Simple Summary
Broiler feeding now faces great challenges because of the rising cost of poultry diets, with corn being the main component. Developing and utilizing unconventional feed resources is an effective method of feeding broilers, thereby alleviating the problem of a high-cost diet. This study investigated how supplementation with jujube powder influences the growth performance, nutrient apparent utilization, serum immune indices, antioxidant indices, and intestinal microbiota of Cobb broilers. Adding jujube powder to the diet significantly improved the average daily gain, the apparent utilization rate of organic matter, and the apparent metabolic energy of broilers; it enhanced the body’s immunity and antioxidant performance and improved the intestinal microbiota of broilers. The most appropriate percentage of jujube powder to be supplemented was 8%. This indicates that jujube powder can act as a new feed type to replace some basic broiler diets.
Abstract
In total, 576 Cobb broilers were randomized into 6 treatment groups, with 8 replicates in each treatment group and 12 broilers in each replicate. Each treatment group was fed six different experimental diets containing 0%, 2%, 4%, 6%, 8%, and 10% jujube powder. The group receiving 0% jujube powder was considered the blank control group. The experimental period was 42 days and was divided into two periods: starter (0–21 days) and finisher (22–42 days). Compared with the control group, the addition of 8% jujube powder significantly improved the ADG of broilers (p < 0.05), and 8% and 10% jujube powder significantly improved the total tract apparent digestibility of organic matter in broilers (p < 0.05). Adding 10% jujube powder significantly improved the apparent metabolic energy of broilers (p < 0.05). Compared with the control group, 4–10% jujube powder significantly increased IgA, IgG, IgM, and sCD4 levels (p < 0.05) and T-AOC and SOD contents, and it reduced the MDA content in the serum of broilers (p < 0.05). In addition, the relative abundance of Firmicutes, Bacteroidetes, Lactobacillus, and Romboutsia significantly increased in the broiler ileum, whereas that of Proteobacteria and Enterobacter decreased significantly (p < 0.05) when 8% jujube powder was added to the diet. The relative abundance of Proteobacteria, Bacteroides, and Faecalibacterium in the cecum increased significantly (p < 0.05), whereas that of Bacteroidetes decreased significantly (p < 0.05).
1. Introduction
Chickens are the most common domesticated animal. Compared with other types of meat, chicken meat is rich in proteins, trace elements, carnosine, creatinine, and amino acids [1,2]. Thus, broiler feeding is irreplaceable. However, the cost of poultry feed, with corn being the main component, has recently been increasing. To promote the sustainable development of the poultry industry and effectively alleviate the problem of high feed costs, developing and utilizing unconventional feed resources has become an inevitable new trend [3,4,5,6].
Red jujube is abundant in China [7], and a large amount of poor-quality jujube is discarded as agricultural waste. Small, scarred, and defective jujubes can be used to prepare jujube powder as an unconventional feed for animals, thereby achieving a win–win situation for fruit farmers and poultry producers. The basic nutrient content of jujube is similar to that of corn [8,9]. Thus, jujube can be used as a high-energy feed, replacing parts of corn in corn–soybean-based animal diets [10]. Furthermore, jujube is rich in minerals, vitamins, and various active substances, such as polysaccharides, phenols, terpenes, nucleosides, dietary fiber, and rutin, which exert dual regulatory effects of nutrition and physiology [11,12,13,14,15]. A diet supplemented with jujube powder can significantly improve the antioxidant capacity of serum, improve body metabolism, and enhance the immunity of laying hens [16]. A diet supplemented with yeast selenium and jujube powder can also maintain the structural stability of the chicken myofibrillar protein after slaughter, improve its digestion characteristics, and inhibit protein oxidation [17,18]. Jujube powder added to the diet can also enhance the growth performance, nutrient digestibility, and meat quality of goats [19]. Jujube also exerts inhibitory effects on cancer cells at different growth stages [20], and its bioactive components (ursolic acid and oleanolic acid) have antiproliferative and apoptotic effects on cancer cells (ovarian cancer cells and normal human fibroblasts BJ1-hTERT and nonmalignant breast epithelial MCF-10A cells) [21,22]. Jujube powder is currently known to mainly improve the meat quality and blood biochemistry in broilers, and the action mechanism of this powder on intestinal microorganisms in broilers is rarely reported. Therefore, in this experiment, defective jujubes were processed into a powder and added to the broiler diet to evaluate the effects of the addition of different amounts of jujube powder on the growth performance, nutrient utilization, blood physical and chemical indices, and intestinal microbiota of broilers. This information would provide a theoretical basis for the rational application of jujube powder in poultry production.
2. Materials and Methods
2.1. Test Materials
The jujube used in this experiment was collected from the jujube garden in Minqin County, Wuwei City, Gansu Province. The dehiscent jujube, ground jujube, and small jujube were selected manually. Moth-eaten and moldy jujubes were removed. The selected jujubes were then sun-dried in a well-ventilated place. The jujube powder was obtained by freezing the jujubes and then crushing the frozen jujubes using a freeze crusher.
The contents of nutrients in jujube powder were determined using conventional methods. The crude protein content was determined by the Kjeldahl method (GB/T 6432-2018 [23]), the crude fat content was determined by the Soxhlet extraction method (GB/T 6433-2006 [24]), the crude fiber content was determined by the filtration method (GB/T 6434-2006 [25]), the crude ash content was determined by the high-temperature burning method (GB/T 6438-2007 [26]), the calcium content was determined by the potassium permanganate titration method (GB/T 6436-2018 [27]), and the phosphorus content was determined by the spectrophotometric method (GB/T 6437-2018 [28]). The results of the measurements are presented in Table 1.

Table 1.
Nutrition content of jujube powder.
2.2. Test Design
In total, 576 1-day-old male Cobb broilers were randomized into 6 treatment groups, with 8 replicates in each group and 12 chickens in each replicate. They were fed corn–soybean meal-based diets supplemented with 0%, 2%, 4%, 6%, 8%, or 10% jujube powder. The test diet was formulated by referring to the agricultural industry standard of the People’s Republic of China chicken feeding standard (NY-T33-2004 [29]). Table 2 presents the composition and nutritional level of diets. The diets used in the present study were calculated to meet or exceed the National Research Council (NRC) nutrient requirements for broilers [30]. The experiment lasted for 42 days, which was divided into two stages: starter (0–21 days) and finisher (22–42 days). Because of winter feeding, the animal house was warmed up 2 days before the chickens entered the houses. The temperature was controlled using a water-heating boiler. It was maintained at 35 °C to 38 °C for 3 days and gradually cooled down to 20 °C at a rate of 1 °C every day. Then, the same temperature was maintained until the end of the experiment. Within 2 h after the experimental chicks arrived, low-concentration (5%) glucose aqueous solution was used for drinking, and the water temperature was moderate. In total, 12 chickens were reared in three-layer cages (length: 140 cm, width: 70 cm, and height: 40 cm), with 8 cages in each group. During the experimental period, the chickens were allowed free access to food and drink and exposed to light for 24 h. The relative humidity of the animal house was 50%. The animal house was ventilated normally, and the environment was kept clean. The animals were immunized in strict accordance with the Cobb broilers’ immunization management manual.

Table 2.
Composition and nutritional level of diets (air-dry basis, %).
2.3. Observations
2.3.1. Growth Performance
The broilers were weighed by pens at 0, 21, and 42 days of age. The amount of feed consumed by each pen was recorded over the starter and finisher periods. Mortality was recorded daily. Any bird that died was weighed, and the weight was used to adjust the feed/gain ratio (F/G). The F/G was calculated by dividing the total feed intake by the weight of live plus dead birds. The average daily feed intake (ADFI), average daily gain (ADG), and F/G were calculated as follows:
2.3.2. Coefficient of Total Tract Apparent Nutrient Digestibility or Retention
On the 35th day of the feeding experiment, three broilers in good body condition and of similar body weights were randomly selected from each group. They were fed in a single cage for the complete fecal metabolism test. On the 37th day, fasting was started at 20:00 to eliminate the effect of the intestinal contents of the broilers on the metabolism test. Free drinking water was made available during fasting, with no changes in other feeding conditions. At 08:00 of the 38th to 41st day, fresh excreta (removing debris, feed, and other debris) were collected for 4 consecutive days. Then, 10 mL of 10% hydrochloric acid was immediately added per 100 g of excreta, and the mixture was immediately refrigerated at −20 °C. Later, the frozen excrement was collected, thawed, mixed evenly, and dried at 65 °C to a constant weight. The excreta were weighed after being maintained at room temperature for 24 h to regain moisture. Then, the excreta were crushed through a 40-mesh sieve, added to a bag, and sealed for the subsequent test and analysis. The contents of total tract apparent dry matter and organic matter digestibility and total tract nitrogen retention in the excreta were determined by referring to the AOAC method [31]. Energy was measured using the oxygen and nitrogen calorimeter (ikc2000, IKA, Staufen, Germany). Titanium dioxide was determined by referring to the methods of Short et al. [32]. The total tract apparent nutrient digestibility or retention was calculated as follows:
2.3.3. Serum Immune and Antioxidant Indices
At the end of the experimental period, 8 chickens (one for each repetition) were randomly selected from each treatment group. Blood was withdrawn from the chicken wing vein. After the blood sample was centrifuged at 4000 r/min for 15 min, the serum was separated, packed in a 2 mL centrifuge tube, and stored at −20 °C to determine immune and antioxidant indices. The serum immunoglobulin IgA, IgG, and IgM and the soluble CD4 surface antigen (sCD4) were determined using the ELISA kit (mlbio, Shanghai, China). The serum glutathione peroxidase (GSH-Px), malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) were determined using the kit of Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China.
2.3.4. Intestinal Chyme Sample Collection and Microbial Sequencing
Six broilers with the best growth performance on day 42 were randomly selected from the control and treatment groups. After slaughtering these broilers, the ileum and cecum were stripped. The midgut chyme was collected, subpacked in a 2 mL cryopreservation tube, and stored at −80 °C for the high-throughput sequencing of microbial bacteria 16S V3-V4. Sequencing was completed by Beijing Biomarker Biotechnology Co., Ltd., Beijing, China. Total DNA was extracted from ileal and cecal digesta samples using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany); bacterial DNA was quantified using a Microvolume UV-Vis Spectrophotometer (NanoDrop™ One, Thermo Fisher Scientific, Waltham, MA, USA) and standardized at 5 ng/μL. PCR amplification was then performed; the conditions for the amplification were set up as follows: 3 min at 95 °C, 30 s at 95 °C (25 cycles), 30 s at 55 °C, 30 s at 72 °C, 5 min at 72 °C, and then held at 4 °C. The PCR products were cleaned, and the library was combined with the sequencing adapters and dual indices using the Nextera XT Index Kit (Illumina, San Diego, CA, USA). The PCR assay conditions were set up as follows: 3 min at 95 °C, 30 s at 95 °C (eight cycles), 30 s at 55 °C, 30 s at 72 °C, 5 min at 72 °C, and held at 4 °C. Next, for purification of the PCR products, AMPure XP beads were used. The libraries were quantified using a KAPA Illumina library quantification kit (KAPA Biosystems, Bellevue, WA, USA). Individual concentrations of the DNA libraries were calculated in nM based on the size of the DNA amplicons as determined by an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The original microbial sequencing data were imported into FLASH v1.2.7 software for overlap data splicing to obtain original tags. The splices were then removed using Trimmomatic v0.33 software to obtain clean tags from among low-quality fragments. Finally, the chimera was removed using UCHIME v4.2 software to obtain the final effective tags. Uparse v7.0.1001 was used to cluster the effective tags with 97% similarity, and representative operational taxonomic unit (OTU) sequences were screened. The SSUrRNA database was used to annotate the representative bacterial OTUs to obtain the bacterial composition at phylum and genus levels. Finally, the data were normalized through α diversity and β diversity analyses. The dilution curve and species distribution histogram were drawn using R software R-4.2.2.
2.4. Data Statistics and Analysis
Raw data, preliminarily processed using Excel 2023, were normalized by centering around the mean and homogenized to meet analysis requirements. One-way ANOVA was performed for data analysis by using SPSS 21.0 (IBM Corporation, New York, NY, USA). Differences between the experimental groups were compared using Duncan’s test. For microorganisms between the two groups, α statistical analysis of diversity and species distribution differences were completed using the SPSS 21.0 independent t-test module, and the significance level was set as p < 0.05.
3. Results
3.1. Effect of Jujube Powder on the Growth Performance of Broilers
Compared with the control group, the diet supplemented with 2%, 4%, 6%, 8%, and 10% jujube powder had no significant effect on the ADG, ADFI, and F/G of broilers in the starter and finisher periods (p > 0.05). However, the addition of 8% jujube powder significantly improved the ADG during days 0–42 (p < 0.05) (Table 3).

Table 3.
Effects of jujube powder on the growth performance of broilers.
3.2. Effect of Jujube Powder on Apparent Nutrient Utilization in Broilers
Compared with the control group, the diet supplemented with 8% and 10% jujube powder significantly improved the apparent utilization of organic matter in the broilers (p < 0.05); it increased by 6.7% and 6.8%, respectively. Then, 10% jujube powder significantly improved the apparent metabolic energy of the broilers (p < 0.05), which was increased by 0.41%. The added jujube powder had no significant effect on the total tract apparent digestibility of dry matter and total tract nitrogen retention in the broilers (p > 0.05) (Table 4).

Table 4.
Effects of jujube powder on the total tract apparent nutrient utilization digestibility or retention of broilers.
3.3. Effect of Jujube Powder on the Serum Immune Indices of Broilers
Compared with the control group, the diet supplemented with 4%, 6%, 8%, and 10% jujube powder significantly increased serum IgA, IgG, IgM, and sCD4 levels in the broilers. Only the 2% jujube powder had no significant effect (Table 5; p < 0.05).

Table 5.
Effects of jujube powder on serum immune indices in broilers.
3.4. Effect of Jujube Powder on the Serum Antioxidant Performance of Broilers
Compared with the control group, the diet supplemented with 2%, 4%, 6%, 8%, and 10% jujube powder significantly increased serum T-AOC and SOD contents in the broilers (Table 6; p < 0.05) and significantly reduced the serum MDA content (p < 0.05). In addition to the diet supplemented with 2% jujube powder, the diet supplemented with 4%, 6%, 8%, and 10% jujube powder significantly increased the serum GSH-Px content in the broilers (p < 0.05), of which the 10% diet led to the most improvement.

Table 6.
Effects of jujube powder on the serum antioxidant properties of broilers.
3.5. Effect of Jujube Powder on the Intestinal Microbiota of Broilers
3.5.1. Ileal and Cecal Microbiota Sequencing and α Diversity Analysis
In total, 1,919,975 pairs of reads were obtained through the sequencing of 12 ileal and cecal samples from the control group and the jujube powder group (8%). A total of 1,602,596 clean tags were obtained after removing the splices, filtering out impurities, and removing chimeras, with an average of 133,549 clean tags per sample. Ileal and cecal microbiota α diversity results revealed that, the ACE and Chao 1 indices of the ileum and cecum (Table 7) were significantly higher in the jujube powder group than in the control group (p < 0.05). This indicated that the number of species in the ileum and cecum increased significantly after jujube powder was added to the diet.

Table 7.
Effects of jujube powder supplementation on the ileal and cecal microbiota α diversity of broilers.
3.5.2. Effect of Jujube Powder on Microbial Species Composition in the Ileum and Cecum of Broilers
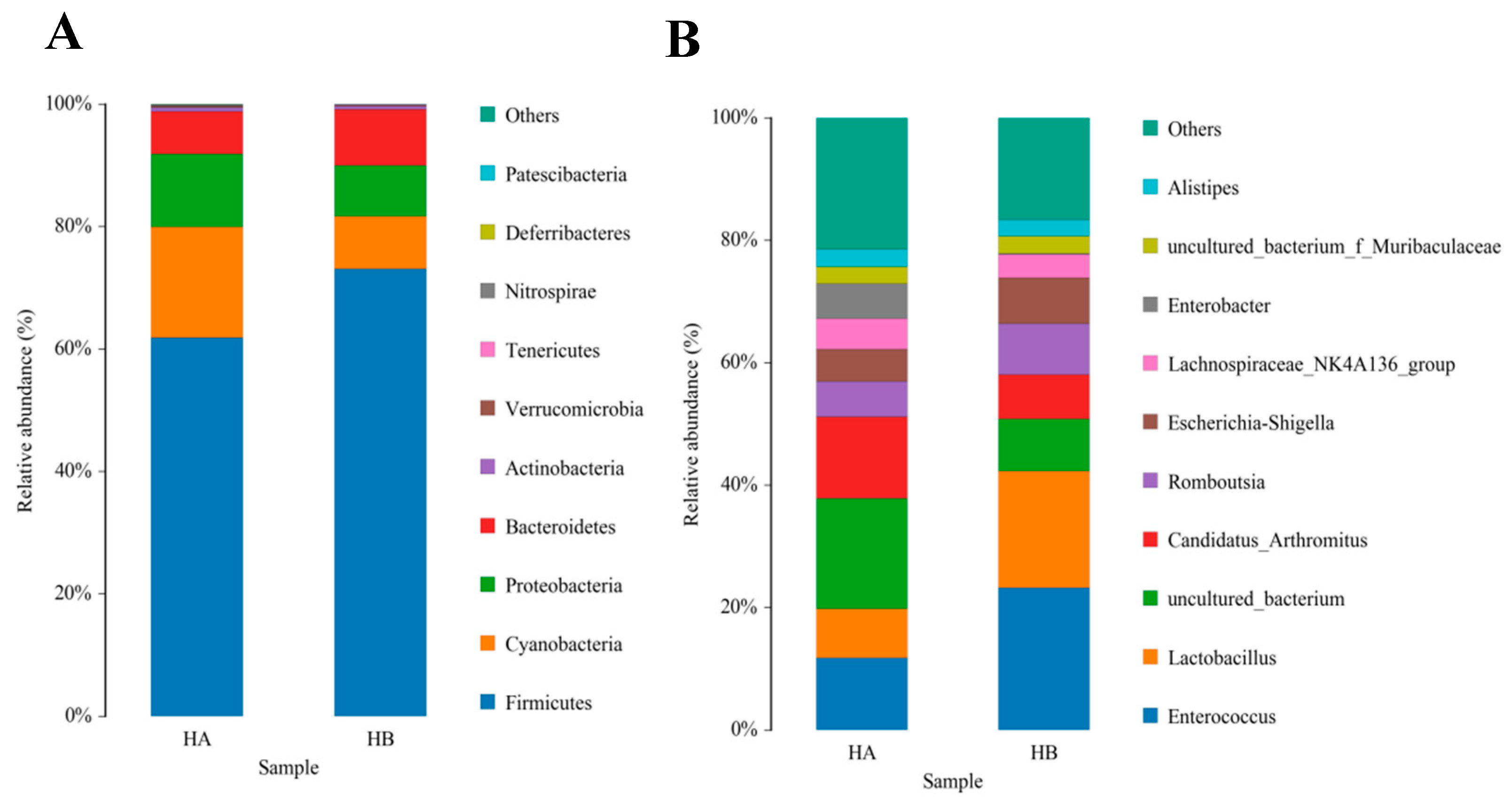
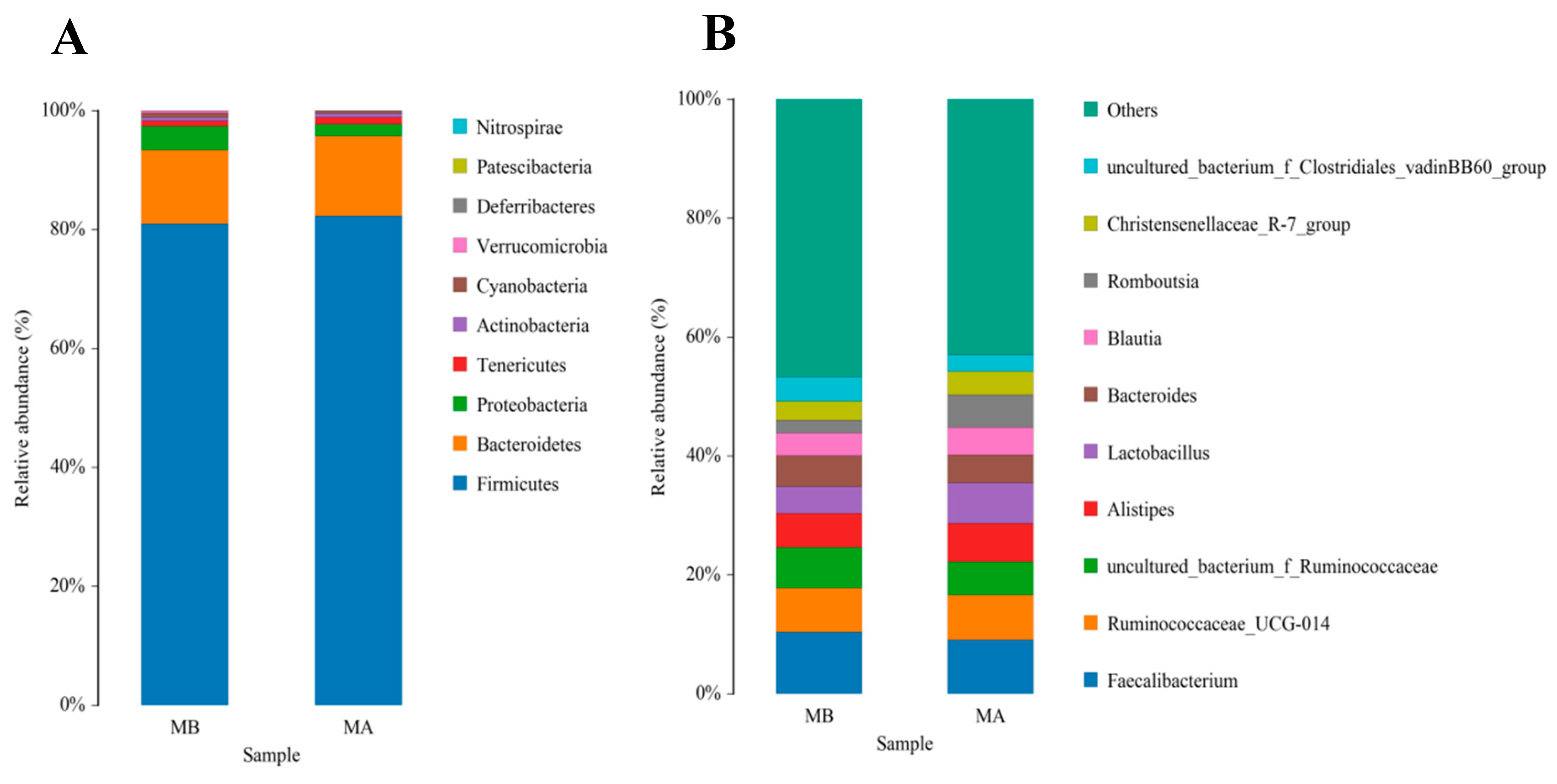
The top 10 species with a high relative abundance were screened according to the relative abundance ranking from high to low, and the results are shown through histograms (Figure 1 and Figure 2). The dominant phyla with a high relative abundance in the ileum of the control group and the jujube powder group were Firmicutes (60.72%, 73.13%), Cyanobacteria (19.08%, 8.35%), Proteobacteria (11.58%, 7.70%), and Bacteroidetes (7.34%, 9.93%) (Figure 1A). The dominant phyla with a high relative abundance in the cecum were Firmicutes (80.83%, 82.56%) and Bacteroidetes (13.48%, 12.52%) (Figure 2A). At the genus level, the abundance of Enterococcus (11.15%, 22.49%), Lactobacillus (8.45%, 18.82%), and Candidatus_Arthromitus (12.74%, 7.73%) (Figure 1B) was relatively higher in the control group and jujube powder group. The dominant genera with a high relative abundance in the cecum were Faecalibacterium (1.39%, 9.08%), Ruminococcaceae_UCG-014 (7.36%, 7.54%), and Alistipes (5.68%, 6.43%) (Figure 2B).
Figure 1.
Effects of diet supplemented with 8% jujube powder on the ileal microbial composition of broilers. (A) is the ileal microbiological histogram at the phylum level; (B) is the ileal microbiological histogram at the genus level. HA is the control group; HB is the jujube powder addition group.
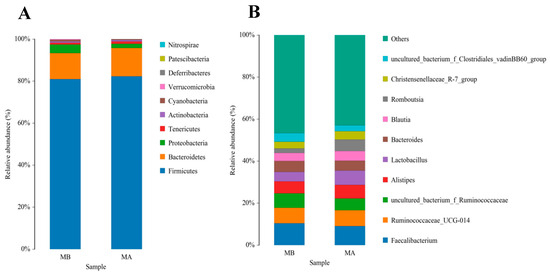
Figure 2.
Effects of diet supplemented with jujube powder on the cecal microbial composition of broilers. (A) is the cecal microbiological histogram at the phylum level; (B) is the cecal microbiological histogram at the genus level. MA is the control group; MB is the jujube powder addition group.
3.5.3. Effect of Jujube Powder on the Microbial Community Composition in the Ileum and Cecum of Broilers
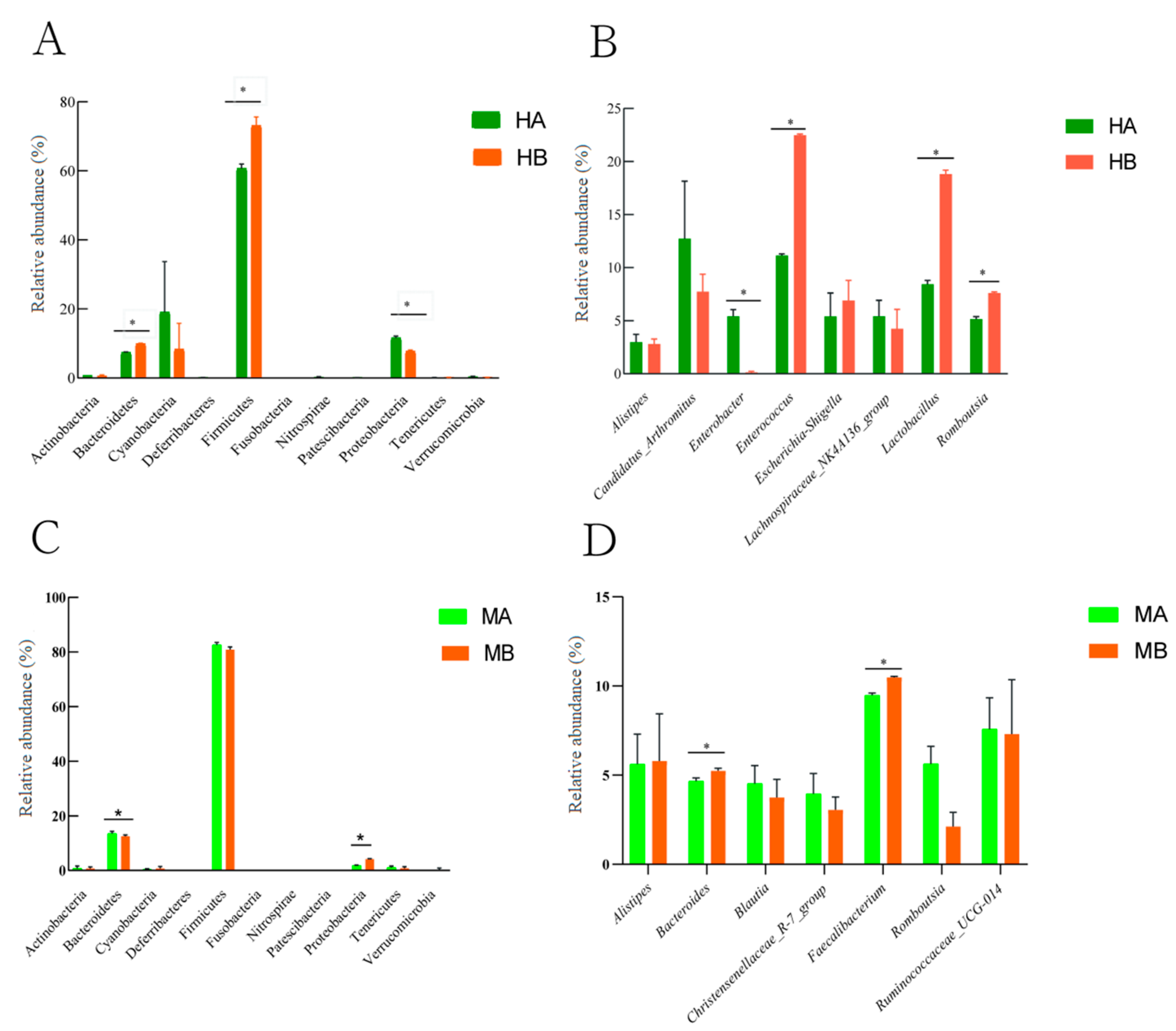
Figure 3 presents the difference in the relative abundance at the level of ileal and cecal microbiota and genera within the top ten. At the phylum level, compared with the control, the relative abundance of Firmicutes and Bacteroidetes in the ileum significantly increased, whereas that of Proteobacteria significantly decreased (Figure 3A) (p < 0.05). The relative abundance of Bacteroidetes in the cecum decreased significantly, whereas that of Proteobacteria increased significantly (Figure 3C) (p < 0.05). At the genus level, compared with the control group, the diet supplemented with 8% jujube powder significantly increased the relative abundance of Lactobacillus and Romboutsia in the ileum, whereas it significantly decreased the relative abundance of Enterobacter (Figure 3B) (p < 0.05). The relative abundance of Bacteroides and Faecalibacterium in the cecum increased significantly (Figure 3D) (p < 0.05).
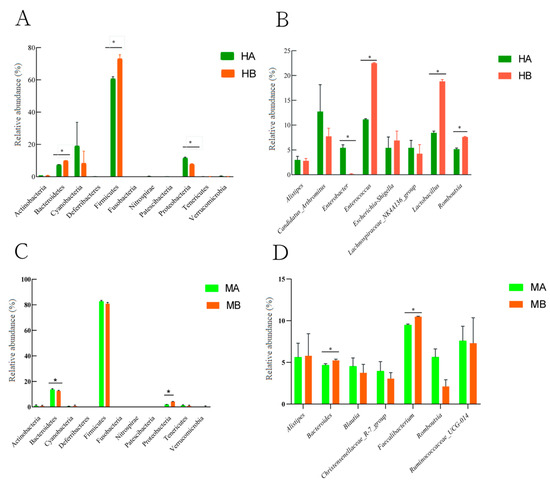
Figure 3.
Differences in the microbial community composition between the ileum and cecum. (A) is the difference in the relative abundance of ileal microorganisms at the phylum level; (B) is the difference in the relative abundance of ileal microorganisms at the genus level; (C) is the difference in the relative abundance of cecal microorganisms at the phylum level; (D) is the difference in the relative abundance of cecal microorganisms at the genus level; * is the significant difference between the two groups (p < 0.05). HA and MA are the control groups; HB and MB are the jujube powder addition groups.
4. Discussion
Most studies have proven that jujube powder has various biological activities. It can provide nutrients for the body’s growth and development as well as regulate the body’s metabolism as a bioactive substance [33,34,35]. As a new feed material, jujube powder contains crude protein (7.43%), ether extract (7.22%), calcium (0.37%), phosphorus (0.18%), and gross energy (17.10 MJ/kg), which can effectively improve the performance of animal production. Diet supplemented with jujube powder can improve the growth performance of broilers [16]. Similar results have been observed in other animals [19,36,37] This study’s results are basically consistent with those of the aforementioned reports. The dietary addition of 8% jujube powder significantly improved the ADG of broilers during the whole period (0–42 days). This improvement is caused by the presence of active substances such as polysaccharides, vitamins, and flavonoids in jujube powder [38]. Of them, polysaccharides make jujube powder palatable and thus improve the feed intake of broilers. Second, flavonoids in jujube powder can promote insulin hormone release [39], which is beneficial for the digestive function of intestines in broilers [40]. These factors also affect the utilization efficiency of nutrients in broilers. The apparent nutrient utilization rate is among the most crucial indicators for measuring whether livestock and poultry can fully utilize the nutritional value of the diet. The apparent nutrient utilization rate is affected by factors such as the feeding level, feed processing technology, animal growth stage, and animal intestinal health. A diet supplemented with 15% jujube powder improved the apparent digestibility of the crude protein, neutral detergent fiber, and acid detergent fiber of goats [41]. Supplementation with 10% jujube powder significantly improved the apparent digestibility of crude protein, calcium, and phosphorus of layers [42]. This result is consistent with the results of the present study. The addition of 10% jujube powder significantly improved the apparent utilization rate of organic matter and apparent metabolic energy in broilers. When 2–8% jujube powder was added, the total tract nitrogen retention utilization rate of broilers improved [43]. However, this experiment found no improvement in the apparent digestibility of total tract nitrogen retention with the addition of jujube powder, which may be related to the variety, nutritional level, dietary type, and feeding environment of experimental animals.
IgA, IgG, and IgM are crucial indicators for measuring humoral immunity [44]. sCD4, which belongs to the immunoglobulin superfamily, is an auxiliary molecule of the T cell differentiation antigen and participates in the body’s cellular immunity. Polysaccharides in jujube powder can promote the maturation of cellular and humoral immune systems, significantly enhance the phagocytic capacity in low-immunity mice [45], significantly increase serum IgA, IgG, and IgM levels in broilers [46,47], and have the same effect on layers [48]. The results of the aforementioned studies are consistent with those of this experiment. The diet supplemented with 4–10% jujube powder significantly increased serum IgG, IgM, and IgA levels in broilers, and the sCD4 content exhibited a significant upward trend. The underlying mechanism may be that polysaccharides, cyclic nucleotides, phenolic compounds, and organic acids are abundant in jujube powder and can affect the expression of pro-inflammatory cytokines, reduce blood lipid activity, and improve immunity [34,38,49,50]. Some studies have suggested that the immune regulatory mechanism of jujube inhibits the phosphorylation of P-38 and JNK signal proteins. Jujube plays an anti-inflammatory role through the NF-kBand P38/JNK-MAPK signaling pathway, in which polysaccharides have a major role [35].
Many studies have reported that blood T-AOC, GSH-Px, SOD, and MDA contents can reflect the antioxidant capacity of the body [51,52,53]. GSH-Px and SOD are crucial peroxidase enzymes that can eliminate excessive free radicals and improve the antioxidant performance of the body [54,55]. Free radicals can increase the plasma lipoprotein level and stimulate cell membrane lipid oxidation [56]. Jujube powder can increase serum T-AOC and GSH-Px contents in laying hens and reduce the MDA content [57]. It can improve SOD, CAT, GPX, and T-AOC activities in the breast muscle of broilers [16] and the antioxidant activity of goat milk yogurt [58]. In this study, the diet supplemented with jujube powder also significantly increased the serum T-AOC and SOD contents and reduced the serum MDA content. The rich polyphenols in jujube powder can serve as a reductant [59] and have a strong antioxidant effect [60], which can reduce free radicals and transform them into a more stable state [61], followed by the improvement in the antioxidant capacity of broiler blood. Vitamin C and phenolic compounds also have a good ability to scavenge free radicals and prevent lipid oxidation [33,62,63]. Whether a single active substance or a combination of multiple active substances has an effect needs to be further explored. In a word, the active substances present in jujube powder can improve the body’s immunity and antioxidant capacity through some mechanisms (polyphenols in particular serve as reducing agents to reduce cell membrane lipid oxidation), which are worthy of recognition.
Intestinal microorganisms have a crucial regulatory role in host intestinal health and function [64]. Normally, intestinal microorganisms are in a dynamic balance, but factors such as diet, environment, temperature, and humidity can cause an imbalance of intestinal flora and affect the performance of animal production [65]. An increasing number of studies have proved that the bioactive substances of jujube powder can enrich intestinal probiotics, thereby improving the body’s immunity [66]. The underlying mechanism may be that water-soluble polysaccharides and insoluble fibers in jujube powder can enhance the immune function by influencing the intestinal microbiota αPD-L1 response rate and immune efficiency [67,68,69]. Similar to the previous results [70], 8% jujube powder added to the diet significantly increased the ACE and Chao 1 indices of broiler ileum and cecum. This indicates that the active substances in jujube powder and inulin can improve the intestinal microbial diversity and abundance of broilers. The increase in the number and diversity of microbes is conducive to the body’s health [71,72].
This experimental result is consistent with those of earlier studies [73,74]. Firmicutes, Proteobacteria, and Bacteroidetes are the dominant bacteria in the broiler ileum and cecum. Firmicutes are involved in short-chain fatty acid (SCFA) production, and SCFAs can synthesize cholesterol, reduce intestinal pH, and help enhance the immune response [75]. Moreover, Firmicutes are abundant in the intestines of many mammals. Butyric acid, produced during carbohydrate fermentation [76], can improve parenteral nutrition-induced intestinal mucosal immune injury [77] and regulate the proliferation of intestinal immune cells [78]. Proteobacteria are considered a marker of intestinal flora imbalance and can cause intestinal inflammation in some intestinal environments [79]. Bacteroidetes and Lactobacillus can regulate the intestinal microbial balance [80]. Bacteroidetes digest carbohydrates and use polysaccharides to produce acetic acid and propionic acid [81]. On the one hand, Lactobacillus can produce lactic acid, hydrogen peroxide, and bacteriocin to exert an antibacterial effect. On the other hand, it can compete with pathogenic bacteria for adsorption sites to resist their proliferation [82]. Romboutsia contributes to the health and development of the human intestine. The abundance of Romboutsia is high in the intestines of healthy people, whereas it is low in the intestines of patients with enteritis. Therefore, Romboutsia is closely related to host health [83]. Enterobacter is a common pathogenic bacterium that can produce adhesins and exotoxins, causing lesions at the host site and diarrhea [84]. A diet supplemented with the Achyranthes bidentata extract significantly increased the number of Lactobacillus in the broiler cecum, whereas it reduced the number of Escherichia coli and Salmonella. This is consistent with this study’s results [85]. This study found that 8% jujube powder added to the diet significantly increased the relative abundance of Firmicutes and Bacteroidetes at the lleal hilum level, whereas it significantly decreased the relative abundance of Proteobacteria. It also significantly increased the relative abundance of Lactobacillus and Romboutsia at the lleal level, whereas it significantly decreased the relative abundance of Enterobacter. This indicated that adding jujube powder significantly promoted beneficial bacteria and significantly inhibited harmful bacteria. Faecalibacterium is generally expressed in high abundance in patients with chronic enteritis and stage enteritis. Faecalibacterium is also associated with the occurrence of the broiler enterotoxicosis syndrome [86]. However, the 8% jujube powder added to the diet in this study significantly reduced the relative abundance of Bacteroidetes at the cecal hilum level, whereas it significantly increased the relative abundance of Proteobacteria. It also significantly increased the relative abundance of Bacteroides and Faecalibacterium at the cecal level. This result may depend on the test chicken variety, diet type, feeding environment, and sampling time, which needs to be further verified. The aforementioned report confirms our previous conjecture that active substances in jujube powder indeed improve the body’s overall immunity by affecting the intestinal microbiota.
5. Conclusions
This study found that adding 8% jujube powder to the diet significantly improved the ADG. Moreover, 8% and 10% jujube powder significantly increased the total tract of apparent organic matter digestibility, and 10% jujube powder significantly increased the apparent metabolic energy. The diet supplemented with jujube powder increased serum IgA, IgG, IgM, sCD4, T-AOC, SOD, and GSH-Px contents and reduced the serum MDA content. Moreover, the 8% jujube powder added to the diet significantly improved the intestinal microbiota of broilers. Jujube powder may enhance the immune level of broilers by improving their intestinal microbiota.
Author Contributions
Conceptualization, J.L. and D.T.; methodology, S.Q.; software, Z.N.; validation, Y.Z.; formal analysis, J.L.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, D.T.; supervision, S.Q.; project administration, F.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Gansu Province University Industry Support Plan under Grant (No. 2023CYZC-47), the Key R&D Plan of Gansu Province under Grant (No. 22YF7NA113), the Discipline Team Project of Gansu Agricultural University under Grant (No. GAU-XKTD-2022-24), and the Fuxi Young Talent Cultivation project of Gansu Agricultural University under Grant (No. GAUFX-02Y07).
Institutional Review Board Statement
This study was conducted at a poultry research farm after receiving approval from the Faculty Animal Policy and Welfare Committee of Gansu Agricultural University (approval no. GSAU-Eth-AST-2021-001, 12 January 2021).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; He, R.; Tsoi, B.; Kurihara, H. Bioactivities of chicken essence. J. Food Sci. 2012, 77, R105–R110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-S.; Thakur, M.; Peng, M.-S.; Jiang, Y.; Frantz, L.A.F.; Li, M.; Zhang, J.-J.; Wang, S.; Peters, J.; Otecko, N.O. 863 genomes reveal the origin and domestication of chicken. Cell Res. 2020, 30, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.; Alagawany, M.; Patra, A.; Abdel-Latef, M.; Ashour, E.; Arif, M.; Farag, M.; Dhama, K. Use of brewers dried grains as an unconventional feed ingredient in the diets of broiler chickens: A review. Adv. Anim. Vet. Sci. 2019, 7, 218–224. [Google Scholar] [CrossRef]
- El Naggar, S.; El-Mesery, H.S. Azolla pinnata as unconventional feeds for ruminant feeding. Bull. Natl. Res. Cent. 2022, 46, 66. [Google Scholar] [CrossRef]
- Lushnikov, N.A.; Alekseeva, E.I.; Tovkalo, M.V.; Pozdnyakova, N.A. Use effect of unconventional feed and mineral additives on animal and poultry productivity. IOP Conf. Ser. Earth Environ. Sci. 2021, 720, 012020. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Wang, L.; Han, C.; Zhang, X. Development and utilization of unconventional feed resources for poultry in China. Chin. J. Anim. Nutr. 2015, 27, 1–7. [Google Scholar]
- Wang, L.; Hu, R. Technical and cost efficiency of jujube growers in Henan province, China. Custos E@ Gronegocio on Line 2016, 12, 279–297. [Google Scholar]
- Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Comparison of nutritional composition of jujube from xinjiang. Food Nutr. China 2018, 24, 31–35. [Google Scholar]
- Zhiping, H. Analysis and evaluation on nutritive composition of chinese dates in different producing areas of north shaanxi. J. Anhui Agric. Sci. 2008, 35, 9830. [Google Scholar]
- Xu, T.; Zhou, X.; Degen, A.; Yin, J.; Zhang, S.; Chen, N. The inclusion of jujube by-products in animal feed: A review. Sustainability 2022, 14, 7882. [Google Scholar] [CrossRef]
- Cheng, D.; Zhu, C.; Cao, J.; Jiang, W. The protective effects of polyphenols from jujube peel (Ziziphus jujube mill.) on isoproterenol-induced myocardial ischemia and aluminum-induced oxidative damage in rats. Food Chem. Toxicol. 2012, 50, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, B.; Zhao, A.; Wei, L.; Shao, Y.; Wang, Y.; Cao, B.; Zhang, F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019, 277, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Xie, L.; Shishir, M.R.I.; Xu, Y.; Chen, W. In vitro study of bioaccessibility, antioxidant, and α-glucosidase inhibitory effect of pelargonidin-3-o-glucoside after interacting with beta-lactoglobulin and chitosan/pectin. Int. J. Biol. Macromol. 2020, 154, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, Y.; Jiao, Y.; Yu, L.; Yang, S.; Yang, X. Antioxidative and hepatoprotective effects of the polysaccharides from Zizyphus jujube cv. Shaanbeitanzao. Carbohyd Polym. 2012, 88, 1453–1459. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, Z.; Liu, Z.; Zhao, Z.; Zhou, G.; Liu, M.; Liu, P. Variations of the nutritional composition of jujube fruit (Ziziphus jujuba mill.) during maturation stages. Int. J. Food Prop. 2020, 23, 1066–1081. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, X.; Liu, W.; Huang, J.; Xie, Z.; Yang, F.; Zhang, L.; Wei, Y. Dietary dried jujube fruit powder (djfp) supplementation improves growth performance, antioxidant stability, and meat composition in broilers. Foods 2023, 12, 1463. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Tang, D.; Yang, X.; Zhang, L.; Yu, Q. Effects of selenium yeast and jujube powder dietary supplements on conformational and functional properties of post-mortem chicken myofibrillar protein. Front. Nutr. 2022, 9, 954397. [Google Scholar] [CrossRef]
- Yang, X.; Yang, C.; Tang, D.; Yu, Q.; Zhang, L. Effects of dietary supplementation with selenium yeast and jujube powder on mitochondrial oxidative damage and apoptosis of chicken. Poult. Sci. 2022, 101, 102072. [Google Scholar] [CrossRef]
- Xie, B.; Wang, P.; Yan, Z.; Ren, Y.; Dong, K.; Song, Z.; Zhang, J.; Zhang, C. Growth performance, nutrient digestibility, carcass traits, body composition, and meat quality of goat fed chinese jujube (Ziziphus jujuba mill.) fruit as a replacement for maize in diet. Anim. Feed Sci. Technol. 2018, 246, 127–136. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ahn, J.-B.; Kim, H.-J.; Im, N.-K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef]
- Plastina, P.; Bonofiglio, D.; Vizza, D.; Fazio, A.; Rovito, D.; Giordano, C.; Barone, I.; Catalano, S.; Gabriele, B. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol. 2012, 140, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Abedini, M.R.; Mitra, M.; Fard, M.H.; Beydokhti, H. “Ziziphus jujuba”: A red fruit with promising anticancer activities. Pharmacogn. Rev. 2015, 9, 99. [Google Scholar] [PubMed]
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. State Administration of Market Supervision and Administration and China National Standardization Administration: Beijing, China, 2018.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and China National Standardization Administration Commission: Beijing, China, 2006.
- GB/T 6434-2006; Feeding Stuffs-Determination of Crude Fiber Content-Method with Intermediate Filtration. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and China National Standardization Administration Commission: Jinan, China, 2006.
- GB/T 6438-2007; Animal Feeding Stuffs-Determination of Crude Ash. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China and China National Standardization Administration Commission: Wuhan, China, 2007.
- GB/T 6436-2018; Determination of Calcium in Feeds. State Administration of Market Supervision and Administration and China National Standardization Administration: Wuhan, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds—Spectrophotometry. State Administration of Market Supervision and Administration and China National Standardization Administration: Shanghai, China, 2018.
- NY-T33-2004; Feeding Standard of Chicken. Industry Standards–Agriculture: Beijing, China, 2004.
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, Association of Official Analytical Chemists, 15th ed.; AOAC Press: Gaithersburg, MA, USA, 1990. [Google Scholar]
- Short, F.; Gorton, P.; Wiseman, J.; Boorman, K. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996, 59, 215–221. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from ziziphus jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef]
- Zhan, R.; Xia, L.; Shao, J.; Wang, C.; Chen, D. Polysaccharide isolated from chinese jujube fruit (Zizyphus jujube cv. Junzao) exerts anti-inflammatory effects through mapk signaling. J. Funct. Foods 2018, 40, 461–470. [Google Scholar]
- Cellat, M.; Alaşahan, S.; Etyemez, M.; Gökçek, İ.; Kutlu, T.; Türkmen, M.; Güvenç, M.; Çiftçi, M. The effects of jujube fruit (Ziziphus jujuba mill.) added in the mixed feed on growth performance and oxidative stress parameters in quails raised in different stocking densities. Int. J. Vet. Anim. Res. 2022, 5, 19–26. [Google Scholar]
- Hoseinifar, S.H.; Zou, H.K.; Van Doan, H.; Harikrishnan, R.; Yousefi, M.; Paknejad, H.; Ahmadifar, E. Can dietary jujube (Ziziphus jujuba mill.) fruit extract alter cutaneous mucosal immunity, immune related genes expression in skin and growth performance of common carp (Cyprinus carpio)? Fish. Shellfish. Immun. 2019, 94, 705–710. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Wang, Y.; Liu, G.; Zhang, Z.; Zhao, Z.; Cheng, H. In vitro antioxidative and immunological activities of polysaccharides from Zizyphus jujube cv. Muzao. Int. J. Biol. Macromol. 2017, 95, 1119–1125. [Google Scholar] [CrossRef]
- Prihambodo, T.R.; Sholikin, M.M.; Qomariyah, N.; Jayanegara, A.; Batubara, I.; Utomo, D.B.; Nahrowi, N. Effects of dietary flavonoids on performance, blood constituents, carcass composition and small intestinal morphology of broilers: A meta-analysis. Anim. Biosci. 2021, 34, 434. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z. Effects of Supplementation of Different Level Substandard Chinese Jujube in Diet on Productive Performance and Indexes of Physiological and Biochemical of Blood in Bucks; Shanxi Agricultural University: Jinzhong, China, 2016. [Google Scholar]
- Zhao, Y.X. Study of Application of Jujube Powder in Laying Hens Diet; Agricultural University of Hebei: Baoding, China, 2012. (In Chinese) [Google Scholar]
- Jia, S.A.; Gao, Q.; Du, B.L.; Nian, F.; Tang, D.F. Effects of organic selenium and jujube powder on growth performance and nutrient utilization of broilers. China Feed. 2021, 1, 124–128. [Google Scholar]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.; Kelly, A.L. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Sun, N.-X.; Liu, H.-P.; Liu, X.-H.; Zhang, Y.; Liu, X.-Q.; Wang, S.; Xu, X.-X.; Tian, W.-T. Immunological activities of polysaccharide extracted from elaeagnus angustifolial. CyTA—J. Food 2018, 16, 995–1002. [Google Scholar] [CrossRef]
- Long, L.; Kang, B.; Jiang, Q.; Chen, J. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020, 99, 744–751. [Google Scholar] [CrossRef]
- Wu, S. Effect of dietary astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Zhang, R.; Wang, L.; Shi, W.; Xu, T.; Zhang, H. Effects of chinese herbal medicines on lipid metabolism and immunity function in laying hens. Indian J. Anim. Res. 2020, 54, 1291–1295. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, X.; Han, A.; Chen, P.; Bai, H. In vitro immunological and anti-complementary activities of two water-soluble lignins from zizyphus jujube cv. Jinchangzao. Int. J. Biol. Macromol. 2017, 105, 204–212. [Google Scholar] [CrossRef]
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42. [Google Scholar] [CrossRef]
- Dai, P.-y.; Huang, C.-l. Influence of percutaneous stimulation of hepatic region with mid-frequency pulse current on the activity of serum gsh-px, sod, t-aoc and the content of malondialdehyde in exercise-induced fatigued soldiers. Med. J. Chin. People’s Lib. Army 2014, 39, 245–248. [Google Scholar]
- Xu, Z.; Regenstein, J.M.; Xie, D.; Lu, W.; Ren, X.; Yuan, J.; Mao, L. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish. Shellfish. Immun. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, R.; Wang, L.; Zhang, H. The antioxidative effects of probiotic lactobacillus casei zhang on the hyperlipidemic rats. Eur. Food Res. Technol. 2010, 231, 151–158. [Google Scholar] [CrossRef]
- Alirezaei, M.; Gheisari, H.R.; Ranjbar, V.R.; Hajibemani, A. Betaine: A promising antioxidant agent for enhancement of broiler meat quality. Brit. Poul. Sci. 2012, 53, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, A.; Alirezaei, M.; Birjandi, M. Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept. 2010, 162, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamada, T.; Terahara, N.; Yamaguchi, M. Two acylated anthocyanins from purple sweet potato. Phytochemistry 1997, 44, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhao, Y.; Liu, Y.; Zhang, Z.; Hao, Y.; Zhao, X. Effects of jujube powder on lipid metabolism, antioxidant ability and immune function in laying hens. China Feed. 2017, 19, 5–8. [Google Scholar]
- Kou, X.; Chen, Q.; Li, X.; Li, M.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef]
- Karasawa, K.; Otani, H. Anti-allergic properties of a matured fruit extract of the date palm tree (Phoenix dactylifera L.) in mite-sensitized mice. J. Nutr. Sci. Vitaminol. 2012, 58, 272–277. [Google Scholar] [CrossRef]
- Ouyang, K.; Xu, M.; Jiang, Y.; Wang, W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2016, 96, 332–341. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as immunomodulatory compounds in the tumor microenvironment: Friends or foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Mohammed, H.A.; Elsisi, H.A.; Sajid, S.; Almogbel, Y.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharm. J. 2022, 30, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Sekelja, M.; Rud, I.; Knutsen, S.; Denstadli, V.; Westereng, B.; Naes, T.; Rudi, K. Abrupt temporal fluctuations in the chicken fecal microbiota are explained by its gastrointestinal origin. Appl. Environ. Microb. 2012, 78, 2941–2948. [Google Scholar] [CrossRef]
- Zhuang, H.; Yang, Z.; Chen, T.; Jing, N.; Zhou, Y.; Jiang, G.; Wang, Y.; Wang, Z.; Liu, Z. Boosting hsa vaccination with jujube powder modulating gut microbiota favorable for arginine metabolism. Nutrients 2023, 15, 1955. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Kang, C.; Zhang, Y.; Tang, L.; Guo, H.; Li, H.; Zhang, K. Immunomodulating and antioxidant effects of polysaccharide conjugates from the fruits of Ziziphus jujube on chronic fatigue syndrome rats. Carbohydr. Polym. 2015, 122, 189–196. [Google Scholar] [CrossRef]
- Wang, L.; Jing, N.; Liu, X.; Jiang, G.; Liu, Z. Nurturing and modulating gut microbiota with jujube powder to enhance anti-pd-l1 efficiency against murine colon cancer. J. Funct. Foods 2020, 64, 103647. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wang, D.; Hu, Y.; Zhang, C.; Qin, T.; Liu, C.; Sheng, X.; Nguyen, T.L. Immune-enhancing activity comparison of sulfated ophiopogonpolysaccharide and sulfated jujube polysaccharide. Int. J. Biol. Macromol. 2013, 52, 212–217. [Google Scholar] [CrossRef]
- Tian, K.; Liu, J.; Sun, Y.; Wu, Y.; Chen, J.; Zhang, R.; He, T.; Dong, G. Effects of dietary supplementation of inulin on rumen fermentation and bacterial microbiota, inflammatory response and growth performance in finishing beef steers fed high or low-concentrate diet. Anim. Feed Sci. Technol. 2019, 258, 114299. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M.W. Exploring the avian gut microbiota: Current trends and future directions. Front. Microbiol. 2015, 6, 673. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Yen, G.-C.; Sheu, F.; Chau, C.-F. Effects of water-soluble carbohydrate concentrate from Chinese jujube on different intestinal and fecal indices. J. Agric. Food Chem. 2008, 56, 1734–1739. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Murakoshi, S.; Fukatsu, K.; Omata, J.; Moriya, T.; Noguchi, M.; Saitoh, D.; Koyama, I. Effects of adding butyric acid to pn on gut-associated lymphoid tissue and mucosal immunoglobulin a levels. Jpen—Parenter. Enter. 2011, 35, 465–472. [Google Scholar] [CrossRef]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.; Hamilton, J.; Keefe, D.M. Gastrointestinal microflora and mucins may play a critical role in the development of 5-fluorouracil-induced gastrointestinal mucositis. Exp. Biol. Med. 2009, 234, 430–441. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Liu, J. Mechanism of action of probiotic function of lactobacilli. Chin. J. Anim. Nutr. 2012, 24, 389–396. [Google Scholar]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; de’Angelis, N.; de’Angelis, G.L.; van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Aoki, K.; Ohkushi, D.; Okamoto, K.; Takehana, K.; Akatsuchi, T.; Ida, K.; Shoji, D.; Ishii, Y.; Doi, Y. Institutional outbreak involving multiple clades of imp-producing Enterobacter cloacae complex sequence type 78 at a cancer center in Tokyo, Japan. BMC Infect. Dis. 2021, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, I. Effects of dietary achyranthes japonica extract supplementation on the growth performance, total tract digestibility, cecal microflora, excreta noxious gas emission, and meat quality of broiler chickens. Poultry Sci. 2020, 99, 463–470. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Vaneechoutte, M.; Doerffel, Y. Active crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm. Bowel Dis. 2008, 14, 147–161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).