Simple Summary
Harmful fungi that contaminate food and feed can negatively impact human and animal health. We measured the ability of safer food-grade and feed-grade materials to remove several mycotoxins that contaminate food and feed. Some Aspergillus fungi produce aflatoxins that can contaminate corn, peanuts, and tree nuts. Certain Aspergillus and Penicillium fungi produce ochratoxin A, which can contaminate cereal grains and fruits. Some Fusarium fungi produce zearalenone, which can contaminate corn. Charcoal and biochar materials derived from coconuts and pine tree wood can remove aflatoxin B1, ochratoxin A, and zearalenone under conditions that simulate digestion. These carbon-based materials show promise as greener methods to help reduce exposure to the effects of toxins found in food and feed.
Abstract
Mycotoxin sequestration materials are important tools to reduce mycotoxin illness and enable proper handling of mycotoxin-contaminated commodities. Three food-grade bentonite clays and four generally recognized as safe (GRAS) charcoal/biochar carbon materials that are marketed as feed additives and supplements were evaluated for their ability to sequester the mycotoxins aflatoxin B1, ochratoxin A, and zearalenone. The surface area of the clays varied between 32.1 to 51.4 mg2/g, and the surface area of the carbon-based materials varied from 1.7 to 1735 mg2/g. In vitro, gastric fluid studies indicated that certain pine biochar and activated coconut charcoal could sequester high amounts (85+%) of the mycotoxins at 1 ppm levels or below. However, some biochar materials with lower surface area properties lacked binding capacity. The coconut shell charcoal and pine biochar utilize agricultural waste products in a manner that significantly reduces carbon emissions and provides valuable materials to minimize exposure to toxins found in food and feed.
Keywords:
feed safety; biochar; sequestration; animal feed; mycotoxin; aflatoxin; ochratoxin; zearalenone; food safety 1. Introduction
Various methods are utilized to reduce exposure to natural contaminants in foods, including early detection, prevention, control, and remediation [1]. Recent surveys have found a greater than 70% chance that agricultural commodities can be contaminated with detectable levels of mycotoxins using the most sensitive detection methods [2,3]. New sorbent materials have recently been investigated for their ability to bind or sequester mycotoxins and other natural toxins in fruit juices and other aqueous beverages, fermentations, and during digestion [4,5]. An aqueous environment for binding interactions is critical when using these materials. Several classes of materials have been shown to promote animal health and reduce the effects of mycotoxins in contaminated feed [6]. However, most studies only focus on one mycotoxin, which complicates the evaluation of a given mycotoxin-binding material’s ability to sequester multiple mycotoxins [7]. Multiple mycotoxins have been found in animal feed [8,9]. This study examines the mycotoxin-binding properties of food-grade and feed-grade materials for aflatoxin B1, ochratoxin A, and zearalenone.
Aflatoxin B1 (1) was discovered during the Turkey X disease outbreak in the 1960s in England, in which 100,000 turkeys died suddenly due to high-level liver necrosis and intestinal inflammation (see Figure 1). The source was a nut-based feed contaminated with aflatoxins from Aspergillus flavus [10]. Aflatoxin B1 is hydrophobic and absorbed through the small intestine and other sites of exposure. Aflatoxin B1 rapidly absorbs into the blood stream [1,4,10]. The International Agency for Research on Cancer (IARC) has classified aflatoxin B1 as a carcinogen, and its exposure is widely regulated. In the U.S., aflatoxin B1 levels are regulated at 20 ppb for corn-related products [11].
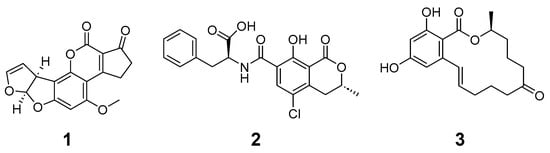
Figure 1.
Regulated mycotoxins aflatoxin B1 (1), ochratoxin A (2), and zearalenone (3) were investigated in this study.
Ochratoxin A (2) is a commonly found mycotoxin in various commodities and is shown to be nephrotoxic, hepatotoxic, teratogenic, and immunotoxic to animals. Ochratoxin A production is most commonly associated with Penicillium verrucosum and Aspergillus ochraceous; however, several Aspergillus and Penicillium species are producers, and a wide variety of commodities and commodity-based products are at risk of exposure [12]. Ochratoxin A is absorbed through the gastrointestinal tract and forms a complex with albumin which increases it time of effect [1,12]. Ochratoxin A contains carboxylic acid and phenolic hydroxyl functional groups that can become deprotonated under pH environments associated with digestion. Ochratoxin A exists as monoanionic and dianionic forms under physiological conditions (carboxylic acid pKa at approximately 4.3 and phenolic hydroxyl pKa at approximately 7.2). Over 60 countries have set regulations and regulatory limits varying from 0.100 to 1 ppm in feed products [13]. Reducing ochratoxin A levels from food and feed using chemical-based detoxification is challenging due to its chemical and physical stability. Ochratoxin A is widely regarded as a mycotoxin of concern due to its chemical stability and the wide range of products in which this mycotoxin can occur.
Zearalenone (3) is produced by Fusarium species that occasionally contaminate agricultural commodities. This estrogenic resorcylic acid lactone derivative possesses a chemical structure similar to 17β-estradiol and acts at sites associated with estrogenic activities. Specifically, zearalenone and its metabolites interact with mammalian estrogen receptors, negatively impacting hormonal regulations. Zearalenone, also known as F2 toxin, is commonly found in wheat and corn [13]. Zearalenone is absorbed through the gastrointestinal tract and can become deprotonated under physiological conditions to exist in anionic forms (pKa of the phenolic hydroxyl is approximately 7.6) [13,14]. Zearalenone is relatively stable, and detection and binding materials are the primary approaches to reducing exposure [14]. Swine are particularly adversely affected by exposure, resulting in reproductive issues detrimental to production.
Recently, several novel approaches have been investigated to reduce exposure to mycotoxins and their metabolites in food and feed, including nutritional supplements. Increasing levels of aflatoxin B1 have been reported in Europe with concerns over the formation of toxic metabolites in dairy milk. The effects of natural phytochemical turmeric powder were investigated to reduce levels of the toxic metabolites aflatoxin M1 and aflatoxicol in dairy cow milk [15]. The study found that formulations need to be further investigated to improve the bioavailability of turmeric powder. In vitro studies have shown the natural product quercetin can reduce the transformation of aflatoxin B1 to more carcinogenic metabolites by increasing production of glutathione [16].
Other approaches to remove mycotoxins include a variety of physical and chemical methods, including sorting/separations, washing, heating, irradiation, and chemical treatments [2,17,18]. However, these methods leave decomposition products and adducts, or can be costly for certain commodities. Clays, modified clays, carbohydrates, polymers, surfactants, and absorbing bacteria have been shown to remove levels of certain mycotoxins [2,5,17,18]. The adsorption properties and applications of these mycotoxin-binding materials are known, and there is a need for greener materials with high capacities for a range of important mycotoxins.
In this study, we investigate the utilization of several commercially available food-grade and feed-grade clay and charcoal materials to bind the regulated mycotoxins aflatoxin B1, ochratoxin A, and zearalenone. Biochar is a black, light weight, solid carbon-based form of charcoal that is formed by pyrolysis under oxygen-limited environments [19]. Carbon sequestration is the primary interest of biochar for its ability to reduce carbon emissions by half with minimal efforts. Biochar materials are often marketed as horticulture charcoals and are generally recognized as safe (GRAS) carbonized biomass. Several types of biochar have improved agricultural productivity as soil amendment products through supporting soil aeration, nutrient availability, and water filtration and retention [19,20,21]. The properties of biochar can vary considerably depending on the type of feedstock, temperature of pyrolysis, and any additional chemical/physical steps used to modify the properties of the materials [19]. Recent studies have investigated the use of biochar and other charcoal-related materials to sequester toxins or aid digestion, including reduction of methane production [20]. For centuries, charcoal-related materials have been used to improve health in animal production [21]. With the recent interest in biochar over the past decade, many producers have included biochar in animal feed [22]. The USDA recently released a report on using plant-based activated carbon as a feed additive to reduce exposure to toxins in feed, and several commercially available charcoal and biochar feed additives are of interest for this purpose [6,23].
2. Materials and Methods
2.1. Reagents
Aflatoxin B1 (1), ochratoxin A (2), and zearalenone (3), HPLC-grade acetonitrile, phosphoric acid, sodium phosphate, and disodium phosphate were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Deionized water was used to prepare all reagents (Nanopure II, Sybron/Barnstead). All solvents were HPLC grade. Food-grade calcium montmorillonite was sourced from 5326 Partners, LLC (Sheridan, WY, USA). Sodium montmorillonite was purchased from Spark Naturals (Orem, UT, USA). Food-grade sodium bentonite was purchased from Belle Chemical, LLC (Billings, MT, USA). Food-grade activated charcoal from coconut shells was purchased from NusaPure, Gmax Central, LLC (Orlando, FL, USA). Feed-grade Pine biochar was purchased from High Plains Biochar LLC (Laramie, WY, USA). Horticulture biochar was purchased from Alleo (Bay Minette, AL, USA). Olive wood biochar was purchased from Olivette Atlas Olive Oils (Miami, FL, USA). Chemical structures were created using ChemDraw v22.2.0.3300 (PerkinElmer Software).
2.2. Steady-State Fluorescence
A Varian Cary Eclipse instrument (Palo Alto, CA, USA) was used to determine mycotoxins’ excitation and emission wavelengths. Cary Eclipse Scan and Simple Read software v1.1(132) (Palo Alto, CA, USA) were used to record the spectra. A Fisher Scientific Accumet AP71 pH/mV/Temperature Meter (Pittsburgh, PA, USA) was used to determine pH. Steady-state fluorescence spectra were recorded in a 10 × 10 mm quartz cell at room temperature (23–25 °C). The slit widths were set to 5 nm for the entrance and exit slits. A scan rate of 600 nm/min was used for the measurements. Ochratoxin A has an excitation wavelength of 333 nm and an emission of 475 nm. Aflatoxin B1 has an excitation wavelength of 365 nm and an emission of 415 nm. Zearalenone has an excitation wavelength of 315 nm and an emission of 460 nm.
2.3. Surface Area
Surface area measurements were made using a Quantachrome autosorbIQ automated gas sorption analyzer. Samples were outgassed under dynamic vacuum at 80 °C for 0.5 h, followed by 200 °C for 2 h, and lastly, 300 °C for 4 h. Measurements were performed with N2 at 77 K, and surface areas were determined using the BET method over a pressure range of 0.025 < P/Po < 0.20. When necessary, the pressure range used to calculate surface areas was reduced, as described by Rouquerol et al., to account for the filling of micropores [24].
2.4. FT-IR
The FT-IR experiments were conducted using modifications to previously published procedures [25,26]. The FT-IR spectra were obtained using a Shimadzu FT-IR instrument equipped with an ATR attachment. The apodization was for square triangles. The scans were set at 60. The resolution was set at 4 1/cm. The FT-IR spectra were recorded within the 340–4000 1/cm fingerprint region.
2.5. Equilibrium Sorption Assays
Mycotoxin sorption studies of the clays, charcoals, and biochars were carried out using a Max Q4450 incubating shaker with the rpm set at 60 and the temperature at 37 °C. Various concentrations (0.1 ppm and 1 ppm) of single evaluations of aflatoxin B1, ochratoxin A, and zearalenone were incubated with biochar (1 mg) dispersed in 1 mL solutions (pH 2.0, 100 mM sodium phosphate buffer, and pH 5.5, 100 mM sodium phosphate buffer) in 1.8 mL vials using variations of previously published procedures [5,20,27,28,29,30]. Following the two hours incubation, the samples were centrifuged for ten min at 3800 rpm. Experiments were performed in triplicate. The bound mycotoxin was calculated by subtracting the amount of mycotoxin free in the solution in the presence of the sorbent from standard solutions run without sorbent. The final concentrations were determined using standard curves and LC chromatography.
The percent adsorption of aflatoxin B1, ochratoxin A, and zearalenone by sequestering materials was calculated using the following equation:
where the initial concentration is the amount of mycotoxin concentration at the beginning of the experiment (0.1 ppm or 1 ppm) and the amount unbound is the mycotoxin concentration in the supernatant at the end of the experiment. The concentration of the unbound mycotoxin was calculated by comparing the mycotoxin levels with a control group of experiments that did not have mycotoxin-binding materials.
% Sorption: (Initial Amount − Amount Unbound)/Initial Amount × 100
2.6. In Vitro Gastric Model Evaluation
The efficacies of the mycotoxin-binding materials were evaluated using a popular gastric model that enabled comparison between other types of materials [5,31,32,33]. A vial (50 mL) containing 2.5 mL of 0.1 M sodium phosphate buffer pH 6.0, 12.5 mg of sequestration agent, and 5 mL of mycotoxin solution (1 ppm in 0.1 M sodium phosphate buffer pH 6.0) was prepared. The pH of the mixture was adjusted to 2.0 using 300 µL of 1 M hydrochloric acid solution (aq) to simulate stomach acid conditions. The 50 mL vials were placed on a Max Q4450 incubating shaker with the rpm set at 60 and the temperature at 39 °C for 2 h. Next, the small intestine was simulated by adding 1 mL of 0.2 M sodium phosphate buffer (pH 6.8) and 300 µL 1 M sodium hydroxide solution. The incubation was continued for an additional 4 h at 39 °C. Following incubation, the solution was centrifuged for 10 min at 3800 rpm. The supernatant was collected to measure the amount of free mycotoxin using LC analysis.
The percent adsorption of aflatoxin B1, ochratoxin A, and zearalenone by sequestering materials was calculated using the procedure described in Section 2.6.
2.7. LC Analysis
LC analysis was used to quantify levels of aflatoxin B1, ochratoxin A, and zearalenone following modifications to previously published procedures [20,29,34,35]. Buffered samples were filtered twice through 0.02 mm PTFE syringe filters, and (100 µL) samples were added to 900 µL of the filtered mobile phase. The LC system included a Shimadzu LC-20AT pump, an RA-10 fluorescence detector (excitation at 365 and emission at 415 for aflatoxin B1, excitation at 333 nm; emission at 475 nm for ochratoxin A, and excitation at 315 nm and emission at 460 nm for zearalenone). The system operated with a flow rate of 1 mL/min. The mobile phase for aflatoxin B1 was water: methanol (7:3, v/v). The mobile phase for ochratoxin A levels was acetonitrile/water/acetic acid (495:495:10). The mobile phase for zearalenone, the mobile phase was 1:1 acetonitrile: water. A CBM-20A communication bus controlled the system. A Rheodyne 775 manual injector equipped with a 20 µL loop was used for sample injection. A Phenomenex Luna 5 mm C18 (2) 100A column (250 × 4.6 mm) was used to achieve separation.
2.8. Statistics
Sorption binding data are presented as mean of triplicate experiments ± standard deviation values. The variation between samples was analyzed using MicroSoft Excel with the statistical analysis tool and the XLMiner Analysis ToolPak v2.0.0.0 (Frontline Systems). The p value was calculated from the T score (www.statology.org, accessed on 18 September 2023). A p value of less than 0.05 was determined to be statistically significant for the evaluation of samples.
3. Results
3.1. Surface Areas of Food-Grade and Feed-Grade Sequestering Agents
The surface areas of the food-grade and feed-grade sequestering agents investigated in this study are provided in Table 1. There is significant variability between the surface areas of charcoal/biochar materials investigated in this study. Calcium montmorillonite (4a) had a surface area of 51.4 m2/g, which is the higher than the surface areas of the sodium montmorillonite (4b) and sodium bentonite (4c). The biochar and charcoal samples investigated in this study have a wide range in surface areas from 1.7 to 1735 m2/g.

Table 1.
Surface areas of sequestering agents.
3.2. In Vitro Percent Adsorption of Mycotoxins under Simulated Digestion Conditions
The binding properties of the three clays and four biochar/charcoals for the in vitro gastric model assays are provided in Table 2. In vitro binding of aflatoxin B1, ochratoxin A, and zearalenone in simulated digestion experiments was conducted at the 1 ppm level. The simulated digestion model consists of two-hour incubation at pH 2.0 to simulate stomach acid digestion and four-hour incubation at pH 6.8 to simulate the small intestine. Activated coconut charcoal (5a) and pine biochar (5b) were capable of binding significant percentages of aflatoxin B1, ochratoxin A, and zearalenone at the 1 ppm level in the in vitro simulated digestion assay. Olive wood biochar (5d) was capable of lowering mycotoxin concentrations significantly, but not as well as activated coconut charcoal (5a) and pine biochar (5b).

Table 2.
In vitro percent adsorption of mycotoxins under simulated digestion conditions.
3.3. Aflatoxin B1 Sorption at pH 2.0
Table 3 shows the sorption studies for aflatoxin B1 after two-hour incubation in simulated gastric fluid (pH 2.0) to simulate the effectiveness of the sequestration agents in an environment that simulates the stomach. Activated coconut charcoal (5a), pine biochar (5b), and olive wood biochar (5d) bind greater than 93% of aflatoxin B1 at the 0.1 ppm level. Increasing the amount of aflatoxin B1 (1) to the 1 ppm level reduced the percentage of aflatoxin B1 absorbed for all materials evaluated. Calcium montmorillonite (4a), sodium montmorillonite (4b), sodium bentonite (4c), and horticulture biochar (5c) bound significantly lower percentages of initial aflatoxin B1 at the 1 ppm level compared to activated coconut charcoal (5a) and pine biochar (5b).

Table 3.
Aflatoxin B1 (1) sorption at pH 2.0.
3.4. Ochratoxin A Sorption at pH 2.0
Table 4 shows the sorption studies for ochratoxin A after two-hour incubation in simulated gastric fluid (pH 2.0) to study the efficacy of the materials to bind ochratoxin A in the stomach. Activated coconut charcoal (5a), pine biochar (5b), and olive wood biochar (5d) bind greater than 91% of ochratoxin A at the 0.1 ppm level. All of the sequestration agents investigated bound over 61% of ochratoxin A at the 0.1 ppm level. For all materials investigated, the percentage of ochratoxin A bound decreased at the 1 ppm level compared to the 0.1 ppm level. Activated coconut charcoal (5a) and pine biochar (5b) were able to bind greater than 97% of ochratoxin A at the 1 ppm level after incubation for two hours under conditions that simulate stomach digestion.

Table 4.
Ochratoxin A (2) sorption at pH 2.0.
3.5. Zearalenone Sorption at pH 2.0
Table 5 shows the sorption studies for zearalenone after two-hour incubation in simulated gastric fluid (pH 2.0). All materials evaluated could bind greater than 46% of zearalenone at the 0.1 ppm and 1 ppm levels under conditions that simulate stomach acid digestion. Increasing the levels of zearalenone from 0.1 ppm to 1 ppm decreased the percentage of zearalenone bound. Activated coconut charcoal (5a), pine biochar (5b), and olive wood biochar (5d) bind greater than 93% of ochratoxin A at the 0.1 ppm level. Activated coconut charcoal (5a) and pine biochar (5b) could bind over 97% of zearalenone at the 0.1 ppm and 1 ppm levels.

Table 5.
Zearalenone (3) Sorption at pH 2.0.
4. Discussion
Mycotoxins in the diet cause harmful effects on swine and other animal health and production. In vitro, equilibrium binding assays provide economical screening methods to evaluate toxins’ capacity and affinity, including mycotoxins. In this study, we investigated a wide range of commercially available and generally recognized as safe materials to bind and sequester several mycotoxins of concern in simulated gastric juice and small intestine [32,33]. Seven toxin-binding candidates were investigated, including three bentonite clays and four charcoal products. Montmorillonite is a specific type of bentonite clay. Previous studies have shown that clay and charcoal-related materials bind various mycotoxins well at low concentrations of mycotoxins (0.010 ppm) [5,32,33].
4.1. Surface Areas of Food-Grade and Feed-Grade Sequestering Agents
The surface areas of the seven toxin-binding materials are shown in Table 1. The food-grade calcium montmorillonite (4a) has 60% more surface area compared to sodium montmorillonite (4b) and sodium bentonite (4c). Montmorillonite and bentonite are soft clay minerals formed during crystallization from water. Calcium montmorillonite (4a) is known to have superior adsorption properties in water and is widely used in cosmetics, detoxification agents, agriculture, and waste treatments [36].
Interestingly, the biochar and charcoal-based products vary in surface area from 1.7 mg2/g to 1735 mg2/g, depending on the feedstock and degree of activation [37]. The activated coconut charcoal (5a) possessed micropores with a surface area of 988 m2/g, and pine biochar (5b) had micropores with a surface area of 463 m2/g. Previous studies have shown that the increased temperature of pyrolysis can increase the surface area of biochars/charcoals [38]. The surface area is associated with feedstock and any chemical activation. It has also been demonstrated that surface area is not necessarily related to binding effectiveness [20,39,40].
4.2. In Vitro Percent Adsorption of Mycotoxins under Simulated Digestion Conditions
The binding properties of the three clays and four biochar/charcoals are provided in Table 2. In vitro binding of aflatoxin B1, ochratoxin A, and zearalenone in simulated digestion experiments were conducted at the 1 ppm level. This toxin level is much higher than previous studies that show near-complete binding of mycotoxins. However, the 1 ppm level is frequently used in animal studies to assess the harmful effects of these mycotoxins in animals [41].
The clay materials (4a, 4b, and 4c) in this study do not bind most of the three mycotoxins at the 1 ppm level after 2 h of incubation in simulated stomach digestion (pH 2.0) and 4 h in the simulated small intestine (pH 6.8). The charcoals/biochars in this study vary significantly in sorption efficacy. Carbon materials with high surface areas bind most of each mycotoxin at the 1 ppm level (86.7–97.3%). In contrast, horticulture biochar (5c) possesses the lowest surface area by BET analysis and binds the lowest amount of each of the mycotoxins of the carbon-based materials evaluated in this study. Activated coconut charcoal (5a) and pine biochar (5b) show promise to reduce animal exposure to the effects of mycotoxins. The harmful effects extend beyond animal production and have been shown to include contamination of animal milk [42,43]. In addition, charcoals have been shown to be essential components in multi-component binders to aid in reducing the effects of ochratoxin A in broiler breeders [44].
4.3. Aflatoxin B1, Ochratoxin A, and Zearalenone Sorption at pH 2.0
To gain insight into the binding properties of the sequestration agents for aflatoxin B1, ochratoxin A, and zearalenone in the initial breakdown of food during digestion, we conducted a series of sorption experiments under conditions that simulate the stomach. Experiments were conducted with mycotoxin levels of 0.1 ppm and 1 ppm. Table 3 shows the sorption studies for aflatoxin B1 after two hours of incubation in simulated gastric fluid (pH 2.0). Interestingly, activated coconut charcoal and pine biochar bind aflatoxin B1 at high concentrations of 0.1 ppm and 1 ppm. It is also interesting that olive wood biochar (5d) and horticulture biochar (5c) bind less. Olive wood biochar (5d) binds greater than 90% of the aflatoxin B1 at 0.1 ppm but only 63% at the 1 ppm level. Olive wood biochar from pruning waste has been shown to bind other small organic molecules, including promazine and promethazine [45].
Table 4 shows the efficacy of sequestration materials to bind ochratoxin A (2) at pH 2.0. All materials exhibit an increase in capacity for ochratoxin A compared to aflatoxin B1. Activated coconut charcoal and pine biochar demonstrate high binding capabilities at up to 1 ppm for ochratoxin A. Biochars produced from cashew nut shells have been shown to exhibit ochratoxin A-binding properties. However, that published study did not allow comparisons with other types of binding materials [45]. The surface areas of the cashew nutshell biochar were significantly smaller than the pine biochar and activated coconut charcoal in this study (5b and 5a). In addition, several biochar products and clays had improved binding efficacy compared to ground nut shells [46].
Interestingly, the clays (4a, 4b, and 4c) exhibit similar binding properties for ochratoxin A. These similar binding properties may be due to the complex structure of ochratoxin A and the multiple formations it achieves through intramolecular interactions. In contrast, aflatoxin B1 is more rigid and has been shown to accommodate the binding sites of calcium montmorillonite clays.
The sorption studies of the mycotoxin zearalenone (3) binding to the sequestration agents at pH 2.0 are shown in Table 5. The sorbents in this study exhibit increased zearalenone binding compared to ochratoxin A and aflatoxin B1. Activated coconut charcoal and pine biochar exhibit excellent potential for binding aflatoxin B1, ochratoxin A, and zearalenone and may address the need for binding materials that can bind more than one mycotoxin. Several research groups have reported that clays, carbohydrate-based sorbents, and algae biomaterials are limited in the types of mycotoxins that these materials can sequester [2,17,18,47,48].
An FT-IR analysis was conducted to gain insight into the structural features of the mycotoxin-binding features of the sorbents in this study. Specific functional groups, such as amines, exhibit strong molecular recognition capabilities for zearalenone and ochratoxin A [49,50]. Ochratoxin A and zearalenone have weak acid functional groups capable of forming hydrogen bond interactions. The FT-IR spectra of the biochar and charcoal in this study are similar. Notable peaks in both biochars were the 3400–3600 1/cm region O–H stretching peak, the peaks at 1200–1500 1/cm are associated with C–C and C–O vibrations, and the bending peak at 1400–1500 1/cm is related to the CH2 group. The peaks near 1500–1700 1/cm may also represent aromatic carbon-carbon alkene groups, common in biochar.
The FT-IR analysis provide information on the structural features the bentonite clays in this study. The clays in this study are marketed for food-grade applications and cosmeceutical use, and not necessarily intended to bind the specific mycotoxins evaluated here. Other researchers have reported bentonite clays have exhibited favorable mycotoxin-binding properties [32]. Other studies report that calcium montmorillonite (4a) and sodium montmorillonite (4b) exhibit characteristic FT-IR Si–O–Si in-plane stretching peaks [50,51], with the sodium montmorillonite (4b) Si–O–Si frequency band red-shifted ca. 25 1/cm compared to calcium montmorillonite (4a), 1000 1/cm vs. 975 1/cm respectively. Both calcium montmorillonite (4a) and sodium montmorillonite (4b) show O-H stretching peaks at 3555 1/cm, broad hydration O–H stretching peaks at 3450 1/cm, and H–O–H deformation bands at 1625–1630 1/cm as other researchers have observed in montmorillonite [51]. Sodium montmorillonite (4b) exhibited a stronger, 10 1/cm blue shifted Si–O stretching out-of-plane peak compared to calcium montmorillonite (4a), 1120 vs. 1110 1/cm, respectively. Characteristic Al–Al–OH bending frequencies for calcium montmorillonite (4a) and sodium montmorillonite (4b) were observed at 910 1/cm, with the sodium montmorillonite (4b) intensity slightly stronger. The Al–Mg–OH bending frequency was observed at 845 1/cm for calcium montmorillonite (4a) and sodium montmorillonite (4b), which is consistent with results from the literature [52]. The Si–O stretching of quartz/silica was observed for both calcium montmorillonite (4a) and sodium montmorillonite (4b) at 790 1/cm, with the sodium montmorillonite (4b) peaks being slightly weaker [53]. Overall, the cationic effect of Na+ vs. Ca2+ resulted in relatively little difference in the stretching and bending frequencies of the respective forms of the cationic montmorillonite clays, as was observed previously [54].
Sodium bentonite (4c) exhibited FT-IR frequency bands similar to those of calcium montmorillonite (4a) and sodium montmorillonite (4b). An O–H stretching band for sodium bentonite (4c) was observed at 3555 1/cm, slightly more intense than the broad O–H hydration band at 3450 1/cm compared to both calcium montmorillonite (4a) and sodium montmorillonite (4b). Sodium bentonite (4c) also exhibited a H–O–H deformation band at 1640 1/cm. An out-of-plane Si–O stretching band was observed for sodium bentonite (4c) at 1120 1/cm, similar to the montmorillonite clays. A large Si–O–Si in-plane stretching peak for sodium bentonite (4c) at 975 1/cm was blue-shifted ca. 25 1/cm, similar to sodium montmorillonite (4b), compared to for sodium bentonite (4c). The FT-IR peak at 915 1/cm and the shoulder observed at 850 1/cm were attributed to Al–Al–OH bending and Al–Mg–OH bending in previous studies [54], similar to the cationic montmorillonite clays. The peak observed at 800 1/cm in the sodium bentonite (4c) was assigned to the Si–O stretching of quartz/silica, which was blue-shifted ca. 10 1/cm compared to the same peaks for calcium montmorillonite (4a) and sodium montmorillonite (4b). The peaks observed at ≤ 650 1/cm were attributed to the maximum absorption band of layered aluminosilicates with the shoulder at 625 1/cm and the peak at 510 1/cm assigned to Si–O bending frequencies, similar to those observed by other researchers for calcium montmorillonite (4a) and sodium montmorillonite (4b) [55,56,57].
4.4. Related Applications and Considerations
This study investigates several food/feed-grade and related materials for their ability to sequester three important mycotoxins under conditions that simulate digestion. The utilization of materials that are currently used as feed/food additives has the benefit of being more readily adaptable to real-world applications compared to materials that are not already food/feed-grade additives. Activated coconut charcoal (5a) and pine biochar (5b) are the most promising materials in this study. The potential non-specific binding of nutrients to carbon-based sequestration agents has led to several recent studies on the effects of charcoals and biochars on animal production to better understand the merits and limitations of the uses of charcoal and biochar as feed additives. Biochar-supplemented feed did not affect the performance of pigs during the two weeks before slaughter [58]. Biochar additives at the 2% levels have been shown to reduce poultry diseases associated with Gallibacterium anatis and campylobacters [59]. Charcoal and biochar from a variety of sources have been shown to increase feed conversion and increase body weight in broilers and strengthen eggshells, depending on the amount of material included in the feed supply [60,61,62]. Sheep demonstrated increased digestibility of feed that included biochar without an impact on weight gain [63]. Recently, it was shown that activated carbon could bind aflatoxin B1, deoxynivalenol, and zearalenone [5]. This study shows that certain food-grade and feed-grade coconut charcoal and pine biochar products can sequester aflatoxin B1, ochratoxin A, and zearalenone at 1 ppm levels. These results demonstrate that selected carbon-based materials marketed as feed additives may have the additional benefit of sequestering toxins that occasionally contaminate animal feed.
5. Conclusions
GRAS clays and biochars were evaluated for their ability to bind the important mycotoxins aflatoxin B1, ochratoxin A, and zearalenone. The influence of surface award on mycotoxin binding was investigated. It was found that surface area has a significant impact on binding properties. Pine biochar was shown to bind high levels of mycotoxins in gastric fluid during in vitro studies, and the results were comparable to food-grade activated coconut charcoal marketed as a detoxification supplement. Certain biochar-based materials show promise to sequester toxins and may be suitable for further activation to enhance mycotoxin-binding properties.
Author Contributions
Conceptualization, methodology, M.A. and E.C.W.; investigation M.A., E.C.W., B.K.S., F.J.E., K.O.E. and D.L.C.; writing original draft M.A., E.C.W. and D.L.C.; writing review and editing M.A., E.C.W., B.K.S., F.J.E., K.O.E. and D.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the U.S. Department of Agriculture, Agricultural Research Service.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the study are available.
Acknowledgments
The authors wish to thank Paige N. Pierson for her excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Ahmed, S.; Islam, M.N.; Maitra, P.; Islam, M.M.; Yu, D. Critical Assessment of Mycotoxins in Beverages and Their Control Measures. Toxins 2021, 13, 323. [Google Scholar] [CrossRef]
- Rodríguez, M.; Núñez, F. Novel Approaches to Minimizing Mycotoxin Contamination. Toxins 2020, 12, 216. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, J.; Cheong, D.H.; Hong, H.; Jeong, J.Y.; Kim, B.G. An In Vitro Study on the Efficacy of Mycotoxin Sequestering Agents for Aflatoxin B1, Deoxynivalenol, and Zearalenone. Animals 2022, 12, 333. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 7, e7373. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.S.; Connolly, L.; Elliott, C. Comparative In Vitro Assessment of a Range of Commercial Feed Additives with Multiple Mycotoxin Binding Claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef]
- Jedziniak, P.; Panasiuk, Ł.; Pietruszka, K.; Posyniak, A. Multiple mycotoxins analysis in animal feed with LC-MS/MS: Comparison of extract dilution and immunoaffinity clean-up. J. Sep. Sci. 2019, 42, 1240–1247. [Google Scholar] [CrossRef]
- Raj, J.; Farkaš, H.; Jakovčević, Z.; Medina, A.; Magan, N.; Čepela, R.; Vasiljević, M. Comparison of multiple mycotoxins in harvested maize samples in three years (2018–2020) in four continents. Food Addit. Contam. Part A 2022, 39, 599–608. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 235289. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, S.; Spalenza, V.; Dellafiora, L.; Badino, P.; Barbarossa, A.; Dall’Asta, C.; Nebbia, C.; Girolami, F. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicol. Vitr. 2019, 57, 174–183. [Google Scholar] [CrossRef]
- Deng, J.; Huang, J.-C.; Xu, Z.-J.; Liu, Y.; Karrow, N.A.; Liu, M.; Sun, L.-H. Remediation Strategies for Mycotoxins in Animal Feed. Toxins 2023, 15, 513. [Google Scholar] [CrossRef]
- Kihal, A.; Rodríguez-Prado, M.; Calsamiglia, S. The efficacy of mycotoxin binders to control mycotoxins in feeds and the potential risk of interactions with nutrient: A review. J. Anim. Sci. 2022, 100, skac328. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Peterson, S.C.; Appell, M.; Jackson, M.A.; Boateng, A.A. Characterization and evaluation of corn stover and switchgrass biochar for removal of estrogenic compounds from water. Can. J. Agric. Sci. 2013, 5, 1–8. [Google Scholar]
- Toth, J.D.; Dou, Z. Use and Impact of Biochar and Charcoal in Animal Production Systems. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, MA, USA, 2016. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Alwan, L.H.; Abdulqader, M.A. Thermodynamic and kinetic studies of Eriochrome black adsorption on activated charcoal prepared from lemon leaves. Mater. Res. Express 2020, 6, 1250h8. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Activated Charcoal Livestock. 2021. Available online: https://www.ams.usda.gov/sites/default/files/media/USDANOPActivatedCharcoalTechnicalReport.pdf (accessed on 18 September 2023).
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the bet equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar] [CrossRef]
- Melo, L.L.A.; Ide, A.H.; Duarte, J.L.S.; Zanta, C.L.P.S.; Oliveira, L.M.T.M.; Pimentel, W.R.O.; Meili, L. Caffeine removal using Elaeis guineensis activated carbon: Adsorption and RSM studies. Environ. Sci. Pollut. Res. 2020, 27, 27048–27060. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, H.; Çalışkan, Ç.E.; İçtüzer, Y.; Arslanoğlu, H. Application of activated carbon obtained from waste vine shoots for removal of toxic level Cu(II) and Pb(II) in simulated stomach medium. Biomass Conv. Bioref. 2023, 1–13. [Google Scholar] [CrossRef]
- Mishra, A.P.; Bajpai, A.; Chandra, S. A Comprehensive Review on the Screening Models for the Pharmacological Assessment of Antiulcer Drugs. Curr. Clin. Pharmacol. 2019, 14, 175–196. [Google Scholar] [CrossRef]
- Alhogbi, B.G.; Al Balawi, G.S. An Investigation of a Natural Biosorbent for Removing Methylene Blue Dye from Aqueous Solution. Molecules 2023, 28, 2785. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Sorption of Ochratoxin A from Aqueous Solutions Using β-Cyclodextrin-Polyurethane Polymer. Toxins 2012, 4, 98–109. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater. Colloid Interface Sci. Commun. 2021, 41, 100380. [Google Scholar] [CrossRef]
- Costa, J.; Ahluwalia, A. Advances and Current Challenges in Intestinal in vitro Model Engineering: A Digest. Front. Bioeng. Biotechnol. 2019, 7, 144. [Google Scholar] [CrossRef]
- Kong, C.; Shin, S.Y.; Kim, B.G. Evaluation of mycotoxin sequestering agents for aflatoxin and deoxynivalenol: An in vitro approach. SpringerPlus 2014, 3, 346. [Google Scholar] [CrossRef]
- Boisen, S.; Fernandez, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed. Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Herzallah, S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009, 114, 1141–1146. [Google Scholar] [CrossRef]
- Mahfuz, M.; Gazi, M.A.; Hossain, M.; Islam, M.R.; Fahim, S.M.; Ahmed, T. General and advanced methods for the detection and measurement of aflatoxins and aflatoxin metabolites: A review. Toxin Rev. 2020, 39, 123–137. [Google Scholar] [CrossRef]
- Mapossa, A.B.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Mhike, W. Thermal, Morphological and Mechanical Properties of Multifunctional Composites Based on Biodegradable Polymers/Bentonite Clay: A Review. Polymers 2023, 15, 3443. [Google Scholar] [CrossRef]
- Ahuja, R.; Kalia, A.; Sikka, R.; Chaitra, P. Nano Modifications of Biochar to Enhance Heavy Metal Adsorption from Wastewaters: A Review. ACS Omega 2022, 7, 45825–45836. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.S. Mercury adsorption characteristics of carbon sorbent with low surface area. J. Air Waste Manag. Assoc. 2021, 71, 1445–1452. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Fawzy, S.; Rooney, D.W.; Osman, A.I. Competitive adsorptive removal of promazine and promethazine from wastewater using olive tree pruning biochar: Operational parameters, kinetics, and equilibrium investigations. Environ. Sci. Pollut. Res. 2023, 30, 82387–82405. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research progress in toxicological effects and mechanism of aflatoxin B1 toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef]
- Bodas, R.; Giráldez, F.J.; Olmedo, S.; Herrera, M.; Lorán, S.; Ariño, A.; López, S.; Benito, A.; Juan, T. The Effects of Aflatoxin B1 Intake in Assaf Dairy Ewes on Aflatoxin M1 Excretion, Milk Yield, Haematology and Biochemical Profile. Animals 2023, 13, 436. [Google Scholar] [CrossRef]
- Falkauskas, R.; Bakutis, B.; Jovaišienė, J.; Vaičiulienė, G.; Gerulis, G.; Kerzienė, S.; Jacevičienė, I.; Jacevičius, E.; Baliukonienė, V. Zearalenone and Its Metabolites in Blood Serum, Urine, and Milk of Dairy Cows. Animals 2022, 12, 1651. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Song, D.; Chang, S.; An, J.; Nam, J.; Lee, B.; Kim, S.; Kim, W.K.; Cho, J. Effects of Combinations of Toxin Binders with or without Natural Components on Broiler Breeders Exposed to Ochratoxin A. Animals 2023, 13, 2266. [Google Scholar] [CrossRef]
- Ahmadou, A.; Brun, N.; Napoli, A.; Durand, N.; Montet, D. Effect of pyrolysis temperature on ochratoxin A adsorption mechanisms and kinetics by cashew nut shell biochars. J. Food Sci. Technol. 2019, 4, 877–888. [Google Scholar]
- Loffredo, E.; Scarcia, Y.; Parlavecchia, M. Removal of ochratoxin A from liquid media using novel low-cost biosorbents. Environ. Sci. Pollut. Res. Int. 2020, 27, 34484–34494. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, A.; Apajalahti, J.; Kettunen, H.; Ojanperä, S.; Bell, A.N.W.; Keegan, J.D.; Moran, C.A. Efficient Aflatoxin B1 Sequestration by Yeast Cell Wall Extract and Hydrated Sodium Calcium Aluminosilicate Evaluated Using a Multimodal In-Vitro and Ex-Vivo Methodology. Toxins 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Le, L.H.T.; Tran-Lam, T.-T.; Nguyen, H.Q.; Quan, T.C.; Nguyen, T.Q.; Nguyen, D.T.; Dao, Y.H. A study on multi-mycotoxin contamination of commercial cashew nuts in Vietnam. J. Food Compos. Anal. 2021, 102, 104066. [Google Scholar] [CrossRef]
- Urraca, J.L.; Marazuela, M.D.; Merino, E.R.; Orellana, G.; Moreno-Bondi, M.C. Molecularly imprinted polymers with a streamlined mimic for zearalenone analysis. J. Chromatogr. A 2006, 1116, 127–134. [Google Scholar] [CrossRef]
- Ramos, A.J.; Hernández, E.; Plá-Delfina, J.M.; Merino, M. Intestinal absorption of zearalenone and in vitro study of non-nutritive sorbent materials. Int. J. Pharm. 1996, 128, 129–137. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-Assembly Processes in Hydrated Montmorillonite by FTIR Investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef]
- Cukrowicz, S.; Grabowska, B.; Kaczmarska, K.; Bobrowski, A.; Sitarz, M.; Tyliszczak, B. Structural studies (FTIR, XRD) of sodium carboxymethyl cellulose modified bentonite. Arch. Foundry Eng. 2020, 20, 119–125. [Google Scholar] [CrossRef]
- Adikary, S.U.; Wanasinghe, D.D. Characterization of Locally Available Montmorillonite Clay Using FTIR Technique. 2012. Available online: https://www.academia.edu/4173843/Characterization_of_locally_available_Montmorillonite_clay_using_FTIR_technique (accessed on 18 September 2023).
- Elkhalifah, A.E.I.; Murugesan, T.; Bustam, M.A. Characterization of different cationic forms of montmorillonite by FTIR, XRD and TGA techniques. In Proceedings of the 2011 National Postgraduate Conference, Perak, Malaysia, 19–20 September 2011; pp. 1–6. [Google Scholar] [CrossRef]
- Krzaczkowska, J.; Fojud, Z.; Kozak, M.; Jurga, S. Spectroscopic Studies of Poly(ε-Caprolactone)/Sodium Montmorillonite Nanocomposites. In Proceedings of the XXI International Meeting on Radio and Microwave Spectroscopy RAMIS 2005, Poznan-Bedlewo, Poland, 24–28 April 2005. [Google Scholar]
- Natkański, P.; Kuśtrowski, P.; Białas, A.; Surman, J. Effect of Fe3+ ions present in the structure of poly(acrylic acid)/montmorillonite composites on their thermal decomposition. J. Therm. Anal. Calorim. 2013, 113, 335–342. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.C.; Chuppava, B.; Witte, F.; Terjung, N.; Visscher, C. Evaluation of Coated Biochar as an Intestinal Binding Agent for Skatole and Indole in Male Intact Finishing Pigs. Animals 2021, 11, 760. [Google Scholar] [CrossRef]
- Willson, N.L.; Van, T.T.H.; Bhattarai, S.P.; Courtice, J.M.; McIntyre, J.R.; Prasai, T.P.; Moore, R.J.; Walsh, K.; Stanley, D. Feed supplementation with biochar may reduce poultry pathogens, including Campylobacter hepaticus, the causative agent of Spotty Liver Disease. PLoS ONE 2019, 14, e0214471. [Google Scholar] [CrossRef]
- Jiya, E.Z.; Ayanwale, B.A.; Ijaiya, A.T.; Ugochukwu, A.; Tsado, D. Effect of Activated Coconut Shell Charcoal Meal on Growth Performance and Nutrient Digestibility of Broiler Chickens. Br. J. Appl. Sci. Technol. 2013, 3, 268–276. [Google Scholar] [CrossRef]
- Kana, J.R.; Teguia, A.; Mungfu, B.M.; Tchoumboue, J. Growth performance and carcass characteristics of broiler chickens fed diets supplemented with graded levels of charcoal from maize cob or seed of Canarium schweinfurthii Engl. Trop. Anim. Health Prod. 2011, 43, 51–56. [Google Scholar] [CrossRef]
- Kutlu, H.R.; Unsal, I.; Gorgulu, M. Effects of providing dietary wood (oak) charcoal to broiler chicks and laying hens. Anim. Feed. Sci. Technol. 2001, 90, 213–226. [Google Scholar] [CrossRef]
- McAvoy, D.J.; Burritt, B.; Villalba, J.J. Use of biochar by sheep: Impacts on diet selection, digestibility, and performance. J. Anim. Sci. 2020, 98, skaa380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).