Simple Summary
Prototheca algae are among the primary agents of bovine mastitis, incurring significant losses to the dairy industry related to a decrease in milk quality and yield, premature culling, and early replacement of infected cows. To date, there is no effective therapy for bovine mammary protothecosis. Here, the in vitro algaecide activity of quaternary ammonium (QA), a sanitizer used for animal and human purposes, was observed in Prototheca algae isolated from mastitic cows, indicating the potential use of this compound as an antiseptic/disinfectant for milking facilities and surrounding environmental areas.
Abstract
The in vitro algaecide activity of quaternary ammonium (QA) against Prototheca isolated from bovine clinical mastitis was investigated, in which the clinical severity was scored, milk samples were subjected to microbiological culture, and algal species were identified by molecular typing. A total of 4275 milk clinical samples of different cows from ten large dairy farms were used. Forty-four (1%) samples of cows from three dairy farms yielded growth of Prototheca, of which 88.6% (39/44) were identified as Prototheca bovis and 11.3% (5/44) as Prototheca sp. by MALDI-TOF MS, whereas 100% of the isolates were identified as P. bovis using PCR sequencing of the cytb gene. Among cows for which clinical severity scoring was available, 78.8% (26/33) and 21.2% (7/33) had mild and moderate infections, respectively, whereas no animal showed severe clinical signs. The algaecide activity of QA in Prototheca was observed in low concentrations among all isolates, in 20.4% (9/44) at 35 ppm, 36.4% (16/44) at 17 ppm, and 43.2% (19/44) at an 8 ppm, in addition to activity on three reference Prototheca strains. Overall, the study highlights the predominance of P. bovis as the causative agent of algal mastitis in bovines. Prototheca induced abnormalities preponderantly in the milk and mammary gland tissue of cows, and to our knowledge, our study is the first to apply clinical severity scoring in protothecal mastitis. In addition, the study underlines the activity of QA in low concentrations against Prototheca, indicating its potential use as an antiseptic/disinfectant in milking facilities and dairy environments.
1. Introduction
Microalgae of the genus Prototheca are eukaryotic, saprophytic, and ubiquitous organisms that reproduce asexually by endosporulation [1]. These algae lost their photosynthetic ability during evolution, which induced heterotrophic metabolism, allowing the development of opportunistic infections in animals and humans [2].
The current taxonomy of the algae has been built based on the mitochondrial cytb gene, subdividing the genus Prototheca into 14 well-defined species, namely: P. ciferrii (formerly P. zopfii genotype 1 or biovar 1), P. bovis (formerly P. zopfii genotype 2 or biovar 2), P. blaschkeae (formerly biovar 3), P. wickerhamii, P. cutis, P. miyajii, P. moriformis (formerly P. ulmea), P. stagnora, P. tumulicola, P. cookei, P. pringsheimii, P. xanthoriae, and P. cerasi [1]. Recently, another four Prototheca species have been described, namely P. paracutis, P. fontanea, P. lentecrescens, and P. vistulensis [3,4]. Among all Prototheca species, P. bovis and, less frequently, P. blaschkeae have been recognized as the causative agents of bovine mammary infections worldwide [5].
Bovine mastitis is the most common clinical manifestation of protothecosis among domestic animals [1]. An increasing occurrence of intramammary protothecosis has been reported in several countries [1,6,7,8,9,10,11,12,13], including those having modern dairy herds with well-controlled management of contagious agents [14].
Prototheca species inhabit wet sources containing feces, decomposing organic matter, stagnant water, and animal bedding, as well as contaminating milking utensils, which favor mammary infections in cows from the dairy environment and surrounding areas [15]. Mammary protothecosis causes significant losses to dairy industries, through a significant reduction in milk yield and quality, increased milk cell counts, loss of mammary quarters, premature culling of animals, costs of veterinary services, and early replacement of animals [5].
Mammary protothecosis has been associated predominantly with clinical infections and, less frequently, subclinical cases, with a chronic evolution, usually restricted to clinical abnormalities in the milk and mammary gland [16].
In the last decade, the clinical severity of bovine mammary infections has been scored as mild, moderate, and severe cases [17] and investigated mainly among environmental agents [18,19]; although, this scoring method for clinical cases has not been investigated in protothecal mammary infections.
To date, no therapy protocol has been fully effective in controlling Prototheca-induced infections in dairy cows; a fact that has prompted a set of in vitro studies focused on the algaecide activity of a wide range of antimicrobials [20,21], antifungals [22], sanitizers [6,15,23,24,25,26], natural extracts [27], essential oils [28], polypeptides [29], nanoparticles [30], photodynamic therapy [31], and herbicides [32].
Quaternary ammonium compounds (QAC) are cationic surfactants commonly used as antiseptics and disinfectants in human and veterinary medicine due to their microbicidal action [33], which denatures proteins of microbial cell membranes and cytoplasm [34]. In addition, they possess other properties, such as low toxicity, good stability, biodegradability, lack of odor, little corrosiveness, and low skin irritation potential toward the skin and mucous membranes [35]. These characteristics have enabled their use in domestic, agricultural, and hospital disinfection applications, as well as in food industries, animal facilities, and swimming pools [36]. Nonetheless, despite their well-known microbicidal action, no in vitro studies have investigated the algaecide effect of quaternary ammonium (QA) used in swimming pools against Prototheca species molecularly identified and isolated from clinical mammary infections in cattle.
Given the increase in protothecal mastitis worldwide, the lack of effective therapy for mammary infections, and severe losses to the dairy industry related to bovine mastitis-related Prototheca species, we investigated the in vitro algaecide activity of QA against Prototheca isolated from clinical bovine mastitis, identified at the species-level based on mass spectrometry and PCR sequencing of the cytb gene.
2. Results and Discussion
Of the total milk samples cultured, 1% (44/4275) yielded growth of Prototheca isolates. The algae originated from only three (3/10 = 30%) farms studied (Figure 1), giving within-farm prevalences of mammary protothecosis of 3.7% (8/212), 4.6% (33/721), and 4.5% (3/66) in farms B, E, and J, respectively (Table 1).
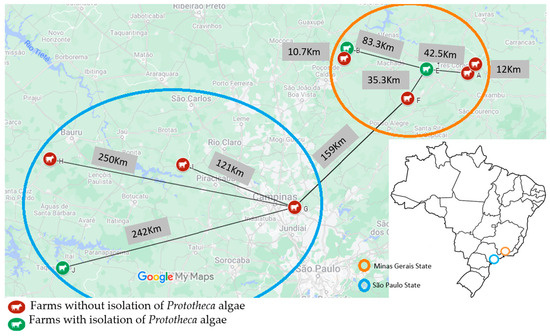
Figure 1.
Geographical location and distance between ten dairy farms sampled in Sao Paulo and Minas Gerais states, Brazil, and isolation of Prototheca algae among cows with clinical mastitis mammary infections (2017–2022).

Table 1.
Frequency of Prototheca bovis identification based on PCR-sequencing of the cytb gene marker in bovine clinical mastitis among farms in Sao Paulo and Minas Gerais states, Brazil (2017–2022).
All Prototheca isolates were cultured from quarters of single cows (n = 44).
Clinical severity score data were obtained for 75% (33/44) of the Prototheca-positive cows. Of these, mild (score 1) and moderate (score 2) clinical severity scores were observed in 78.8% (26/33) and 21.2% (7/33) of cows, respectively, showing abnormalities exclusively in milk and mammary glands. None of the animals showed a severe infection (score 3) or systemic signs.
Among the 44 isolates initially diagnosed as Prototheca (i.e., upon colony macro- and micromorphology), 84.4% (38/44) and 13.6% (5/44) were identified either as P. bovis or Prototheca sp. using MALDI-TOF MS, respectively. In contrast, PCR sequencing of the cytb gene allowed all 44 Prototheca isolates to be identified as P. bovis.
The in vitro algicide activity of QA was observed at low concentrations against all 44 Prototheca mastitis isolates and three type strains, at three concentrations: 35 ppm, 17 ppm, and 8 ppm (Table 2; Figure 2 and Figure 3).

Table 2.
In vitro algaecide effect of quaternary ammonium in Prototheca isolated from cows with clinical mastitis (2017–2022).
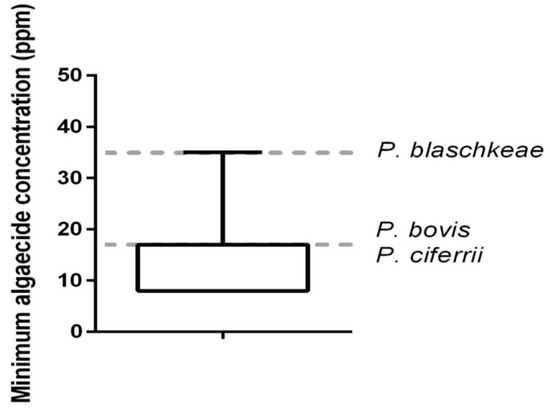
Figure 2.
In vitro minimum algaecide concentration of quaternary ammonium on 44 Prototheca bovis isolated from clinical bovine mastitis and reference strains of Prototheca (P. bovis, P. blaschkeae, and P. ciferrii). Reference strains provided by Dr. Tomasz Jagielski, University of Warsaw, Poland [1].
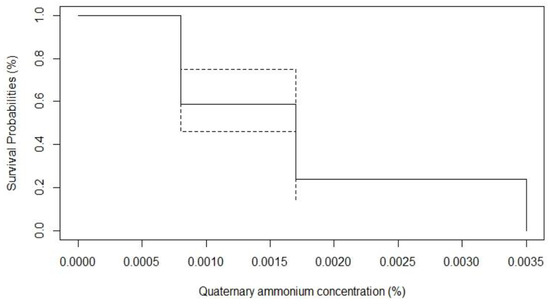
Figure 3.
Survival probabilities of 44 P. bovis isolated from bovine clinical mastitis in face of in vitro algaecide effect of quaternary ammonium.
Mammary infections caused by Prototheca species pose a contemporary and serious challenge to the control of bovine mastitis [37,38], particularly among modern dairy herds with well-established control measures for traditional contagious pathogens [14]. These difficulties are due to a wide distribution and adaptation of the algae to a variety of biomes [16], including milking environments and surrounding areas that favor intramammary infections [39], in addition to a lack of effective protocols for intramammary or systemic treatment of algal infections [37,40], a fact that may be considered the primary motivation of the current study.
A substantial increase in cases and outbreaks of Prototheca-related bovine mammary infections has recently been described in different countries, including Canada [13], Italy [11,41], Poland [1,9], Korea [12], China [10] and Ecuador [8]. In Brazil, mastitis was first described in 1992 in cows with chronic clinical mastitis in the interior of the state of São Paulo, southeastern Brazil [42]. Subsequently, the algae has been described in several states of the country with expressions in milk production, mainly in clinical, chronic cases, in rural properties with excess humidity and organic matter in the milking environment and deficiency in the practice of milking [6,7,23,24]. Currently, it is estimated that breast infections by Prototheca in the country occur in between 5 and 10% of clinical cases, and have been considered one of the main agents of cases of environmental mastitis [5]. In the present study, of the 4275 milk samples from clinical mastitic cows, the algae were isolated among 1% of the animals, with the within-farm prevalence not exceeding 5%. The low prevalence of clinical mammary protothecosis among dairy herds in this study can be credited to sound knowledge and strict adherence to hygienic milking practices preventing the spread of environmental and contagious pathogens [16].
Clinical severity scoring of cases has been investigated in bovine mammary infections [17], in addition to a set of virulence factors from some pathogens, especially of Enterobacteriaceae origin [19], to gain a better understanding of the pathogenic mechanisms involved in these infections, aiming to adopt proper preventive and control measures and to develop therapeutic interventions targeting the rational use of antimicrobials in dairy herds [43]. Among cows for which clinical severity scoring was available, 78.8% (26/33) and 21.2% (7/33) had mild and moderate infections, respectively. Conversely, no animal showed severe clinical signs, reinforcing the view that bovine mammary protothecosis induces abnormalities preponderantly in milk and mammary gland tissue [16], and rarely manifests with systemic signs [44]. To our knowledge, our study is the first to apply the clinical severity scoring of bovine mammary infections by Prototheca.
Routine diagnosis of protothecal mammary infections in cattle has been performed using milk cultures (farms and laboratories), identification of the algae based on macro- and micromorphology features of the cells, and biochemical testing (e.g., carbohydrate assimilation profile) [1,45]. Recently, a new molecular marker targeting the mitochondrial cytb gene has been proposed for the reliable identification of Prototheca species [1]. In the present study, all 44 Prototheca isolates collected from clinical mammary infections were identified as P. bovis using PCR-sequencing of the cytb marker, confirming P. bovis as the major etiological agent of protothecal bovine mastitis [6,7,8,9,10].
The identification of Prototheca has also been facilitated with the introduction of MALDI-TOF MS technology [7]. In the current study, MALDI-TOF MS allowed a prompt diagnosis of P. bovis in 86% (39/44) of the algae isolates, while the remaining five isolates were identified as Prototheca sp. This inability of species discrimination might refer to some technical inaccuracies in the sample. Nonetheless, the implementation of molecular methods into the diagnosis pathway of Prototheca mastitis has enabled a valuable decision-making process and delivery of proper control measures in affected herds [37,40].
The lack of effective intramammary or systemic therapy for Prototheca-induced infections has prompted several in vitro studies involving a great variety of different compounds with potential algaecide activity [6,15,20,21,22,23,24,25,26,28,29,30]. However, only a few of the compounds approached have been assessed in vivo and provided only a temporary resolution of clinical signs [37,40]. The refractoriness of Prototheca species to conventional treatment under in vivo conditions can be attributed to the development of pyogranulomatous reactions [46], high genetic diversity [40], and mechanisms of virulence and evasion of the immune response [47], such as biofilm production [23,24] and the induction of cellular apoptosis in mammary cells [48,49].
The in vitro algicidal effect of QA, a sanitizer with a well-known microbicidal potential, was investigated here on 44 Prototheca mastitic isolates. The in vitro algicidal activity of QA was observed against all isolates, at low concentrations (i.e., within the range of 8 ppm to 35 ppm, including three type strains of P. bovis, P. blaschkeae, and P. ciferrii). The biocidal effect of QA has been attributed to the direct action of the drug on the plasma membrane, leading to protein denaturation, membrane rupture, and consequent death of the pathogen [33,34]. QAC has long been used a sanitizer for human and animal safety (i.e., hospital, agricultural, and industrial environments) [36], due to its good stability, low toxicity, lack of odor, low corrosiveness, and biodegradability [35].
A similar study with 106 isolates of P. bovis (formerly P. zopfii 2) also investigated the algicidal effect of QA (3%) at three different time and temperature ratios (62 °C/30 s; 72 °C/15 s; and 80 °C/10 s), and observed a high algicidal effectiveness of quaternary ammonium (88–90%) at a dilution of 1:100, indicating that these compounds could be used as sanitizers for the control of protothecal mastitis [50]. Here, the in vitro algaecide activity of QA used as a sanitizer for swimming pools was observed at low concentrations against P. bovis, isolated from clinical mastitic cows. Our results suggest that QA could be used at low concentrations as a sanitizer for the milking environment and surrounding areas of dairy farms, particularly those with persisting protothecal mastitis. In addition, it would be worth testing the algaecide effect of QA as pre- and post-dipping antiseptic solutions to milking cows since this compound exhibits low toxicity and causes low irritation to the mucous membranes [36].
Prototheca algae have zoonotic potential [51]. This applies particularly to P. bovis [1] and P. wickerhamii [5] species in terms of livestock and companion animals. Milk from infected animals should not be consumed by humans or offered to calves, due to the possibility of developing enteric infections [25,37], in addition to severe systemic disorders, mostly in immunosuppressed patients [51]. In general, since Prototheca algae are transmitted from cows to humans by the consumption of milk and its derivatives, they are relevant to human health [38] and require attention to adopt measures aimed at the prevention and control of protothecal mammary infections in herds.
Convenience sampling of Prototheca isolates and the lack of sampling of milking devices and immediate cow surroundings, which would allow for the environmental impact on the occurrence of clinical protothecal mastitis to be assessed, should be considered the main limitations of the study.
Overall, the predominance of P. bovis among cows with clinical protothecosis, supports the leading role of this species in the etiology of algal bovine mastitis. QA used in swimming pools was revealed to have an in vitro algaecide effect in the Prototheca, at low concentrations. This study contributes to the molecular epidemiology of protothecal mastitis and clinical severity scoring of cases, and explores the in vitro activity of QAC sanitizers against Prototheca.
3. Material and Methods
3.1. Animals and Farms
A convenience sampling of clinical mastitis from ten large dairy farms in Brazil was carried out, of which six were located in the south of the State of Minas Gerais (farms A, B, C, D, E, and F) and four in the southwest region of the State of Sao Paulo (farms G, H, I and J).
Farms and cows enrolled in the study were eligible if they met the following inclusion criteria: (1) mastitis control programs with data recording available in management software, (2) bulk tank milk somatic cell count <400,000 cells/mL, (3) Holstein or crossbreed Holstein cows, (4) >20 L milk/cow/day, (5) a minimum of 200 lactating cows, (6) mechanical milking system, and (7) history of clinical mastitis.
3.2. Diagnosis of Clinical Mastitis and Severity Scores
Clinical mastitis cases were classified according to severity scoring as mild (score 1), moderate (score 2), and severe (score 3). Mild cases presented macroscopic abnormalities in milk appearance (e.g., flakes, pus, blood). Moderate cases were recognized as abnormal aspects of milk and udder signs of inflammation (i.e., redness, swelling, pain, abscesses, nodules), while additional signs of fever, inappetence, tachycardia/tachypnea, or decubitus were identified as severe cases [17]. Before the onset of the study, milkers were trained to distinguish the three severity scores of clinical cases [19].
Clinical milk abnormalities were observed using the strip cup test or after depositing the first jets of milk on a black rubber floor.
3.3. Milk Sampling
A total of 4275 milk samples from different cows with clinical mastitis from ten farms studied between 2017 and 2022 were included in this study. After milkers carried out routine pre-milking procedures (i.e., stripping, pre-dipping, and drying of teats using paper towels), antisepsis of the teat end region was performed using 70% alcohol solution. Then, approximately 15 mL of milk was aseptically collected in individual sterile plastic vials and immediately refrigerated (4–8 °C). On each farm, milk samples were kept frozen (−20 °C) for further transport to the laboratory for microbiological culture.
Approximately 10 µL of each milk sample was plated onto defibrinated sheep blood agar (5%) and MacConkey agar (Oxoid™, Basingstoke, UK). The plates were incubated at 37 °C under aerobic conditions for 72 h [52]. Colonies suggestive of Prototheca (i.e., irregular to mucoid, white-to-gray, nonhemolytic yeast-like aspect, and 1–2 mm in diameter) [1,5] were subjected to Gram and Diff-quick staining for micromorphology examination. Compatible isolates (i.e., presence of spherical to oblong or wedge-shaped radially arranged sporangiospores) [5] were stored in brain and heart infusion broth (BHI—Oxoid™, Basingstoke, UK) with glycerol 85% (Merck™, Darmstadt, Germany) at −80 °C, until required for further molecular diagnostic purposes.
Intramammary infection was defined as at least 3 CFU of Prototheca. Milk samples that yielded more than 3 different colony types were considered contaminated and were discarded [52].
3.4. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS)
All compatible Prototheca isolates were identified by MALDI-TOF MS [53], with some modifications in the extraction of proteins [7]. Briefly, 20–40 μL of acetonitrile (100% P.A.) was added to each sample in the same proportion of formic acid 70% (1:1), and centrifuged. Then, 1 μL of the solution for each sample was added to the MSP steel target plate (MSP 96 polished-steel target; Bruker Daltonik™, Bremen, Germany) and allowed to dry for 20 min at room temperature. Next, the samples were recovered with 1 μL of matrix solution (2-cyano-4-hydroxycinnamic acid diluted with 50% acetonitrile and 2.5% trifluoroacetic acid). Finally, the steel target plates were placed in the equipment (Microflex LT/SH MALDI-TOF MS; Bruker and Daltonik™, Bremen, Germany).
The spectra were obtained between the 2000 and 20,000 m/z mass range using FlexControl 3.3 software. The following parameters were configured on the device: ion source 1 set at 20.0 kV; ion source 2 set at 18.2 kV; and lens set at 6.0 kV. For spectral generation, 240 laser shots of each target sample (isolate) were captured. The identification of Prototheca isolates was performed by MALDI-TOF MS Biotyper 4.1.70 software, supplemented with a local library, which had the insertion of P. bovis and P. blaschkeae species.
Scores between ≥1.7 and <2.0 and ≥2.0 were considered reliable for genus and species identification, respectively [7].
3.5. Polymerase Chain Reaction and Sequencing of the cytb Gene
For Prototheca species identification, partial cytb gene sequencing was performed [30]. Briefly, a loopful of Prototheca cells from a single colony grown on SDA agar was used for the DNA extraction procedure. Mechanical cell disruption of DNA was carried out using a purification kit (GeneMATRIX™ Environmental DNA and Amp; RNA purification™, EURx, Gdańsk, Poland) and by vigorous shaking with glass beads in a detergent-rich solution, combined with lysozyme and proteinase K action. All steps, including additional treatment with lyticase (100 g/mL) (Sigma™, Saint Louis, MO, USA) and β-mercaptoethanol (1 L/mL) (Sigma™, Saint Louis, MO, USA) were carried out according to the manufacturer’s instructions. Finally, the purified DNA, dissolved in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0), was quantified using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific™, Waltham, MA, USA) and stored at 20 °C until required for further procedures.
For PCR amplification and sequencing, the cytb partial gene was amplified in 30-μL reaction mixtures containing 18 μL of Color Taq PCR MasterMix (EURx™, Gdańsk, Poland), 1 μL (ca. 10 ng) of template DNA, and 1 μL of each primer cytb-F1 and cytb-R1 (0.2 μM each). PCR was performed based on the following cycle conditions: 3 min of initial denaturation at 95 °C, followed by 35 cycles of 30 s at 95 °C, 30 s at 50 °C, and 30 s at 72 °C, with a final extension period of 5 min at 72 °C. The amplified products were visualized using agarose gel electrophoresis (1%, wt/vol) and stained with ethidium bromide. The amplicons were purified using the Short DNA Clean-up DNA purification kit (EURx™, Gdańsk, Poland) and directly sequenced with the same primers used for PCR amplification.
The resulting sequences were assembled in the Clone Professional Manager Suite 8 program. The results were then compared with Blast (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome accessed on: 21 October 2022) Prototheca ID databases (https://prototheca-id.org accessed on: 21 October 2022) [54].
3.6. In Vitro Algaecide Activity of QA
In vitro algaecide activity of QA against Prototheca isolates was performed as described previously using other sanitizers [6,26].
Two to three Prototheca colonies of each isolate were inoculated in tubes containing 3 mL of BHI (Oxoid™, Basingstoke, UK) and incubated in aerobic conditions at 37 °C for 48 h. The turbidity of the tubes was adjusted by optical density equivalent to the 0.5 standards of the McFarland scale using a sterile 0.9% saline solution [6,26]. For the evaluation of the algaecide activity, a commercial product for swimming pools was used (Oxi Algacida®, Sandet Química Ltd.a., Sao José do Rio Preto, SP, Brazil) containing exclusively QA as the active compound (alkyl amido propyl dimethyl benzyl ammonium chloride) in liquid form at a 15% concentration.
For each Prototheca isolate, a series of 15 dilutions in separate sterile tubes was prepared, with a starting QA solution (1 mL) at a concentration of 15% or 150,000 parts per million (ppm) deposited. In the 14 subsequent tubes, 0.5 mL of sterile Milli-Q water as a diluent was distributed. Then, 0.5 mL of QA was transferred from the first to the second tube, with subsequent homogenization. This same process was carried out successively in the other 14 tubes, discarding 0.5 mL after the homogenization of tube 15. Then, 0.5 mL of the inoculum containing Prototheca cells was added to each tube, at the following concentrations: 75,000, 37,000, 18,000, 9000, 4500, 2250, 1120, 560, 280, 140, 70, 35, 17, 8, and 4 ppm. The tubes were then incubated overnight under aerobic conditions, at 37 °C. After that time, an aliquot of 10 µL of each tube was plated on Sabouraud agar medium and kept under aerobic conditions at 37 °C for 7 days, with readings taken every 24 h, aiming to evaluate the minimum algicidal concentration of the QA. The testing was carried out in duplicate for all isolates.
As a control, suspensions were prepared in sterilized tubes containing 0.5 mL of sterilized Milli-Q water with 0.5 mL of the inoculum, adjusted to the same turbidity (0.5 McFarland standard). The following Prototheca-type strains were used as references: P. bovis (SAG 2021), P. ciferrii (SAG 2063), and P. blaschkeae (SAG 2064).
Kaplan–Meier survival analysis was conducted to determine the antimicrobial effect of QA against Prototheca isolates. For this, the antimicrobial concentration was used as a time variable, and inhibition of bacterial growth was used as an event.
4. Conclusions
P. bovis was the species identified by MALDI-TOF MS and PCR in the sampled animals, reinforcing the predominance of this species of algae as the primary agent of clinical mastitis in cows on large dairy farms in Brazil. The absence of severe cases of mastitis was observed, reinforcing those infections by protothecae, particularly P. bovis, are mainly restricted to the mammary gland, without dissemination or systemic manifestations by the animals. Quaternary ammonium showed an algicidal effect at low concentrations on Prototheca isolates and can be used as an alternative sanitizer for the milking environment or in pre- and post-dipping solutions in the control/prophylaxis of mammary protothecosis.
Author Contributions
M.F.A.F. (Data curation, Formal analysis, Investigation, In vitro algaecide analysis, Writing—original draft, and Writing—review and editing), T.J. (PCR sequencing and Writing—review and editing), A.P. (PCR sequencing), M.V.d.S. and C.E.F. (Molecular diagnosis of mass spectrometry and Writing—review and editing), F.F.G., S.T.G. and S.F.J. (Sampling, milk culture and identification of the agents, Project administration, and Writing—review and editing), M.d.S.R.M. (Writing—review and editing), J.C.d.F.P. (Sampling, Investigation, Formal analysis, and Writing—review and editing), H.L. (Funding acquisition, Resources, Sampling, Project administration, and Writing—review and editing), L.F.G.S. (Writing—original draft and Investigation), and M.G.R. (Conceptualization, Data curation, Methodology, Supervision, Validation, Writing—original draft, and Writing—review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 88887835659/2023-00. Additionally, this research was supported by the Sao Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de Sao Paulo—FAPESP), Brazil, grant 2015/19688-8 and 2023/10548-5.
Institutional Review Board Statement
This study was conducted following the Ethics Committee on Animal Use (CEUA) guidelines, and approved by the School of Veterinary Medicine and Animal Science (FMVZ), Sao Paulo State University (UNESP), Botucatu, SP, Brazil (protocol number 0136/2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq), Brazil, for the productive research fellowship (PQ) given to Márcio Garcia Ribeiro (grant 310345/2020), and Helio Langoni (grant 303890/2016-9).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Jagielski, T.; Bakuła, Z.; Gawor, J.; Maciszewski, K.; Kusber, W.-H.; Dyląg, M.; Nowakowska, J.; Gromadka, R.; Karnkowska, A. The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: Implications from molecular taxonomic studies. Algal Res. 2019, 43, 101639. [Google Scholar] [CrossRef]
- Shave, C.D.; Millyard, L.; May, R.C. Now for something completely different: Prototheca, pathogenic algae. PLoS Pathog. 2021, 17, e1009362. [Google Scholar] [CrossRef]
- Jagielski, T.; Iskra, M.; Bakuła, Z.; Rudna, J.; Roeske, K.; Nowakowska, J.; Bielecki, J.; Krukowski, H. Occurrence of Prototheca microalgae in aquatic ecosystems with a description of three new species, Prototheca fontanea, Prototheca lentecrescens, and Prototheca vistulensis. Appl. Environ. Microbiol. 2022, 88, 0109222. [Google Scholar] [CrossRef] [PubMed]
- Kunthiphun, S.; Endoh, R.; Takashima, M.; Ohkuma, M.; Tanasupawat, S.; Savarajara, A. Prototheca paracutis sp. nov., a novel oleaginous achlorophyllous microalga isolated from a mangrove forest. Mycoscience 2019, 60, 165–169. [Google Scholar] [CrossRef]
- Ribeiro, M.G. Protothecosis. In The Merck Veterinary Manual, 12th ed.; Aiello, S.E., Ed.; Merck & Co.: Duluth, MN, USA, 2021. [Google Scholar]
- Alves, A.; Capra, E.; Morandi, S.; Cremonesi, P.; Pantoja, J.; Langoni, H.; de Vargas, A.; da Costa, M.; Jagielski, T.; Bolaños, C.; et al. In vitro algicidal effect of guanidine on Prototheca zopfii genotype 2 strains isolated from clinical and subclinical bovine mastitis. Lett. Appl. Microbiol. 2017, 64, 419–423. [Google Scholar] [CrossRef]
- Fidelis, C.E.; Franke, M.; de Abreu, L.C.R.; Jagielski, T.; Ribeiro, M.G.; dos Santos, M.V.; Gonçalves, J.L. MALDI-TOF MS identification of Prototheca algae associated with bovine mastitis. J. Vet. Diagn. Investig. 2021, 33, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Huilca-Ibarra, M.P.; Vasco-Julio, D.; Ledesma, Y.; Guerrero-Freire, S.; Zurita, J.; Castillejo, P.; Blasco, F.B.; Yanez, L.; Changoluisa, D.; Echeverría, G.; et al. High prevalence of Prototheca bovis infection in dairy cattle with chronic mastitis in Ecuador. Vet. Sci. 2022, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakuła, Z.; Wlazło, Ł.; Woch, P. Prevalence of Prototheca spp. on dairy farms in Poland—A cross-country study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Jin, E.; Wang, G.; Wu, L.; Shao, Z.; Wan, P.; Hu, C.; Li, J.; Chen, J.; et al. A survey of Prototheca bovis infection in dairy farms of the Hubei province, China. J. Vet. Med. Sci. 2021, 83, 1248–1255. [Google Scholar] [CrossRef]
- Morandi, S.; Cremonesi, P.; Povolo, M.; Capra, E.; Silvetti, T.; Castiglioni, B.; Ribeiro, M.G.; Alves, A.C.; da Costa, G.M.; Luini, M.; et al. Prototheca blaschkeae subsp. brasiliensis subsp. nov., isolated from cow milk. Int. J. Syst. Evol. Microbiol. 2017, 67, 3865–3871. [Google Scholar] [CrossRef]
- Park, H.-S.; Moon, D.C.; Hyun, B.-H.; Lim, S.-K. Short communication: Occurrence and persistence of Prototheca zopfii in dairy herds of Korea. J. Dairy Sci. 2019, 102, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Pieper, L.; Godkin, A.; Roesler, U.; Polleichtner, A.; Slavic, D.; Leslie, K.; Kelton, D. Herd characteristics and cow-level factors associated with Prototheca mastitis on dairy farms in Ontario, Canada. J. Dairy Sci. 2012, 95, 5635–5644. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, V.; Denardi, L.; Weiblen, C.; de Jesus, F.; Dorneles, M.; Ianiski, L.; Santurio, J. Short communication: Algicide activity of antimicrobial peptides compounds against Prototheca bovis. J. Dairy Sci. 2021, 104, 3554–3558. [Google Scholar] [CrossRef]
- Jánosil, S.; Szigeti, G.; Rátz, F.; Laukó, T.; Kerényi, J.; Tenk, M.; Katona, F.; Huszenicza, A.; Kulcsár, M.; Huszenicza, G. Epidemiology: Prototheca zopfii mastitis in dairy herds under continental climatic conditions. Vet. Q. 2001, 23, 80–83. [Google Scholar] [CrossRef]
- Pinzón-Sánchez, C.; Ruegg, P. Risk factors associated with short-term post-treatment outcomes of clinical mastitis. J. Dairy Sci. 2011, 94, 3397–3410. [Google Scholar] [CrossRef]
- Fredebeul-Krein, F.; Schmenger, A.; Wente, N.; Zhang, Y.; Krömker, V. Factors Associated with the Severity of Clinical Mastitis. Pathogens 2022, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.T.; Orsi, H.; Joaquim, S.F.; Guimarães, F.F.; Lopes, B.C.; Dalanezi, F.M.; Leite, D.S.; Langoni, H.; Pantoja, J.C.; Rall, V.L.; et al. Short communication: Investigation of extra-intestinal pathogenic Escherichia coli virulence genes, bacterial motility, and multidrug resistance pattern of strains isolated from dairy cows with different severity scores of clinical mastitis. J. Dairy Sci. 2020, 103, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Buzzini, P.; Lassa, H.; Malinowski, E.; Branda, E.; Turchetti, B.; Polleichtner, A.; Roesler, U.; Lagneau, P.-E.; Marques, S.; et al. Multicentre Etest evaluation of in vitro activity of conventional antifungal drugs against European bovine mastitis Prototheca spp. isolates. J. Antimicrob. Chemother. 2012, 67, 1945–1947. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Cremonesi, P.; Capra, E.; Silvetti, T.; Decimo, M.; Bianchini, V.; Alves, A.; Vargas, A.; Costa, G.; Ribeiro, M.; et al. Molecular typing and differences in biofilm formation and antibiotic susceptibilities among Prototheca strains isolated in Italy and Brazil. J. Dairy Sci. 2016, 99, 6436–6445. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Branda, E.; Goretti, M.; Amici, M.; Lagneau, P.E.; Scaccabarozzi, L.; Bronzo, V.; Moroni, P. Large-scale screening of thein vitrosusceptibility of Prototheca zopfii towards polyene antibiotics. Med. Mycol. 2008, 46, 511–514. [Google Scholar] [CrossRef]
- Ely, V.L.; Da Costa, M.M.; De Oliveira, H.P.; Júnior, F.A.G.D.S.; Pereira, D.I.B.; Soares, M.P.; De Vargas, A.C.; Sangioni, L.A.; Cargnelutti, J.F.; Ribeiro, M.G.; et al. In vitro algicidal effect of polypyrrole on Prototheca species isolates from bovine mastitis. Med. Mycol. 2020, 58, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Lee, S.H.I.; Arruda, E.d.P.; Galles, D.P.; Caetano, V.C.; de Oliveira, C.A.F.; Fernandes, A.M.; dos Santos, M.V. Biofilm-producing ability and efficiency of sanitizing agents against Prototheca zopfii isolates from bovine subclinical mastitis. J. Dairy Sci. 2015, 98, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Melville, P.; Benites, N.; Sinhorini, I.; Costa, E. Susceptibility and features of the ultrastructure of Prototheca zopfii following exposure to copper sulphate, silver nitrate and chlorexidine. Mycopathologia 2002, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salerno, T.; Ribeiro, M.G.; Langoni, H.; Siqueira, A.K.; da Costa, E.O.; Melville, P.A.; Bueno, V.F.F.; Yamamura, A.A.M.; Roesler, U.; da Silva, A.V. In vitro algaecide effect of sodium hypochlorite and iodine-based antiseptics on Prototheca zopfii strains isolated from bovine milk. Res. Vet. Sci. 2010, 88, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Langoni, H.; Troncarelli, M.Z.; Wanderley, G.G.; Salina, A. Prototecose mamária: Um problema nos rebanhos leiteiros. Veterinária Zoo-Tec. 2013, 20, 552–566. Available online: https://rvz.emnuvens.com.br/rvz/issue/view/41/39 (accessed on 15 July 2023).
- Nardoni, S.; Pisseri, F.; Pistelli, L.; Najar, B.; Luini, M.; Mancianti, F. In vitro activity of 30 essential oils against bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae. Vet. Sci. 2018, 5, 45. [Google Scholar] [CrossRef]
- Tomasinsig, L.; Skerlavaj, B.; Scarsini, M.; Guida, F.; Piccinini, R.; Tossi, A.; Zanetti, M. Comparative activity and mechanism of action of three types of bovine antimicrobial peptides against pathogenic Prototheca spp. J. Pept. Sci. 2012, 18, 105–113. [Google Scholar] [CrossRef]
- Jagielski, T.; Bakuła, Z.; Pleń, M.; Kamiński, M.; Nowakowska, J.; Bielecki, J.; Wolska, K.I.; Grudniak, A.M. The activity of silver nanoparticles against microalgae of the Prototheca genus. Nanomedicine 2018, 13, 1025–1036. [Google Scholar] [CrossRef]
- dos Anjos, C.; Sellera, F.P.; Gargano, R.G.; Lincopan, N.; Pogliani, F.C.; Ribeiro, M.G.; Jagielski, T.; Sabino, C.P. Algicidal effect of blue light on pathogenic Prototheca species. Photodiagn. Photodyn. Ther. 2019, 26, 210–213. [Google Scholar] [CrossRef]
- Morello, L.; Tiroli, T.; Aretino, F.; Morandi, S.; Breviario, D. Preliminary results, perspectives, and proposal for a screening Method of in vitro susceptibility of Prototheca Species to antimicrotubular Agents. Antimicrob. Agents Chemother. 2020, 64, e01392-19. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, L.M.; Bessa, M.C.; Cardoso, M.d.I.; Avancini, C.A.M. Sensibilidade e resistência de amostras de Salmonella Typhimurium isoladas de suínos abatidos no Rio Grande do Sul/Brasil frente aos desinfetantes químicos quaternário de amônio e iodofor. Ciênc. Rural 2006, 36, 1474–1479. [Google Scholar] [CrossRef]
- Carvalho, D.; de Moraes, L.B.; Rocha, S.L.d.S.; Moraes, H.L.d.S.; Salle, C.T.P.; Avancini, C.A.M. Atividade antimicrobiana dos desinfetantes Cloreto de Benzalcônio e Iodóforos frente à cepas de Escherichia coli de alta patogenicidade (APEC) de origem aviária. Rev. Bras. Prod. Saúde Anim. 2017, 18, 10–15. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Dimuccio, M.M.; Casalino, G.; Ceci, E.; Corrente, M. New approaches for risk assessment and management of bovine protothecosis. Saudi J. Biol. Sci. 2022, 29, e103368. [Google Scholar] [CrossRef]
- Libisch, B.; Picot, C.; Ceballos-Garzon, A.; Moravkova, M.; Klimesová, M.; Telkes, G.; Chuang, S.-T.; Le Pape, P. Prototheca infections and ecology from a one health perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef]
- Scaccabarozzi, L.; Turchetti, B.; Buzzini, P.; Pisoni, G.; Bertocchi, L.; Arrigoni, N.; Boettcher, P.; Bronzo, V.; Moroni, P. Short communication: Isolation of Prototheca species strains from environmental sources in dairy herds. J. Dairy Sci. 2008, 91, 3474–3477. [Google Scholar] [CrossRef]
- Tashakkori, N.; Rahmani, H.K.; Khoramian, B. Genotypic and phenotypic diversity of Prototheca spp. recovered from bovine mastitis in terms of antimicrobial resistance and biofilm formation ability. BMC Vet. Res. 2022, 18, 452. [Google Scholar] [CrossRef]
- Ricchi, M.; De Cicco, C.; Buzzini, P.; Cammi, G.; Arrigoni, N.; Cammi, M.; Garbarino, C. First outbreak of bovine mastitis caused by Prototheca blaschkeae. Vet. Microbiol. 2013, 162, 997–999. [Google Scholar] [CrossRef]
- Langoni, H.; Domingues, P.F.; Dias, H.L.T. Prototheca zopffi as agent of bovine mastitis: Clinics and therapeutics. Arq. Bras. Med. Veterinária Zootec. 1995, 47, 727–732. [Google Scholar]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 7, 928346. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.; Silva, E.; Marques, S.; Müller, A.; Carvalheira, J. Algaemia in a dairy cow by Prototheca blaschkeae. Med. Mycol. 2009, 47, 527–531. [Google Scholar] [CrossRef]
- Roesler, U.; Möller, A.; Hensel, A.; Baumann, D.; Truyen, U. Diversity within the current algal species Prototheca zopfii: A proposal for two Prototheca zopfii genotypes and description of a novel species, Prototheca blaschkeae sp. nov. Int. J. Syst. Evol. Microbiol. 2006, 53, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.S.; Richard, J.L.; Cheville, N.F. Natural and experimental bovine intramammary infection with Prototheca zopfii. Am. J. Vet. Res. 1984, 45, 592–595. [Google Scholar] [PubMed]
- Marques, S.; Silva, E.; Carvalheira, J.; Thompson, G. Short Communication: In Vitro Antimicrobial Susceptibility of Prototheca wickerhamii and Prototheca zopfii. J. Dairy Sci. 2006, 89, 4202–4204. [Google Scholar] [CrossRef]
- Shahid, M.; Gao, J.; Zhou, Y.; Liu, G.; Ali, T.; Deng, Y.; Sabir, N.; Su, J.; Han, B. Prototheca zopfii isolated from bovine mastitis induced oxidative stress and apoptosis in bovine mammary epithelial cells. Oncotarget 2017, 8, 31938–31947. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, M.; Barkema, H.W.; Xie, X.; Lin, Y.; Khan, S.; Kastelic, J.P.; Wang, D.; Deng, Z.; Han, B. Prototheca bovis induces autophagy in bovine mammary epithelial cells via the HIF-1α and AMPKα/ULK1 pathway. Front. Immunol. 2022, 13, e934819. [Google Scholar] [CrossRef]
- Lassa, H.; Jagielski, T.; Malinowski, E. Effect of different heat treatments and disinfectants on the survival of Prototheca zopfii. Mycopathologia 2010, 171, 177–182. [Google Scholar] [CrossRef]
- Kano, R. Emergence of Fungal-Like Organisms: Prototheca. Mycopathologia 2019, 185, 747–754. [Google Scholar] [CrossRef]
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis; NMC Inc.: Madison, WI, USA, 1999. [Google Scholar]
- Gonçalves, J.L.; Tomazi, T.; Barreiro, J.R.; Braga, P.A.d.C.; Ferreira, C.R.; Junior, J.P.A.; Eberlin, M.N.; dos Santos, M.V. Identification of Corynebacterium spp. isolated from bovine intramammary infections by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Vet. Microbiol. 2014, 173, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dziurzyński, M.; Decewicz, P.; Iskra, M.; Bakuła, Z.; Jagielski, T. Prototheca-ID: A web-based application for molecular identification of Prototheca species. Database 2021, 2021, e.baab073. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).