Identification of Various InDel-II Variants of the White Spot Syndrome Virus Isolated from Frozen Shrimp and Bivalves Obtained in the Korean Commercial Market
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Polymerase Chain Reaction (PCR) Primer Design
2.3. Viral DNA Extraction and WSSV Detection
2.4. Sequence Analysis of InDel-II Region
2.5. Quantitative PCR
2.6. Pathogenicity of WSSV InDel-II Region Variants
3. Results
3.1. Detection of WSSV in Frozen Shrimp and Bivalve Mollusks
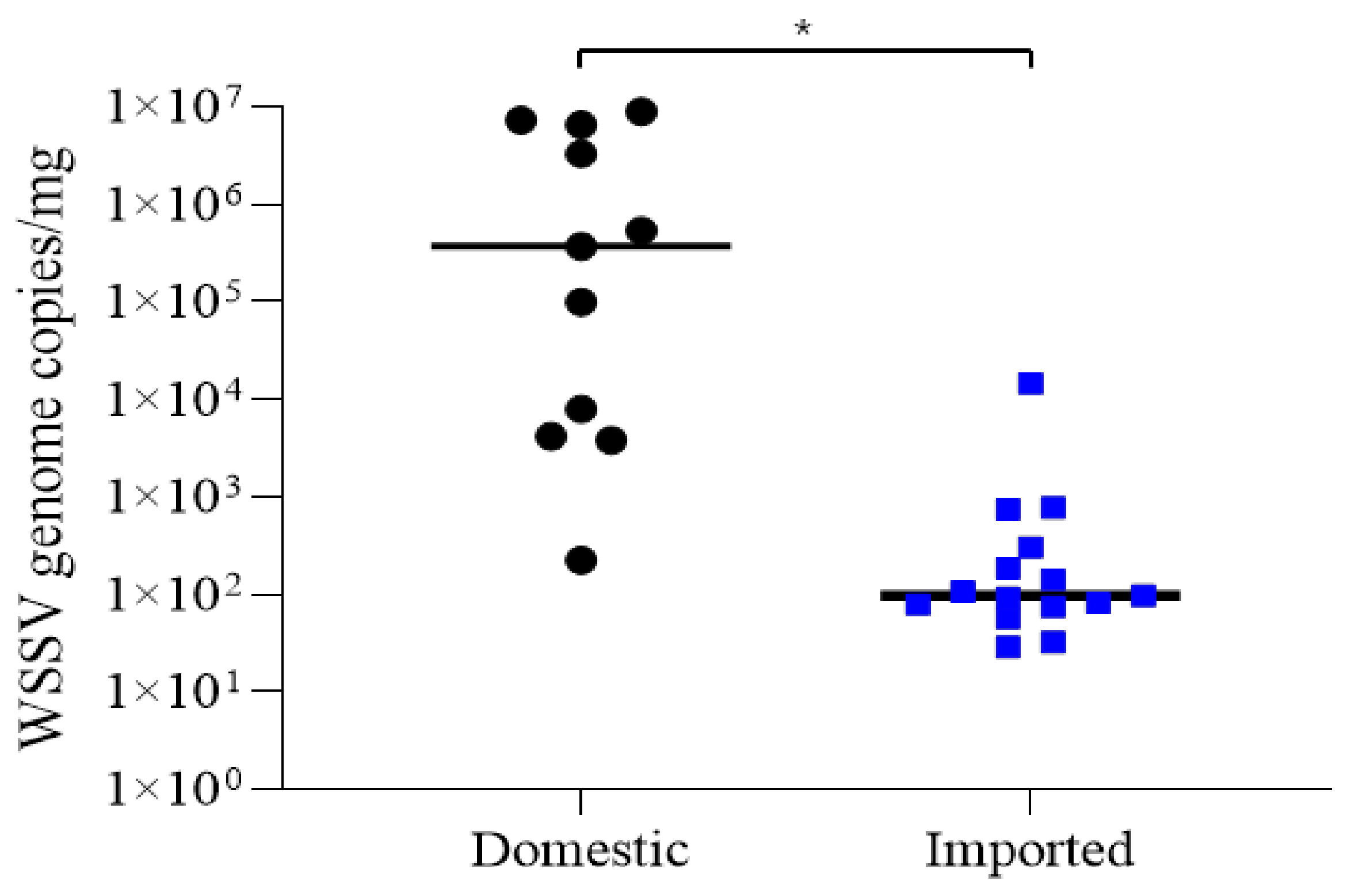
3.2. Quantification of WSSV
3.3. WSSV InDel-II Variants from Frozen Shrimp and Bivalve Mollusks
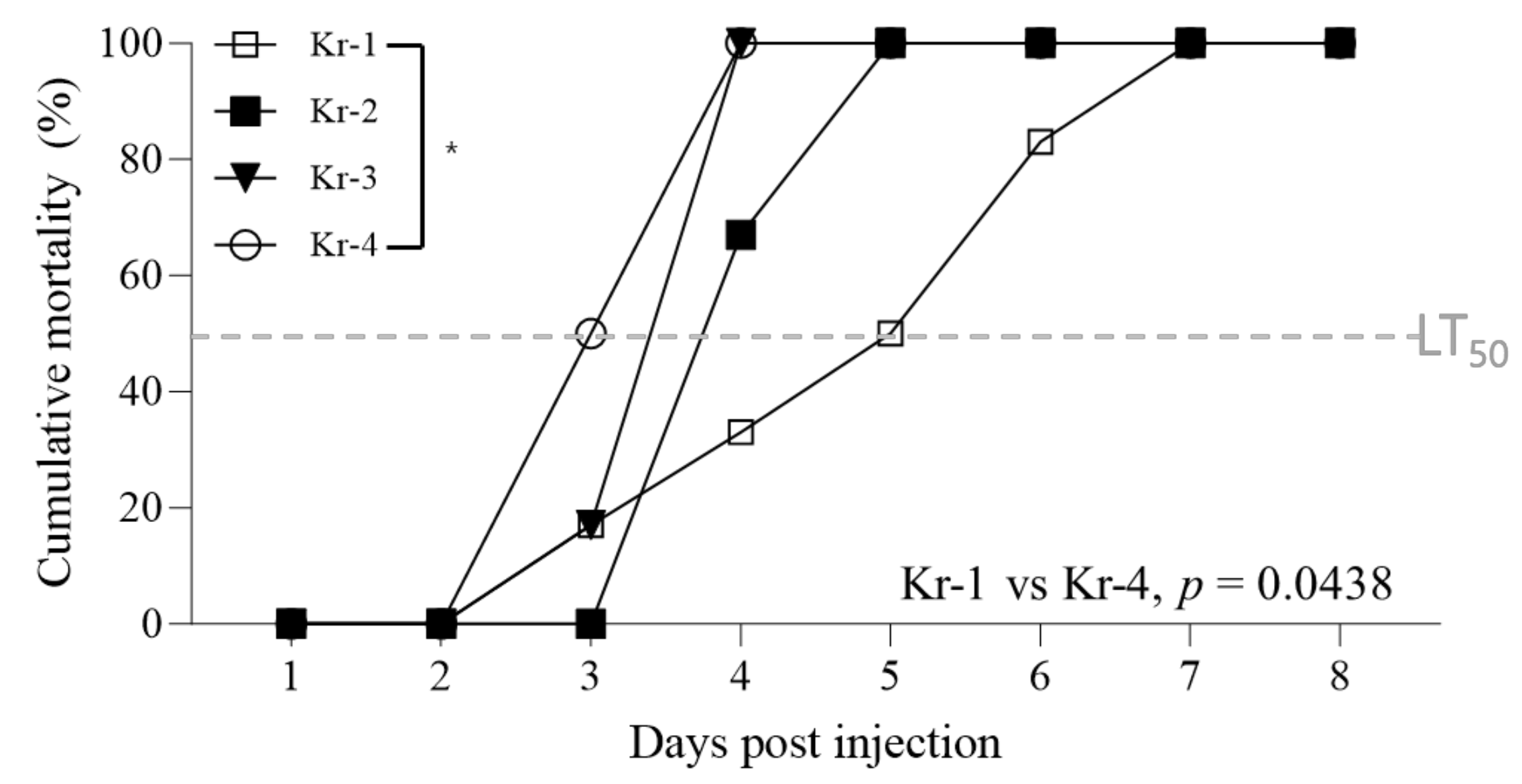
3.4. Pathogenicity of Different InDel-II Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knibb, W.; Le, C.; Katouli, M.; Bar, I.; Lloyd, C. Assessment of the origin of white spot syndrome virus DNA sequences in farmed Penaeus monodon in Australia. Aquaculture 2018, 494, 26–29. [Google Scholar] [CrossRef]
- Glanville, R.; Neville, P.; Walker, P. White Spot Disease of Prawns Queensland Response 2016–17 Scenario Planning Advisory Panel Report; Queensland Department of Agriculture and Fisheries: Brisbane City, Australia, 2017. [Google Scholar]
- Park, S.C.; Choi, S.K.; Han, S.H.; Park, S.; Jeon, H.J.; Lee, S.C.; Kim, K.Y.; Lee, Y.S.; Kim, J.H.; Han, J.E. Detection of infectious hypodermal and hematopoietic necrosis virus and white spot syndrome virus in whiteleg shrimp (Penaeus vannamei) imported from Vietnam to South Korea. J. Vet. Sci. 2020, 21, e31. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Baek, E.J.; Choi, J.Y.; Tae, W.J.; Kim, H.S.; Park, W.S.; Kim, M.J.; Kim, K.I. Genetic relatedness of white spot syndrome virus (WSSV) from imported frozen shrimp. J. Fish Pathol. 2021, 34, 141–147. [Google Scholar]
- Molloy, S.D.; Pietrak, M.R.; Bouchard, D.A.; Bricknell, I. The interaction of infectious salmon anaemia virus (ISAV) with the blue mussel, Mytilus edulis. Aquac. Res. 2014, 45, 509–518. [Google Scholar] [CrossRef]
- Pietrak, M.R.; Molloy, S.D.; Bouchard, D.A.; Singer, J.T.; Bricknell, I. Potential role of Mytilus edulis in modulating the infectious pressure of Vibrio anguillarum 02β on an integrated multi-trophic aquaculture farm. Aquaculture 2012, 326, 36–39. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.O.; Jang, G.I.; Kwon, M.G.; Kim, K.I. Evaluation of the Horizontal Transmission of White Spot Syndrome Virus for Whiteleg Shrimp (Litopenaeus vannamei) Based on the Disease Severity Grade and Viral Shedding Rate. Animals 2023, 13, 1676. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, K.I.; Joh, S.J.; Kang, J.Y.; Kwon, J.H.; Lee, H.S.; Kwon, Y.K. Development of a highly sensitive single-tube nested PCR protocol directed toward the sequence of virion envelope proteins for detection of white spot syndrome virus infection: Improvement of PCR methods for detection of WSSV. Aquaculture 2013, 410, 225–229. [Google Scholar] [CrossRef]
- McColl, K.A.; Slater, J.; Jeyasekaran, G.; Hyatt, A.D.; Crane, M.S. Detection of white spot syndrome virus and yellowhead virus in prawns imported into Australia. Aust. Vet. J. 2004, 82, 69–74. [Google Scholar] [CrossRef]
- Polo, D.; Varela, M.F.; Romalde, J.L. Detection and quantification of hepatitis A virus and norovirus in Spanish authorized shellfish harvesting areas. Int. J. Food Microbiol. 2015, 193, 43–50. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Le Pendu, J. Norwalk virus–specific binding to oyster digestive tissues. Emerg. Infect. Dis. 2006, 12, 931. [Google Scholar] [CrossRef]
- Tian, P.; Bates, A.H.; Jensen, H.M.; Mandrell, R.E. Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Lett. Appl. Microbiol. 2006, 43, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kwon, W.J.; Kim, M.S.; Kim, K.I.; Min, J.G.; Jeong, H.D. High prevalence of betanodavirus barfin flounder nervous necrosis virus as well as red-spotted grouper nervous necrosis virus genotype in shellfish. J. Fish Dis. 2018, 41, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Boucard, C.; Alvarez-Ruiz, P.; Escobedo-Fregoso, C.; Anguiano-Vega, G.; de Jesus Duran-Avelar, M.; Pinto, V.S.; Escobedo-Bonilla, C.M. Detection of white spot syndrome virus (WSSV) in the Pacific oyster Crassostrea gigas. J. Invertebr. Pathol. 2010, 104, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Boucard, C.; Escobedo-Fregoso, C.; Duran-Avelar, M.D.J.; Mercier, L.; Llera-Herrera, R.; Escobedo-Bonilla, C.; Vibanco-Perez, N. Crassostrea gigas oysters as a shrimp farm bioindicator of white spot syndrome virus. Dis. Aquat. Org. 2012, 98, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.; Goldbach, R.W.; Vlak, J.M.; Van Hulten, M.C.W. Genetic variation among isolates of white spot syndrome virus. Arch. Virol. 2004, 149, 673–697. [Google Scholar] [CrossRef] [PubMed]
- Zwart, M.P.; Dieu, B.T.M.; Hemerik, L.; Vlak, J.M. Evolutionary trajectory of white spot syndrome virus (WSSV) genome shrinkage during spread in Asia. PLoS ONE 2010, 5, e13400. [Google Scholar] [CrossRef] [PubMed]
- Dieu, B.T.M.; Marks, H.; Siebenga, J.J.; Goldbach, R.W.; Zuidema, D.; Duong, T.P.; Vlak, J.M. Molecular epidemiology of white spot syndrome virus within Vietnam. J. Gen. Virol. 2004, 85, 3607–3618. [Google Scholar] [CrossRef]
- Dieu, B.T.M.; Marks, H.; Zwart, M.P.; Vlak, J.M. Evaluation of white spot syndrome virus variable DNA loci as molecular markers of virus spread at intermediate spatiotemporal scales. J. Gen. Virol. 2010, 91, 1164–1172. [Google Scholar] [CrossRef]
- Marks, H.; van Duijse, J.J.; Zuidema, D.; van Hulten, M.C.; Vlak, J.M. Fitness and virulence of an ancestral white spot syndrome virus isolate from shrimp. Virus Res. 2005, 110, 9–20. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Li, F.; Xiang, J. One type of VEGFR is involved in WSSV infection to the Pacific whiteleg shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2015, 50, 1–8. [Google Scholar] [CrossRef]
- Chang, Y.S.; Chen, T.C.; Liu, W.J.; Hwang, J.S.; Kou, G.H.; Lo, C.F. Assessment of the roles of copepod Apocyclops royi and bivalve mollusk Meretrix lusoria in white spot syndrome virus transmission. Mar. Biotechnol. 2011, 13, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.S. Emerging viruses in aquaculture. Curr. Opin. Virol. 2019, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (WOAH). Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health, WOAH: Paris, France, 2019. [Google Scholar]
- Claydon, K.; Cullen, B.; Owens, L. OIE white spot syndrome virus PCR gives false-positive results in Cherax quadricarinatus. Dis. Aquat. Org. 2004, 62, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Aranguren Caro, L.F.; Mai, H.N.; Nunan, L.; Lin, J.; Noble, B.; Dhar, A.K. Assessment of transmission risk in WSSV-infected shrimp Litopenaeus vannamei upon cooking. J. Fish Dis. 2020, 43, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.V.; Redman, R.M.; Poulos, B.T.; Nunan, L.M.; Mari, J.L.; Hasson, K.W. Risk of spread of penaeid shrimp viruses in the Americas by the international movement of live and frozen shrimp. Rev. Sci. Tech. Int. Off. Epizoot. 1997, 16, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Sorrento, LA, USA, 1996. [Google Scholar]
- Kim, M.J.; Kim, J.O.; Jang, G.I.; Kwon, M.G.; Kim, K.I. Diagnostic validity of molecular diagnostic assays for white spot syndrome virus at different severity grades. Heliyon 2023, 9, e19351. [Google Scholar] [CrossRef]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C.; Gough, J.; Vogel, C.; Teichmann, S.A. Evolution of the protein repertoire. Science 2003, 300, 1701–1703. [Google Scholar] [CrossRef]
- Emond, S.; Petek, M.; Kay, E.J.; Heames, B.; Devenish, S.R.; Tokuriki, N.; Hollfelder, F. Accessing unexplored regions of sequence space in directed enzyme evolution via insertion/deletion mutagenesis. Nat. Commun. 2020, 11, 3469. [Google Scholar] [CrossRef]
- Hoa, T.T.; Hodgson, R.A.; Oanh, D.T.; Phuong, N.T.; Preston, N.J.; Walker, P.J. Genotypic variations in tandem repeat DNA segments between ribonucleotide reductase subunit genes of white spot syndrome virus (WSSV) isolates from Vietnam. Dis. Asian Aquac. V 2005, 339–351. [Google Scholar]
- Tang, K.F.; Le Groumellec, M.; Lightner, D.V. Novel, closely related, white spot syndrome virus (WSSV) genotypes from Madagascar, Mozambique and the Kingdom of Saudi Arabia. Dis. Aquat. Org. 2013, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.C.; Andrade, T.P.; Tang-Nelson, K.F.; Marques, M.R.; Lightner, D.V. Genotyping of white spot syndrome virus (WSSV) geographical isolates from Brazil and comparison to other isolates from the Americas. Dis. Aquat. Org. 2010, 88, 91–98. [Google Scholar] [CrossRef]
- Chai, C.Y.; Yoon, J.; Lee, Y.S.; Kim, Y.B.; Choi, T.J. Analysis of the complete nucleotide sequence of a white spot syndrome virus isolated from pacific white shrimp. J. Microbiol. 2013, 51, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Krell, P.J. Passage effect of virus infection in insect cells. Cytotechnology 1996, 20, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Onihary, A.M.; Razanajatovo, I.M.; Rabetafika, L.; Bastaraud, A.; Heraud, J.M.; Rasolofo, V. Genotype Diversity and Spread of White Spot Syndrome Virus (WSSV) in Madagascar (2012–2016). Viruses 2021, 13, 1713. [Google Scholar] [CrossRef] [PubMed]
- Hoa, T.T.T.; Zwart, M.P.; Phuong, N.T.; Oanh, D.T.; de Jong, M.C.; Vlak, J.M. Indel-II region deletion sizes in the white spot syndrome virus genome correlate with shrimp disease outbreaks in southern Vietnam. Dis. Aquat. Org. 2012, 99, 153–162. [Google Scholar] [CrossRef]


| Target | Primers | Sequence (5′–3′) | Positions * | Object (Expected Size, bp) | Reference |
|---|---|---|---|---|---|
| VP 28 | W VP28 F1 * W VP28 R1 | CTT TCA CTC TTT CGG TCG TGT C TCG GTC TCA GTG CCA GAG TA | 278,875–278,896 279,456–279,475 | First-step PCR (601) | This study |
| WSSV VP28 F2 WSSV VP28 R2 | CAC TGT GAC CAA GAC CAT CG GGT GCC AAC TTC ATC CTC ATC | 278,957–278,976 279,354–279,374 | Second-step PCR (408) | [8] | |
| WSSV qF WSSV qR | TGT GAC CAA GAC CAT CGA A CCA CAC CTT GAA TGT TC | 278,960–278,978 279,223–279,239 | Quantification (281) | [21] | |
| ORF 23/24 (InDel-II region) | S—F1 S—R1 | TAC ATG GGA GGG AGA GGT GAT TGC GAA ATA CGG GCA ATG TTT | 10,457–10,477 11,982–12,005 | First Discrimination First-step PCR | This study |
| S—F2 S—R2 | TCT GGG GCG CTT GTT ACT TG AAG GAG GAG GTG TTG GAG CTA | 10,515–10,534 11,936–11,956 | First Discrimination Second-step PCR | ||
| M—F1 M—R1 | CGC CAG TAC CTT CTT CCA CT TCT CAA GGA GGA GAG AGC GT | 8112–8131 14,726–14,745 | Second Discrimination First-step PCR | ||
| M—F2 M—R2 | GTC GAC AGG GAC TTC AAT ACC GTG TTG GTA AAT GCA CG | 8185–8202 14,619–14,638 | Second Discrimination Second-step PCR | ||
| L—F1 L—R1 | CCA CTA GCC TTC CAC GTG TT GCA GTC GGC AAC ATC TTG TG | 2208–2227 16,367–16,386 | Third Discrimination First-step PCR | ||
| L—F2 L—R2 | GTG CCC TTT TGC AAG GCA TA ATA CCG GCG AGT CTT GAA CC | 2467–2486 16,180–16,199 | Third Discrimination Second-step PCR |
| PCR | Domestic (Korea) | Imported | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Thailand | Malaysia | Ecuador | Indonesia | Vietnam | Argentina | Saudi Arabia | New Zealand | ||
| First-step PCR | 7/19 * (36.8) ** | 1/19 (5.3) | 0/12 (0.0) | 0/12 (0.0) | 0/10 (0.0) | 0/6 (0.0) | 0/4 (0.0) | 0/2 (0.0) | 0/2 (0.0) |
| Second-step PCR | 11/19 (57.9) | 8/19 (42.1) | 7/12 (58.3) | 7/12 (58.3) | 4/10 (40.0) | 4/6 (66.6) | 0/4 (0.0) | 2/2 (100) | 0/2 (0.0) |
| Total | 11/19 (57.9) | 32/67 (47.8) | |||||||
| Samples | Location | No. of Groups | No. of PCR Positives (%) | |
|---|---|---|---|---|
| First-Step PCR | Second-Step PCR | |||
| Pacific oyster | BS * | 25 | 0 (0.0) ** | 4 (16.0) |
| (Crassostrea gigas) | DC | 12 | 0 (0.0) | 2 (16.7) |
| Mussel | DC | 21 | 2 (9.5) | 3 (14.3) |
| (Mytilus edulis) | BS | 14 | 1 (7.1) | 2 (14.3) |
| GJ | 8 | 0 (0.0) | 1 (12.5) | |
| TY | 7 | 0 (0.0) | 0 (0.0) | |
| Manila clam | BS | 15 | 0 (0.0) | 1 (6.7) |
| (Venerupis philippinarum) | SS | 8 | 1 (12.5) | 3 (37.5) |
| Granular ark (Tegillarca garnosa) | BG | 14 | 0 (0.0) | 4 (28.6) |
| Venus clam (Mercenaria mercenaria) | BA | 12 | 1 (8.3) | 1 (8.3) |
| Common orient clam (Meretrix meretrix) | MS | 5 | 0 (0.0) | 1 (20.0) |
| Scallop (Patinopecten yessoensis) | TY | 6 | 0 (0.0) | 1 (16.7) |
| Total | 147 | 5 (3.4) | 23 (15.6) | |
| Samples | No. of Groups | No. of PCR Positives (%) | |
|---|---|---|---|
| First-Step PCR | Second-Step PCR | ||
| China | |||
| Manila clam (Venerupis philippinarum) | 13 | 0 (0.0) * | 0 (0.0) |
| Venus clam (Mercenaria mercenaria) | 6 | 1 (16.7) | 1 (16.7) |
| Common orient clam (Meretrix meretrix) | 4 | 0 (0.0) | 1 (25.0) |
| Chinese cyclina (Cyclina sinensis) | 3 | 0 (0.0) | 1 (33.3) |
| Scallop (Pationopecten yessoensis) | 2 | 0 (0.0) | 0 (0.0) |
| Purple washington clam (Saxidomus purpurata) | 1 | 0 (0.0) | 0 (0.0) |
| Bittersweet clam (Glycymeris vestita) | 1 | 0 (0.0) | 0 (0.0) |
| Sub total | 30 | 1 (3.3) | 3 (10.0) |
| Japan | |||
| Granular ark (Tegillarca granosa) | 8 | 0 (0.0) | 1 (12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.-G.; Jeong, H.-D.; Kim, K.-I. Identification of Various InDel-II Variants of the White Spot Syndrome Virus Isolated from Frozen Shrimp and Bivalves Obtained in the Korean Commercial Market. Animals 2023, 13, 3348. https://doi.org/10.3390/ani13213348
Min J-G, Jeong H-D, Kim K-I. Identification of Various InDel-II Variants of the White Spot Syndrome Virus Isolated from Frozen Shrimp and Bivalves Obtained in the Korean Commercial Market. Animals. 2023; 13(21):3348. https://doi.org/10.3390/ani13213348
Chicago/Turabian StyleMin, Joon-Gyu, Hyun-Do Jeong, and Kwang-Il Kim. 2023. "Identification of Various InDel-II Variants of the White Spot Syndrome Virus Isolated from Frozen Shrimp and Bivalves Obtained in the Korean Commercial Market" Animals 13, no. 21: 3348. https://doi.org/10.3390/ani13213348
APA StyleMin, J.-G., Jeong, H.-D., & Kim, K.-I. (2023). Identification of Various InDel-II Variants of the White Spot Syndrome Virus Isolated from Frozen Shrimp and Bivalves Obtained in the Korean Commercial Market. Animals, 13(21), 3348. https://doi.org/10.3390/ani13213348





