63 | 2.30 | 86.69 | 89.01 | 5.36 | 10.32 | 10.94 | 14.72 |
| 250 | 50 | 204.21 | 44.53 | 16.96 | 6.57 | 81.68 | 89.07 | 8.30 | 14.76 | 14.99 | 17.14 | |
| 500 | 75 | 488.40 | 74.53 | 8.28 | 4.82 | 97.68 | 99.38 | 1.70 | 6.47 | 5.68 | 10.84 | |
| Tylosin | 100 | 25 | 97.11 | 21.58 | 5.64 | 2.79 | 97.11 | 86.31 | 5.80 | 12.94 | 13.03 | 15.85 |
| 250 | 50 | 263.79 | 44.17 | 12.02 | 6.44 | 105.92 | 88.34 | 4.56 | 14.59 | 8.03 | 17.37 | |
| 500 | 75 | 524.30 | 73.07 | 19.47 | 6.95 | 104.86 | 97.43 | 3.71 | 9.51 | 11.10 | 11.73 | |
| Cephapirin | 100 | 30 | 97.74 | 26.26 | 4.99 | 1.23 | 97.74 | 87.52 | 5.11 | 4.68 | 10.36 | 7.31 |
| 250 | 60 | 259.89 | 61.18 | 12.12 | 6.54 | 103.96 | 101.96 | 4.66 | 10.7 | 14.85 | 13.06 | |
| 500 | 90 | 458.11 | 82.28 | 25.58 | 1.99 | 91.62 | 91.42 | 5.58 | 2.41 | 12.10 | 3.48 | |
| Tetracycline | 100 | 25 | 102.32 | 44.78 | 7.20 | 3.82 | 102.32 | 89.57 | 7.04 | 8.53 | 12.55 | 12.52 |
| 250 | 50 | 230.34 | 94.95 | 12.22 | 6.68 | 92.14 | 94.95 | 5.30 | 7.04 | 11.39 | 9.80 | |
| 500 | 75 | 491.56 | 126.81 | 11.55 | 8.30 | 98.31 | 84.54 | 2.35 | 6.55 | 8.18 | 8.41 | |
| Cloxacillin | 100 | 15 | 105.4 | 14.83 | 3.77 | 1.21 | 105.4 | 98.88 | 3.58 | 8.19 | 6.77 | 10.74 |
| 250 | 30 | 255.39 | 27.54 | 4.95 | 2.56 | 102.16 | 91.81 | 1.94 | 9.28 | 3.98 | 12.12 | |
| 500 | 45 | 488.92 | 39.51 | 11.80 | 4.48 | 97.78 | 87.80 | 2.41 | 11.33 | 5.89 | 17.86 | |
| Oxacillin | 100 | 15 | 89.29 | 14.22 | 4.92 | 2.44 | 89.29 | 94.84 | 5.51 | 17.16 | 9.66 | 22.04 |
| 250 | 30 | 221.37 | 27.36 | 7.98 | 3.08 | 88.55 | 91.22 | 3.61 | 11.26 | 6.01 | 14.33 | |
| 500 | 45 | 450.84 | 40.17 | 6.83 | 5.44 | 90.17 | 89.27 | 1.23 | 13.54 | 4.44 | 16.28 | |
| Cefalexin | 100 | 50 | 85.73 | 41.48 | 5.14 | 5.26 | 85.73 | 82.96 | 6.00 | 12.68 | 7.61 | 15.87 |
| 250 | 100 | 244.44 | 88.48 | 7.19 | 7.11 | 97.77 | 88.48 | 2.94 | 8.04 | 8.21 | 10.97 | |
| 500 | 150 | 484.13 | 123.17 | 5.93 | 9.22 | 96.83 | 82.11 | 1.93 | 7.49 | 7.33 | 10.02 | |
| Ceftiofur | 100 | 50 | 93.60 | 50.49 | 2.13 | 3.67 | 93.6 | 100.97 | 2.28 | 7.27 | 5.73 | 14.39 |
| 250 | 100 | 246.23 | 94.57 | 9.15 | 4.33 | 98.49 | 94.57 | 3.72 | 4.57 | 5.12 | 7.82 | |
| 500 | 150 | 480.64 | 132.32 | 8.85 | 9.27 | 96.13 | 88.21 | 1.84 | 7.00 | 4.30 | 9.89 | |
| Enrofloxacin | 100 | 50 | 82.64 | 54.10 | 8.60 | 7.47 | 82.64 | 108.2 | 10.41 | 13.8 | 13.31 | 15.92 |
| 250 | 100 | 212.54 | 92.52 | 12.42 | 12.08 | 85.01 | 92.52 | 5.84 | 13.06 | 14.81 | 14.25 | |
| 500 | 150 | 432.07 | 141.36 | 27.74 | 17.48 | 86.41 | 98.22 | 6.42 | 11.86 | 14.74 | 14.96 | |
| Ciprofloxacin | 100 | 50 | 89.56 | 45.49 | 3.24 | 3.14 | 89.56 | 90.97 | 3.62 | 6.91 | 8.76 | 9.73 |
| 250 | 100 | 247.88 | 83.10 | 4.79 | 11.6 | 99.15 | 83.10 | 1.93 | 13.96 | 5.61 | 15.46 | |
| 500 | 150 | 495.32 | 131.55 | 3.98 | 16.46 | 99.06 | 87.70 | 0.80 | 12.51 | 2.11 | 15.98 | |
| Oxytetracycline | 100 | 50 | 89.82 | 40.68 | 9.93 | 3.28 | 89.82 | 88.04 | 11.06 | 7.45 | 14.99 | 9.39 |
| 250 | 100 | 209.99 | 96.00 | 14.24 | 10.76 | 83.99 | 96.00 | 6.78 | 11.21 | 14.76 | 13.22 | |
| 500 | 150 | 421.17 | 159.94 | 21.69 | 13.38 | 84.23 | 106.63 | 5.15 | 8.36 | 12.98 | 10.91 | |
| Sulfachlorpyridazin | 100 | 50 | 82.45 | 48.06 | 2.52 | 7.51 | 82.45 | 96.11 | 3.06 | 15.63 | 5.21 | 18.96 |
| 250 | 100 | 219.02 | 91.05 | 4.01 | 15.71 | 87.61 | 91.05 | 1.83 | 17.26 | 4.68 | 19.98 | |
| 500 | 150 | 470.61 | 151.33 | 11.69 | 18.25 | 94.12 | 100.89 | 2.48 | 12.06 | 5.02 | 13.33 | |
| Sulfadiazine | 100 | 50 | 86.08 | 46.89 | 4.51 | 8.74 | 86.08 | 93.79 | 5.25 | 18.63 | 6.90 | 21.68 |
| 250 | 100 | 246.59 | 91.85 | 6.09 | 18.35 | 98.63 | 91.85 | 2.47 | 19.98 | 3.77 | 20.84 | |
| 500 | 150 | 488.60 | 141.33 | 8.44 | 7.33 | 97.72 | 94.21 | 1.73 | 5.18 | 2.58 | 8.18 | |
| Sulfadimethoxine | 100 | 50 | 83.62 | 42.42 | 1.67 | 4.86 | 83.62 | 84.84 | 2.00 | 11.46 | 3.09 | 14.55 |
| 250 | 100 | 226.68 | 87.23 | 7.03 | 10.65 | 90.67 | 87.23 | 3.10 | 12.21 | 6.20 | 13.34 | |
| 500 | 150 | 467.95 | 123.81 | 13.21 | 12.21 | 93.59 | 82.54 | 2.82 | 11.00 | 6.62 | 13.30 | |
| Sulfadimidine | 100 | 50 | 97.40 | 42.50 | 2.75 | 8.98 | 97.40 | 84.99 | 2.83 | 21.12 | 6.29 | 23.44 |
| 250 | 100 | 243.62 | 95.66 | 5.60 | 15.63 | 97.45 | 95.66 | 2.30 | 16.33 | 4.03 | 17.22 | |
| 500 | 150 | 511.71 | 132.17 | 5.54 | 20.01 | 102.34 | 88.11 | 1.08 | 15.14 | 2.61 | 16.77 | |
| Sulfamethoxazole | 100 | 50 | 85.05 | 46.38 | 2.93 | 5.80 | 85.05 | 92.75 | 3.45 | 12.51 | 8.23 | 16.53 |
| 250 | 100 | 236.86 | 88.06 | 11.12 | 9.60 | 94.74 | 88.06 | 4.69 | 10.90 | 6.14 | 13.27 | |
| 500 | 150 | 480.43 | 120.05 | 5.77 | 11.45 | 96.09 | 80.03 | 1.08 | 9.54 | 2.61 | 13.70 | |
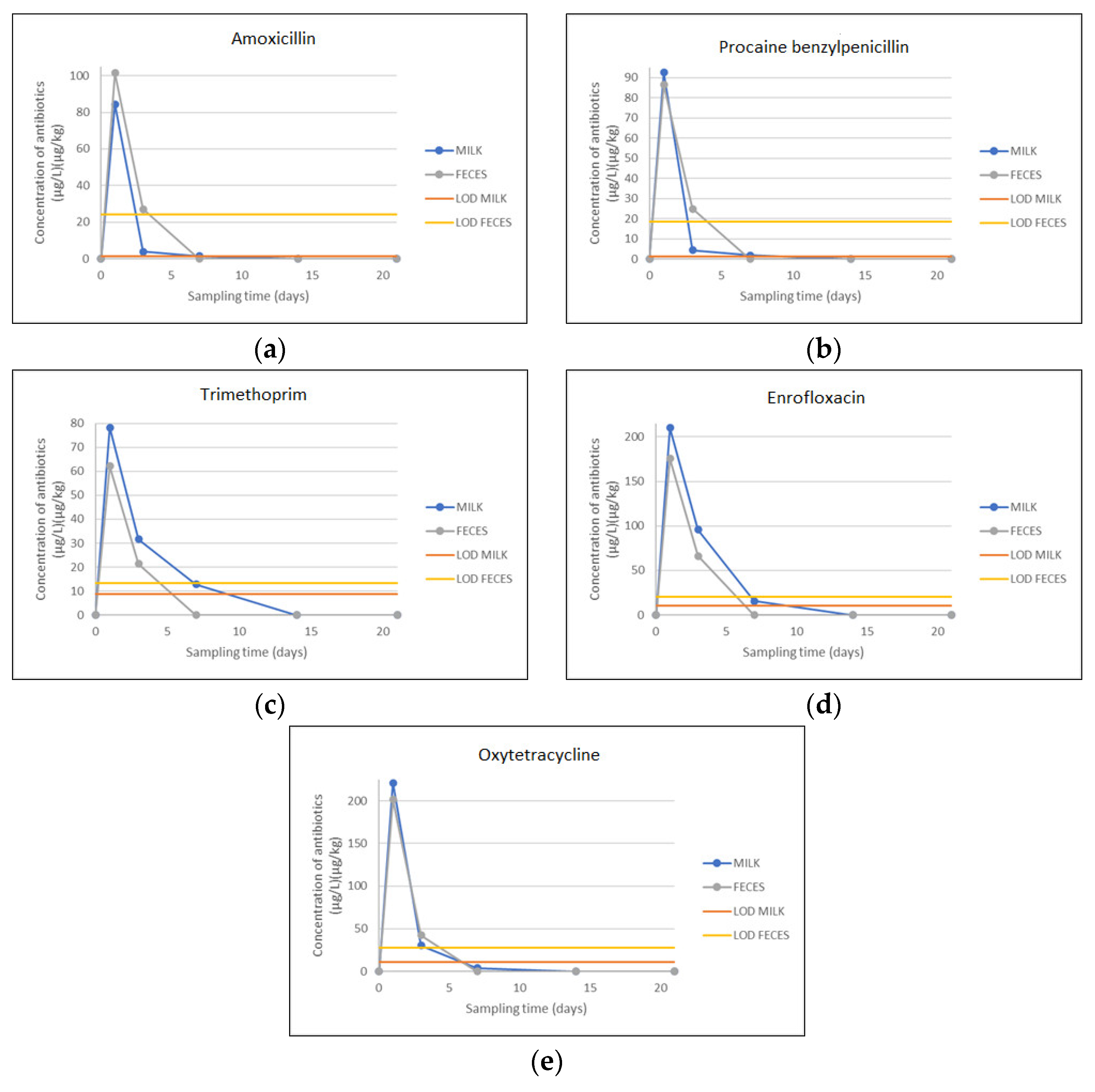
| Antibiotic | Matrix | Sampling Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 7 | 14 | 21 | LOD | CCα | MRL | ||
| Amoxicillin | Milk | ND | 84.35 | 3.80 | 1.35 | <LOD | <LOD | 1.28 | 5.34 | 4.0 |
| µg/L | ||||||||||
| Feces | ND | 101.38 | 27.11 | <LOD | <LOD | <LOD | 24.10 | 42.36 | / | |
| µg/kg | ||||||||||
| Procaine benzylpenicillin | Milk | ND | 92.50 | 4.40 | 1.72 | <LOD | <LOD | 1.36 | 4.80 | 4.0 |
| µg/L | ||||||||||
| Feces | ND | 86.30 | 24.88 | <LOD | <LOD | <LOD | 18.33 | 43.18 | / | |
| µg/kg | ||||||||||
| Trimethoprim | Milk | ND | 78.30 | 31.60 | 12.80 | <LOD | <LOD | 8.68 | 54.74 | 50.0 |
| µg/L | ||||||||||
| Feces | ND | 62.18 | 21.30 | <LOD | <LOD | <LOD | 13.36 | 38.29 | / | |
| µg/kg | ||||||||||
| Enrofloxacin | Milk | ND | 210.10 | 95.60 | 15.40 | <LOD | <LOD | 10.53 | 112.33 | 100.0 |
| µg/L | ||||||||||
| Feces | ND | 175.48 | 66.20 | <LOD | <LOD | <LOD | 20.40 | 47.80 | / | |
| µg/kg | ||||||||||
| Oxytetracycline | Milk | ND | 221.10 | 30.20 | 3.90 | <LOD | <LOD | 10.76 | 113.65 | 100.0 |
| µg/L | ||||||||||
| Feces | ND | 201.30 | 42.45 | <LOD | <LOD | <LOD | 27.60 | 51.48 | / | |
| µg/kg | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajrulai-Musliu, Z.; Uzunov, R.; Krluku, M.; Jovanov, S.; Stojkovski, V.; Arapcheska, M.; Musliu, D.; Sasanya, J.J. Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period. Animals 2023, 13, 3603. https://doi.org/10.3390/ani13233603
Hajrulai-Musliu Z, Uzunov R, Krluku M, Jovanov S, Stojkovski V, Arapcheska M, Musliu D, Sasanya JJ. Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period. Animals. 2023; 13(23):3603. https://doi.org/10.3390/ani13233603
Chicago/Turabian StyleHajrulai-Musliu, Zehra, Risto Uzunov, Maksud Krluku, Stefan Jovanov, Velimir Stojkovski, Mila Arapcheska, Dea Musliu, and James Jacob Sasanya. 2023. "Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period" Animals 13, no. 23: 3603. https://doi.org/10.3390/ani13233603
APA StyleHajrulai-Musliu, Z., Uzunov, R., Krluku, M., Jovanov, S., Stojkovski, V., Arapcheska, M., Musliu, D., & Sasanya, J. J. (2023). Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period. Animals, 13(23), 3603. https://doi.org/10.3390/ani13233603






