Simple Summary
Rabbit meat is considered as a functional food. But, there are few studies directed to determine the influence of natural additives from farm to the table. This study was performed using a fruit obtained from Pithecellobium dulce to follow its effect in rabbits from farm to a fresh meat product. The use of this fruit at 5% of the diet increased the dry and organic matter digestibility of the diet and improved feed conversion rate. Also, it increased acceptance of meatballs prepared with rabbit meat obtained from that animal’s feed.
Abstract
Pithecellobium dulce produces a fruit used in alternative medicine that could be utilized to feed rabbits. The objective of this study was to measure the effect of the P. dulce fruit on productive performance, carcass traits, meat characteristics, and meat product quality as well as shelf-life. Seventy-two California × English pot crossbreed rabbits (35 d age) were randomly distributed into two treatments: a control group without P. dulce and another group fed with 5% of P. dulce, and fattening for 28 d. Productive performance parameters, blood biochemistry and hematology, apparent digestibility, carcass traits, meat characteristics, and meat product shelf-life were measured. The results indicate inclusion of 5% P. dulce improves (p < 0.05) dry and organic matter digestibility and feed conversion rate, but some serum blood enzymes were increased (p < 0.05). The a* value, hardness, and pH decreased (p < 0.05) in the group fed with P. dulce. Antioxidant properties in the meatballs were different (p < 0.05), improving shelf-life and acceptance in sensory analysis. In conclusion, the use of 0.5% of P. dulce fruits to feed fattening rabbits can be used to improve the shelf-life of rabbit meat.
1. Introduction
Rabbit production is improving in productivity, which has led to the design of more efficient diets, since rabbits’ feeds are formulated mainly based on by-products high in fiber, animal and vegetable fats, and other ingredients that contain nutrients sufficient for maintaining an efficient productivity [1]. However, rabbit is a species that produces excellent meat, including nutritional characteristics and potential health properties, with this meat and its derivatives considered as functional foods due to their functional compounds [2]. One of the benefits of this species is that it can be fed with different fibrous material, parts of herbs and spices as alternatives to additives or ingredients [3]. In addition, feed costs associated with producing rabbit meat are high which is why alternatives are being sought in order to decrease them. One ingredient which could be an alternative is the fruit from the tree called Pithecellobium dulce.
P. dulce is a fruit which originates from the Americas in countries including Brazil, Argentina, Colombia, and Mexico, but it is also distributed in several countries around the world, such as India or the tropical regions of Africa. This tree belongs to the Fabaceae family and is one of the 18 species of the genus Pithecellobium [4]. Murugesan et al. [5] reviewed therapeutic and biological properties of P. dulce, indicating that it has insecticide, anti-diabetic, anti-hyperlipidemic, antioxidant, antiulcer, antidiarrheal, antibacterial, and other properties. Dhanisha et al. [6] demonstrated that an extract of P. dulce fruit induced apoptosis in vivo and in vitro. Furthermore, Vargas-Madriz et al. [7] reviewed the antioxidant capacity and phenol profile of P. dulce indicating the main phenolics reported are caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, protocatechuic acid, apigenin, catechin, daidzein, kaempferol, luteolin, quercetin, myricetin, naringin, and rutin. However, it was also mentioned that antioxidant capacity varies according to all the studies reviewed. P. dulce are used in combination with other plants to feed goats, using leaves [8,9] or fruits [10] The above-mentioned findings indicate that the fruit of P. dulce is an ingredient that could be used to elaborate animal feed. The objective of this study was to evaluate the effect of P. dulce fruit on productive performance, carcass traits, meat characteristics, biochemical and hematology analysis; as well as meatballs prepared with rabbit meat; as a potential alternative ingredient to feed fattening rabbits.
2. Materials and Methods
2.1. Raw Material and Proximate Analysis
The fruit of P. dulce was collected in San Miguel de las Palmas, Guerrero, Mexico. The fruits were dried at 28 °C under shadow, and were then grounded in an Antarix grinder model THCF2800M13 (Antarix de México, Mexico City, Mexico). Afterwards, a proximate analysis was performed according to AOAC methodology [11] to determine moisture (930.15), crude protein (945.01), and crude fat (954.02). Regarding fiber fractions (NDF and ADF), the technique described by Van Soest et al. [12] was used.
2.2. Animals and Experimental Design
This study and animal management were carried out according to the institutional committee guidelines on animal care (protocol number CICUA/ICAP 001/2020). The experiments were conducted in a rabbitry located in Tulancingo, Hidalgo, Mexico. Ambiental conditions in the rabbit production house had an average temperature of 17 °C and 70% relative humidity. Seventy-two rabbits were used, which were 35 d of age, unsexed, California × English pot crossbreed, and weighed 650 g on average. The animals were selected and distributed randomly in two treatments, a control group (C, n = 36) and a group (G5, n = 36) fed with 5% of fruit of P. dulce, with nine repetitions (n = 4 rabbits). The fattening period was 29 d. In addition, the animals were housed in cages measuring 45 × 40 × 60 cm which were adapted with automatic drinkers and manual feeders. The rabbits were fed ad libitum.
2.3. Diets
Diets were prepared following the nutritional requirements of the National Research Council [13], while the ingredient composition was based on the guidelines provided by Fundación Española para el Desarrollo de la Nutricion Animal [14]. Diet formulations had to be isoproteic (16%), isoenergetic (2.5 Mcal·kg−1 of digestible energy), and isofibrous (16% Neutro Detergent Fiber) as shown in Table 1. The ingredients were mixed in an ASF model MZ50 double helicoidal mixer (Molinos y Mezcladoras Industriales S. A. de C. V. Mexico), and then pelletized in a SKJ-120 feed pellet machine (Yuezhen Machinery Co., Jinan, China) and finally stored in a hermetic container until use.

Table 1.
Diet formulation and chemical composition.
2.4. Productive Performance
Feed consumption was registered daily (offered and rejected) while live weight was measured every week using a Mettria MTNUV-40 digital scale (Mettria México, Ciudad de México, Mexico). From the data obtained, the average daily feed intake (DFI), daily weight gain (DWG), and feed conversion rate (FCR) were calculated between ages 35 and 63 days. In addition, total weight gain and total feed intake were also determined, including initial and final weight of the experiment.
2.5. Apparent Digestibility
Dry matter, organic matter, neutral detergent fiber, and acid detergent fiber were determined according to Perez et al. [15]. Briefly, 8 rabbits by group were selected to perform apparent digestibility, then feces were collected from each cage every morning during the last 4 days of the fattening period. Afterwards, feces were dried in a Riossa model HCF82D oven (RSU Labsupply, Monterrey, NL, Mexico). Content for moisture, ash, neutral detergent fiber (NDF), and acid detergent fiber (ADF) was determined in both feces and feed as indicated above. Subsequently, the digestibility coefficient was calculated.
2.6. Carcass Traits
After the fattening period, animals (63 days of age, n = 32 rabbits by group) were weighed and transported to the meat laboratory belonging to the Instituto de Ciencias Agropecuarias and then slaughtered without previous fasting and mechanical concussion stunning according to national legislation [16]. Before slaughtering, the dorsal length and the lumbar circumference of the animals was measured (from the atlas to the last ischia vertebra) using a measuring tape. After evisceration, the weights of the skin, feet, hot carcass, viscera (including esophagus, trachea, digestive apparatus, heart, lungs, kidneys, and liver), carcass length, and lumbar circumference were obtained. Carcasses were stored in refrigeration at a temperature of 4 °C for 24 h. Afterwards, cold carcass and main cuts (head, forequarter, thoracic cage, foreleg, and legs) were obtained according to the indications provided by Blasco et al. [17]. Then, legs were dissected into meat, fat, and bone using a Scout Pro model SP402 scale (Ohaus Corporation, Pine Brook, NJ, USA).
2.7. Hematological and Biochemical Analysis
During the exsanguination procedure, 3 mL of blood were collected in vacutainer tubes (n = 9 by treatment) to determine blood biochemical analysis using BA400 Biosystem equipment. Another tube was used to collect blood and perform blood biometry using a hematology analyzer Procyte Dx (Idexx laboratories Inc., Westbrook, ME, USA).
2.8. Meat Characteristics
The carcasses were kept for 24 h under refrigerated conditions, then color was measured using a Minolta colorimeter model CM-580d (Konica-Minolta, Tokyo, Japan) with a CIEL*a*b* color space using an illuminant D65, and 0.8 cm aperture size. The observer was set to 10° according to the American Meat Science Association meat color measurement guidelines [18]. For measuring pH, a Hanna HI99163 meat pHmeter (Hanna Instruments, Cluj-Napoca, Romania) was used. Furthermore, in order to determine water holding capacity (WHC), a technique described by Honikel [19] was employed. Cooking loss was measured by cutting half of a loin which was then weighed and cooked in a hot water bath at 80 °C for 20 min. Subsequently, 1 cm3 meat cubes were analyzed for a texture profile analysis in a Brookfield CT3 texture analyzer (Brookfield, Middleboro, MA, USA). The equipment was adapted with a TA3/1000 probe and set up to compress the sample at 50% using a crosshead speed of 1 mm·s−1. The sample was compressed twice. Force–time graph parameters of hardness, resilience, cohesiveness, springiness, and chewiness were obtained using Texture Pro CT software (Brookfield, Middleboro, MA, USA).
2.9. Analysis of Meatball
Meat obtained from legs was ground in a Torrey grinder (Torrey, Monterrey, NL, Mexico); the meat was separated into two batches. The meatballs were prepared by adding 10 g of salt and 200 mL of water to 1 kg of rabbit meat, which were then mixed. Afterwards, 50 g portions were made and stored on plastic trays, covered with film, and then stored at 4 °C until analysis.
To determine the effect of P. dulce, microbiological and physicochemical analysis was performed on days 0, 7, and 14, with dilutions and bacterial counts analyzed using indications according to national legislation [20]. Total viable counts of bacteria Enterobacteriaceae and Staphylococcus were tested. Antioxidant activity was determined according to Brand-Williams et al. [21] with 2,2-Diphenyil-1-picrylhydrazyl (DPPH) as radical, while antioxidant activity was expressed in mg·mL−1. The pH was measured using a Hanna meat pHmeter model HI99163 (Hanna Instruments, Cluj-Napoca, Romania). Finally, water activity (Aw) was determined with a HP23-aw HygroPalm (Rotronic Measurement Solutions, Bassersdorf, Switzerland).
Meatballs were subjected to a sensory analysis using an affective hedonic test to determine the acceptability of the meat’s taste. A total of 120 consumers with an average of 21.5 years participated, of which 41.7% were female and 57.5% were male. A hedonic 7-point scale affective test (7 like very much and 1 dislike very much) was undertaken to determine acceptability. The test was developed according to the indications provided by Drake [22].
2.10. Statistical Analysis
In this work, a completely randomized design was used to analyze productive performance parameters, including total weight gain, total feed consumption, and feed conversion ratio; for these variables, treatment was the fixed effect and cage was random term. On apparent digestibility of dry matter, organic matter, neutral detergent fiber, and acid detergent fiber, all carcass traits, all meat characteristics, all biochemical and hematology analysis, an analysis of variance following the general linear model procedure was carried out, continuing with a lsmeans option, using treatment as fixed effect and cage as random term. Statistical model was following:
where Yij = dependent variable, μ = mean of the variable, βi = the fixed effect of i-th rabbit of the group, δk = the random effect of k-th cage, and εij = experimental error associated with the observation Yij.
Yij = μ + βi + εij + δijk,
A repeated time one-way design was used to analyze feed consumption and daily weight gain through the fattening period; treatment and time was used as fixed effect, with the nested time in treatment, then cage was utilized as random term. Also, all variables measured to determine meatballs’ shelf-life were analyzed through a repeated time one-way design, where treatment and time was used as fixed effect, with the nested time in treatment, then batch was utilized as random term.
where Yij = dependent variable, μ = mean of the variable, βi = the fixed effect of i-th rabbit of the group, τj = time and βi(τj) time inside treatment, δl = the random effect of l-th cage or batch, and εijk = experimental error associated with the observation Yijk.
Yijk = μ + βi + τj+ βi(τj) + εijk + δijkl,
For sensory analysis, firstly a statistical descriptive analysis was carried out, subsequently a Student’s t-test was conducted to determine differences between samples of meatballs elaborated with meat from rabbits given feed with or without P. dulce, one session in approximately two hours was conducted, then data collected as indicates above.
The statistical models were the following:
where t is t-test, the numerator is the difference between the two consumers groups to taste meat, denominator is an estimate of the standard error. All analyses were performed using SAS software ver. 9.0.
3. Results
3.1. Proximate Analysis
A proximate analysis of P. dulce fruits revealed 89.5% of dry matter, 6.4% ash, 6.2% ethereal extract, 21.5% protein, 74% of NDF, and 32.1% acid detergent fiber. According to the results, P. dulce fruits are a rich source of protein and fiber.
3.2. Productive Performance
Growth performance parameters are shown in Table 2. Supplementing the rabbits’ diets with P. dulce fruits did not affect productive performance (p > 0.05), including body weight, feed intake, and feed conversion rate. These results indicate that P. dulce fruits could be used to feed growing rabbits.

Table 2.
Productive performance of rabbits that consumed Pithecellobium dulce fruits.
3.3. In Vivo Apparent Digestibility
The results of digestibility are shown in Table 3. Dry matter and organic apparent digestibility were higher (p < 0.05) when rabbits consumed 5% of P. dulce fruits. However, NDF and ADF digestibility were similar (p > 0.05).

Table 3.
In vivo total tract apparent digestibility of diets including Pithecellobium dulce fruits consumed by rabbits.
3.4. Biochemical and Blood Analysis
Results are shown in Table 4. Rabbits of both experimental groups showed similar (p > 0.05) compositions from biochemical and blood analyses, except for alanine transferase, aspartate transferase, and phosphorus which were higher (p < 0.05) in the G5 group.

Table 4.
Blood biochemistry and hematology of rabbits fed with Pithecellobium dulce fruits.
3.5. Carcass Traits
Carcass traits are shown in Table 5, nearly all of which were similar (p > 0.05) between treatments. Nevertheless, feet weight was lower (p < 0.05) in G5 group, while drip loss was higher (p < 0.05) in the control group.

Table 5.
Carcass traits of the rabbits fed with Pithecellobium dulce.
3.6. Meat Characteristics
All the carcass characteristics of the rabbits fed with 5% of P. dulce fruits were similar (p > 0.05) to the control group (Table 6), except for a* value (redness) and hardness of meat, as lower values (p < 0.05) for these parameters were presented in the group fed with P. dulce.

Table 6.
Meat characteristics of the rabbits fed with Pithecellobium dulce fruits.
3.7. Meatball Quality
Measurements of meatballs elaborated with rabbit meat from animals fed with P. dulce are shown in Table 7. An interaction treatment with storage time was observed in pH, Aw, and antioxidant activity. However, bacteria count groups were different (p < 0.05) over storage time. The pH value increased during storage time, but it was lower in treatment G5 on day 14. The water activity of meatballs in the control treatment decreased during storage time, but G5 treatments were similar during 14 d of storage. Antioxidant activity increased during storage time; however, G5 treatment was higher after 14 d of storage.

Table 7.
pH, aw, microbial analysis, and antioxidant activity of meatball quality elaborated from rabbits that consumed Pithecellobium dulce fruits during storage time.
3.8. Sensory Analysis of Meatballs
The results from the affective hedonic sensory test on meatballs with meat from rabbits fed with P. dulce are shown at Figure 1, with panelists considering that the samples were similar (p > 0.05). Therefore, P. dulce fruits can be incorporated into rabbit feed because they did not show any adverse effects on sensory analysis.
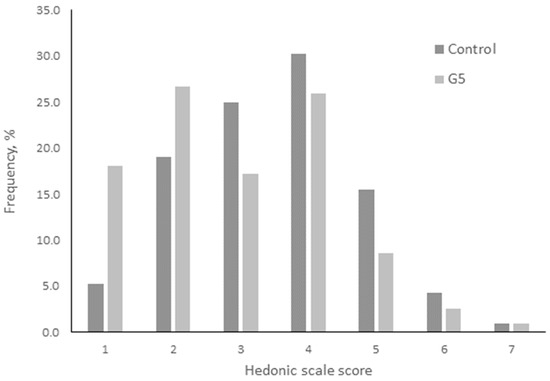
Figure 1.
Frequency of acceptability of meatballs elaborated with meat obtained from rabbits fed with Pithecellobium dulce fruits.
4. Discussion
There is little information about P. dulce fruits’ proximate composition. However, there are some studies that revealed chemical composition is diverse; when the fruit is divided into its main parts (seed, aril, and husk), the seed has 39% protein, while dry aril is between 12 and 15%, and fresh aril is 3%; which indicates that composition is influenced by the part of the fruit analyzed [23,24]. Yet, whole P. dulce fruits have a high quantity of protein (21.5%) and NDF (78%); according to these amounts, those fruits can be used to feed animals as an additive or ingredient.
Productive performance results indicate the use of P. dulce fruits to feed rabbits saw similar results between groups. However, there are several studies on the use of fruits, vegetables, extracts, or essential oils to feed fattening rabbits. Some of these investigations concluded that there are no effects on productive performance, such as the studies by Perna et al. [25] which used cauliflower leaves, Mancini et al. [26] who fed rabbits with quebracho and chestnuts tannins mixes, as well as Kovitvadhi et al. [27] who focused on Lythrum salicaria. Other studies indicated an increase in some productive performance parameters, such as Khalid et al. [28] who observed that Moringa oleifera leaf powder increased daily gain and improved the feed rate conversion. In another study, Elwan et al. [29] fed rabbits with 1 and 2% of Citrus limon powder and found that the parameters related to final weight, weight gain, and average daily gain all increased. Perhaps, P. dulce fruits did not improve productive performance, but they can be used to feed rabbits.
Apparent digestibility coefficients were similar to other ingredients in rabbit feeds, including bilberry pomace [30]. It is notable that cellular content has high digestion influenced by P. dulce fruits. It is possible that this fruit has small quantities of tannins (according to Roselin and Parameshwari [31]); their study also reviewed bioactive compounds in P. dulce which indicated the presence of tannins. Also, according to Mancini et al. [26], tannins did not influence the palatability of the feed. However, in contrast to this study, several studies indicate that dry or organic matter digestibility is not influenced by tannins. For example, Kovitvadhi et al. [27] stated that low levels of tannins did not affect digestibility, while similar results were obtained by Dabbou et al. [30]. Dalle-Zotte et al. [32] did not find any differences in dry matter and organic matter digestibility when feeding rabbits with chestnut hydrolysable tannins.
In general, the reference values for these biochemical parameters were normal with regard to rabbits; however, control groups had low levels for alanine transferase, aspartate transferase, and phosphorus compared to G5 group. It is possible that a hepatic malfunction was a consequence of intensive production or use of P. dulce. However, biochemical parameters were normal as indicated by Brandão et al. [33]. Aljohani and Abduljawad [34] reported similar concentrations of alanine and aspartate transferase in rabbits fed with Moringa oleifera, although values reported in their study were higher than those obtained in this experiment. In contrast, Imbabi et al. [35] found aspartate transferase decreased in rabbits fed with fennel oil, although alanine transferase was similar.
Most of the carcass traits were similar between groups, except feet and drip loss (the growth of animals is related to productive performance; if these last parameters are analogous between groups, it is possible that organs and body composition were similar.) There are other studies that report similar results, such as Wolf and Cappai [36] who used rabbit feed incorporated with acorns (Quercus pubescens Willd.) and there was no difference in carcass traits. However, the use of Moringa olefira leaves increased carcass traits in a study by Aljohani and Abduljawad [34]. Moreover, drip loss is related to protein and the accumulation of lactic acid [37], meaning that it is possible that P. dulce slightly increased protein content while the pH also remained slightly high.
Meat characteristics of rabbits fed with P. dulce fruits were similar between groups, except a* value and hardness. The maximum shear force is correlated with the connective tissue [38]. It is possible that P. dulce induced a lower proportion of collagen instead of structural proteins; Sembiring et al. [39] demonstrated that Muntingia calabura extract leaves increase collagen density. However, other studies using leaves or fruits did not find differences, such as Pałka et al. [40] who supplemented rabbit feed with Urtica dioica L. or Trigonella foenum-graecum L., or Garcia-Vazquez et al. [41] who used an infusion of Chenopodium ambroisoides. Also, the change in redness color value is associated to Fe and anthocyanins content in the aryl of P. dulce fruits, as indicated by Rao et al. [23].
P. dulce fruits feed to rabbits decreases Staphylococcus counts. Koné et al. [42] observed that plant extracts or essential oils supplemented into rabbits’ diets decreased bacterial content in rabbit meat under anaerobic conditions. Mancini et al. [43] used 3.5% of turmeric powder to extend the shelf-life of rabbit burgers.
The acceptance of rabbit meat burgers was similar between treatments. Other studies have observed similar results using bilberry pomace [44] and goji berries [45], while Mancini et al. [46] used Zingiber officinale roscoe powder in rabbit meat burgers to improve sensorial characteristics.
5. Conclusions
The inclusion of P. dulce at a proportion of 5% into the diet for growing rabbits did not affect productive performance, carcass traits, or meat characteristics. Regarding biochemical analysis and hematological analyses, there were no differences in almost all parameters evaluated; however, some enzyme levels (alanine and aspartate transferase) were higher in G5. As the product elaborated from rabbit meat with animals fed P. dulce after 14 d of refrigeration storage showed a low pH value, a slightly low bacteriological group, and a higher antioxidant capacity, then these parameters are indicatives of the increased shelf-life of the meat product, while sensory analysis showed no differences between the control and treatments. Therefore, this study suggests that the incorporation of P. dulce could increase the shelf-life of meat without any deleterious effect on acceptance of the meat by panelists.
Author Contributions
Conceptualization, J.A.-B., M.A.-M. and V.G.A.-R.; methodology, J.A.-B. and J.O.-L.; validation, J.O.-L., S.S.-S., V.G.A.-R. and M.A.-M.; formal analysis, S.S.-S. and J.A.-B.; investigation, J.A.-B. and S.S.-S.; resources, S.S.-S., V.G.A.-R. and M.A.-M.; data curation, S.S.-S.; writing—original draft preparation, J.A.-B. and M.A.-M.; writing—review and editing, S.S.-S. and J.O.-L.; supervision, J.O.-L., S.S.-S., V.G.A.-R. and M.A.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnologia from Mexican Government, grant number 565898 to Jairo Apaez Barrios.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Universidad Autonoma del Estado de Hidalgo (protocol code CICUA/ICAP/001/2020, date of approval: 21 September 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
All original data can be obtained or requested from Maricel Ayala Martinez.
Acknowledgments
The authors would like to say thanks to Christopher Schackley for review of English language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Blas, C.; Mateos, G.G. Feed Formulation. In Nutrition of the Rabbits, 3rd ed.; de Blas, C., Wiseman, J.W., Eds.; CABI: Oxfordshire, UK, 2020; pp. 243–253. [Google Scholar]
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Celia, C.; Szendrő, Z. Herbs and spices inclusion as feedstuff or additive in growing rabbit diets and as additive in rabbit meat: A review. Livest. Sci. 2016, 189, 82–90. [Google Scholar] [CrossRef]
- Rao, G.N. Physico-chemical, mineral, amino acid composition, in vitro antioxidant activity and sorption isotherm of Pithecellobium dulce L. seed protein flour. J. Food Pharm. Sci. 2013, 1, 74–80. [Google Scholar] [CrossRef]
- Murugesan, S.; Lakshmanan, D.K.; Arumugam, V.; Alexander, R.A. Nutritional and therapeutic benefits of medicinal plant Pithecellobium dulce (Fabaceae): A review. J. Appl. Pharm. Sci. 2019, 9, 130–139. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Pithecellobium dulce induces apoptosis and reduce tumor burden in experimental animals via regulating pro-inflammatory cytokines and anti-apoptotic gene expression. Food Chem. Toxicol. 2022, 161, 112816. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Kuri-García, A.; Vargas-Madriz, H.; Chávez-Servín, J.L.; Ferriz-Martínez, R.A.; Hernández-Sandoval, L.G.; Guzmán-Maldonado, S.H. Phenolic profile and antioxidant capacity of Pithecellobium dulce (Roxb) Benth: A review. J. Food Sci. Technol. 2020, 57, 4316–4336. [Google Scholar] [CrossRef]
- Kahindi, R.K.; Abdulrazak, S.A.; Muinga, R.W. Effect of supplementing Napier grass (Pennisetum purpureum) with Madras thorn (Pithecellobium dulce) on intake, digestibility and live weight gains of growing goats. Small Rumin. Res. 2007, 69, 83–87. [Google Scholar] [CrossRef]
- Olivares-Perez, J.; Avilez-Nova, F.; Albarran-Portillo, B.; Castelan-Ortega, O.; Rojas-Hernandez, S. Use of three fodder trees in the feeding of goats in the subhumid tropics in Mexico. Trop. Anim. Health Prod. 2013, 45, 1573–7438. [Google Scholar] [CrossRef]
- Olivares-Pérez, J.; Avilés-Nova, F.; Albarrán-Portillo, B.; Castelán-Ortega, O.A.; Rojas-Hernández, S. Nutritional quality of Pithecellobium dulce and Acacia cochliacantha fruits, and its evaluation in goats. Livest. Sci. 2013, 154, 74–81. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Rabbits, 2nd ed.; National Research Council: Washington, DC, USA, 1977. [Google Scholar] [CrossRef]
- FEDNA. FEDNA/Fundación Española para el Desarrollo de la Nutricion Animal. 2020. Available online: http://www.fundacionfedna.org/ (accessed on 7 July 2022).
- Perez, J.M.; Lebas, F.; Gidenne, T.; Maertens, L.; Xiccato, G.; Parigi-Bini, R.; Dalle Zotte, A.; Cossu, M.E.; Carazzolo, A.; Villamide, M.J.; et al. European reference method for in vivo determination of diet digestibility in rabbits. World Rabbit Sci. 1995, 3, 41–43. [Google Scholar] [CrossRef]
- NOM-033-SAG/ZOO-2014. Métodos para dar Muerte a los Animales Domésticos y Silvestres. Diario Oficial de la Federación, México. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5405210&fecha=26/08/2015#gsc.tab=0 (accessed on 7 July 2022).
- Blasco, A.; Ouhayoun, J.; Masoero, G. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; Available online: https://meatscience.org/publications-resources/printed-publications/amsa-meat-color-measurement-guidelines (accessed on 7 July 2022).
- Honikel, K.O. How to measure the water-holding capacity of meat? Recommendation of standardized methods. In Evaluation and Control of Meat Quality in Pigs; Current Topics in Veterinary Medicine and Animal, Science; Tarrant, P.V., Eikelenboom, G., Monin, G., Eds.; Springer: Dordrecht, Germany, 1987; p. 38. [Google Scholar]
- NOM-210-SSA1-2014. Productos y Servicios. Métodos de Prueba Microbiológicos. Determinación de Microorganismos Indicadores. Determinación de Microorganismos Patógenos. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5398468&fecha=26/06/2015 (accessed on 7 July 2022).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Nagender, A.; Satyanarayana, A.; Rao, D.G. Preparation, chemical composition and storage studies of quamachil (Pithecellobium dulce L.) aril powder. J. Food Sci. Technol. 2011, 4, 90–95. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Traditional knowledge to clinical trials: A review on nutritional and therapeutic potential of Pithecellobium dulce. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Simonetti, A.; Grassi, G.; Gambacorta, E. Effect of a cauliflower (Brassica oleraceae var. Botrytis) leaf powder-enriched diet on performance, carcass and meat characteristics of growing rabbit. Meat Sci. 2019, 149, 134–140. [Google Scholar] [CrossRef]
- Mancini, S.; Moruzzo, R.; Minieri, S.; Turchi, B.; Cerri, D.; Gatta, D.; Sagona, S.; Felicioli, A.; Paci, G. Dietary supplementation of quebracho and chestnut tannins mix in rabbit: Effects on live performances, digestibility, carcase traits, antioxidant status, faecal microbial load and economic value. Ital. J. Anim. Sci. 2019, 18, 621–629. [Google Scholar] [CrossRef]
- Kovitvadhi, A.; Gasco, L.; Ferrocino, I.; Rotolo, L.; Dabbou, S.; Malfatto, V.; Gai, F.; Peirette, P.G.; Falzone, M.; Vignolini, C.; et al. Effect of purple loosestrife (Lythrum salicaria) diet supplementation in rabbit nutrition on performance, digestibility, health and meat quality. Animal 2016, 10, 10–18. [Google Scholar] [CrossRef]
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Yu, D.; Feng, J.; Zhu, X.; Hang, S. Supplementation of Moringa oleifera leaf powder orally improved productive performance by enhancing the intestinal health in rabbits under chronic heat stress. J. Therm. Biol. 2020, 93, 102680. [Google Scholar] [CrossRef]
- Elwan, H.A.; Dawood, D.H.; Abd El-Aziz El-Shafei, S.M.; Abd El-Mohsen Abd El-Rahman, A.; Abdel-Latif, S.A.; Mohany, M.; Alqahtani, F.; Alqahtani, S.; Al-Rejaie, S.S. The potential role of citrus limon powder as a natural feed supplement to boost the productive performance, antioxidant status, and blood biochemistry of growing rabbits. Animals 2019, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Ferrocino, I.; Kovitvadhi, A.; Bergagna, S.; Dezzuto, D.; Schiavone, A.; Cocolin, L.; Gai, F.; Santoro, V.; Gasco, L. Bilberry pomace in rabbit nutrition: Effects on growth performance, apparent digestibility, caecal traits, bacterial community and antioxidant status. Animal 2019, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Roselin, C.; Parameshwari, S. A systematic review on the materialistic use of Pithecellobium dulce in food formulations. Mater Today Proc. 2022, 66, 996–1001. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Cullere, M.; Tasoniero, G.; Gerencsér, Z.; Szendrő, Z.; Novelli, E.; Matics, Z. Supplementing growing rabbit diets with chestnut hydrolyzable tannins: Effect on meat quality and oxidative status, nutrient digestibilities, and content of tannin metabolites. Meat Sci. 2018, 146, 101–108. [Google Scholar] [CrossRef]
- Brandão, J. Basic approach to veterinary care of rabbits. In Ferrets, Rabbits and Rodents Clinical Medicine and Surgery; Quesenberry, K.E., Orcutt, C.J., Mans, C., Carpenter, J.W., Eds.; Elsevier: St. Louis, MO, USA, 2021. [Google Scholar]
- Aljohani, N.E.; Abduljawad, S.H. Efficacy of Moringa oleifera leaf supplementation for enhanced growth performance, haematology and serum biochemistry of rabbits. Food Nutr. Sci. 2018, 9, 1285–1298. [Google Scholar] [CrossRef][Green Version]
- Imbabi, T.; Sabeq, I.; Osman, A.; Mahmoud, K.; Amer, S.A.; Hassan, A.M.; Kostomakhin, N.; Habashy, W.; Easa, A.A. Impact of fennel essential oil as an antibiotic alternative in rabbit diet on antioxidant enzymes levels, growth performance, and meat quality. Antioxidants 2021, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Cappai, M.G. Response of fattening rabbits with acorns (Quercus pubescens willd.) Combined in the diet: First acquaintances on growth performance, carcass traits and perirenal fatty acid profile. Animals 2020, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Wang, Y.; Zhou, L.; Hu, H.; Bai, L.; Wang, J. Weighted gene co-expression network analysis reveals potential candidate genes affecting drip loss in pork. Anim. Genet. 2020, 51, 855–865. [Google Scholar] [CrossRef]
- Ramirez, J. Effect of selection for growth rate on biochemical, quality and texture characteristics of meat from rabbits. Meat Sci. 2004, 67, 617–624. [Google Scholar] [CrossRef]
- Sembiring, I.C.B.; Wardhita, A.A.G.; Adi, A.A.M. Muntingia calabura’s leaves extract ointment increased collagen density and enhanced healing of skin incision wound in hyperglycemic mice. Indones. Med. Veterinus 2021, 10, 189–199. [Google Scholar] [CrossRef]
- Pałka, S.E.; Otwinowska-Mindur, A.; Migdał, Ł.; Kmiecik, M.; Wojtysiak, D. Effect of a diet supplemented with nettle (Urtica dioica L.) or fenugreek (Trigonella foenumgraecum L.) on the post-slaughter traits and meat quality parameters of Termond white rabbits. Animals 2021, 11, 1566. [Google Scholar] [CrossRef]
- García-Vázquez, L.M.; Zepeda-Bastida, A.; Ayala-Martinez, M.; Soto-Simental, S. Infusion of Chenopodium ambrosioides consumed by rabbits: Effects on carcass, meat and burger quality. Food Sci. Technol. 2020, 40, 451–457. [Google Scholar] [CrossRef]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant extracts and essential oil product as feed additives to control rabbit meat microbial quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F.; Paci, G. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gai, F.; Renna, M.; Rotolo, L.; Dabbou, S.; Lussiana, C.; Kovitvadhi, A.; Brugiapaglia, A.; Helal, M.N.; Zoccarato, I.; et al. Inclusion of bilberry pomace in rabbit diets: Effects on carcass characteristics and meat quality. Meat Sci. 2017, 124, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brechia, G.; Miraglia, D. Impact of dietary supplementation with goji berries (Lycium barbarum) on microbiological quality, physico-chemical, and sensory characteristics of rabbit meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Fratini, F.; Torracca, B.; Nuvoloni, R.; Dal Bosco, A.; Paci, G. Qualitative improvement of rabbit burgers using Zingiber officinale Roscoe powder. World Rabbit Sci. 2017, 25, 367–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).