The Raccoon (Procyon lotor) as a Neozoon in Europe
Abstract
Simple Summary
Abstract
1. Introduction
2. The Raccoons as a Neozoon
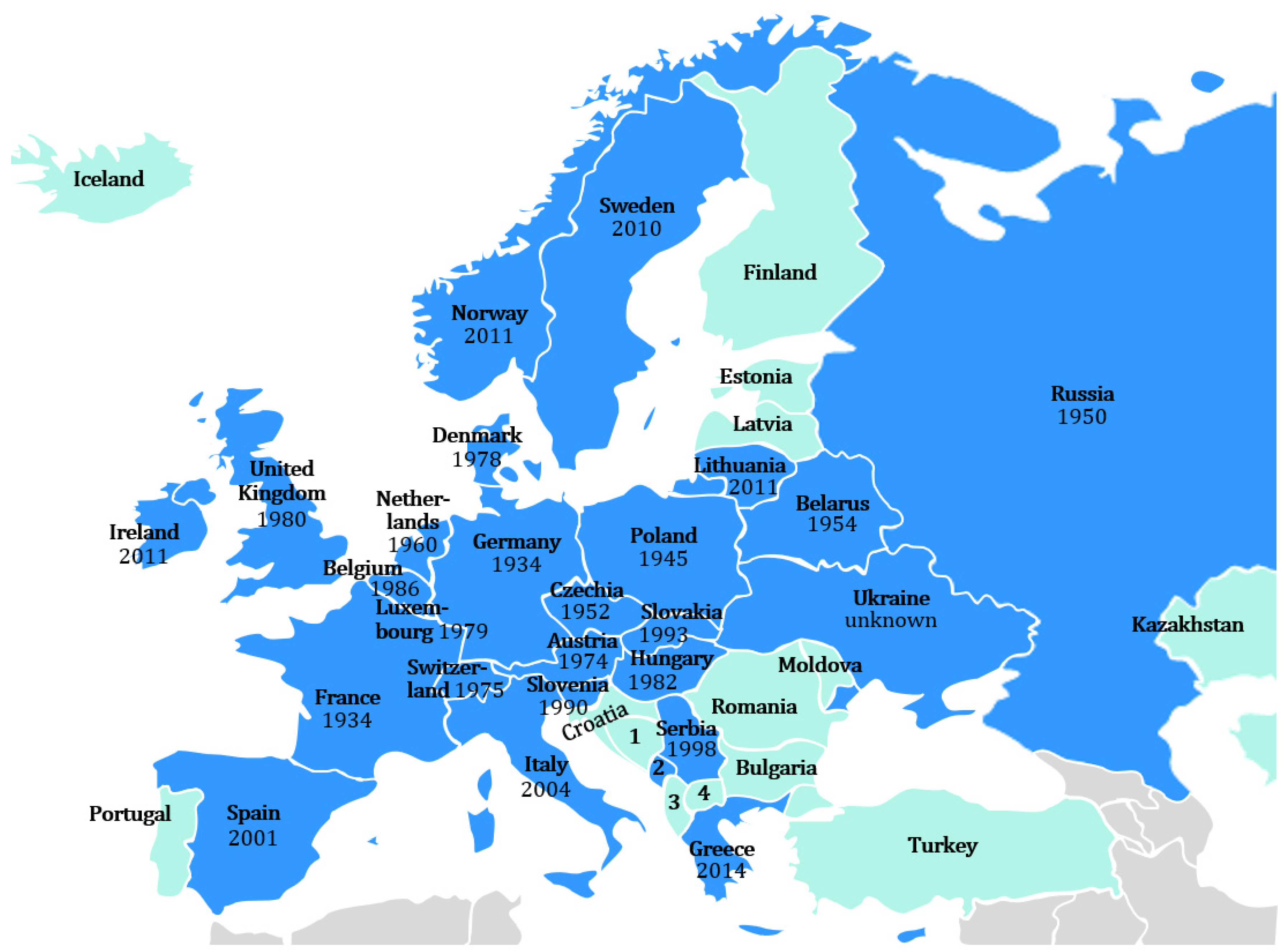
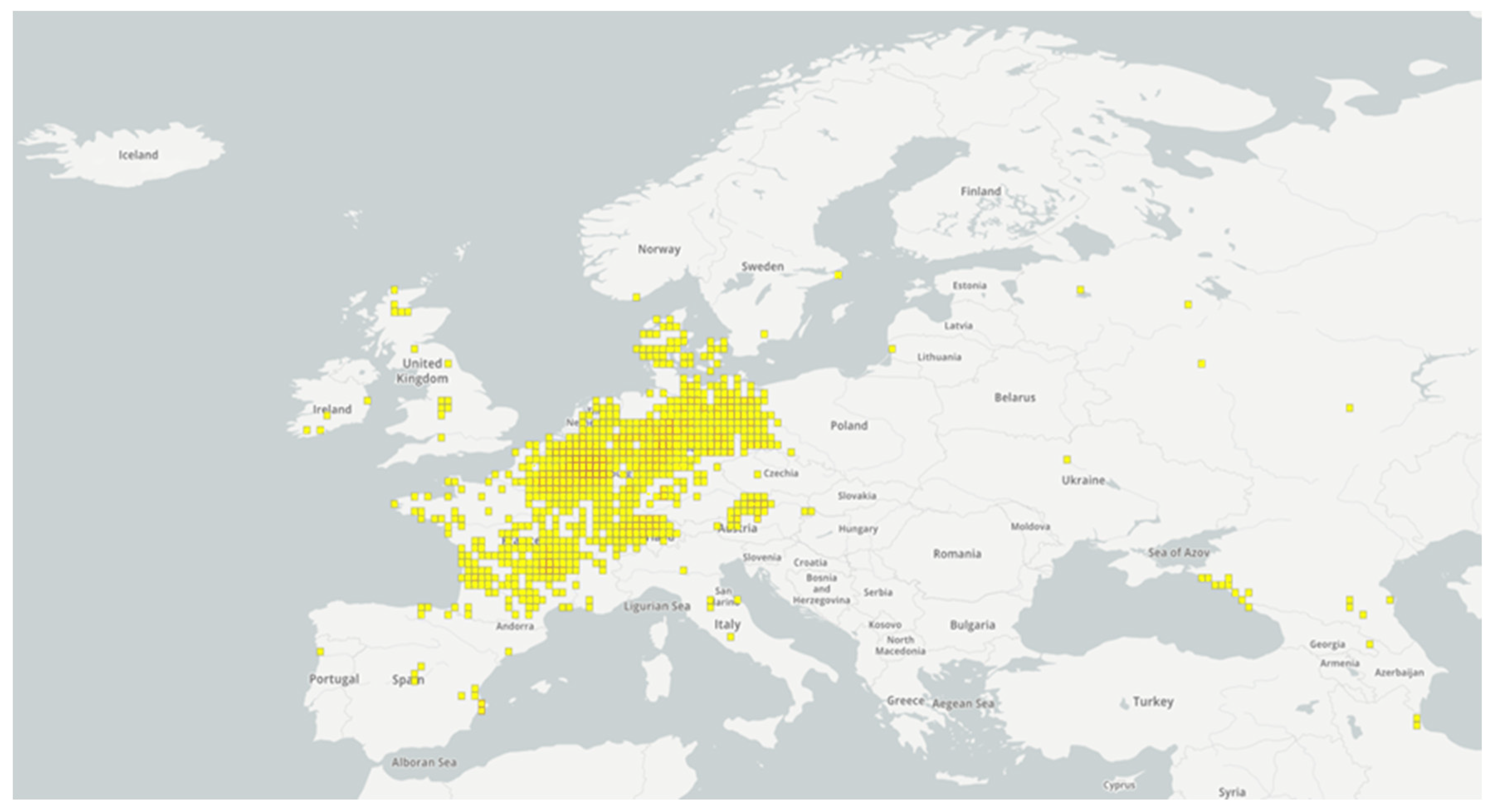
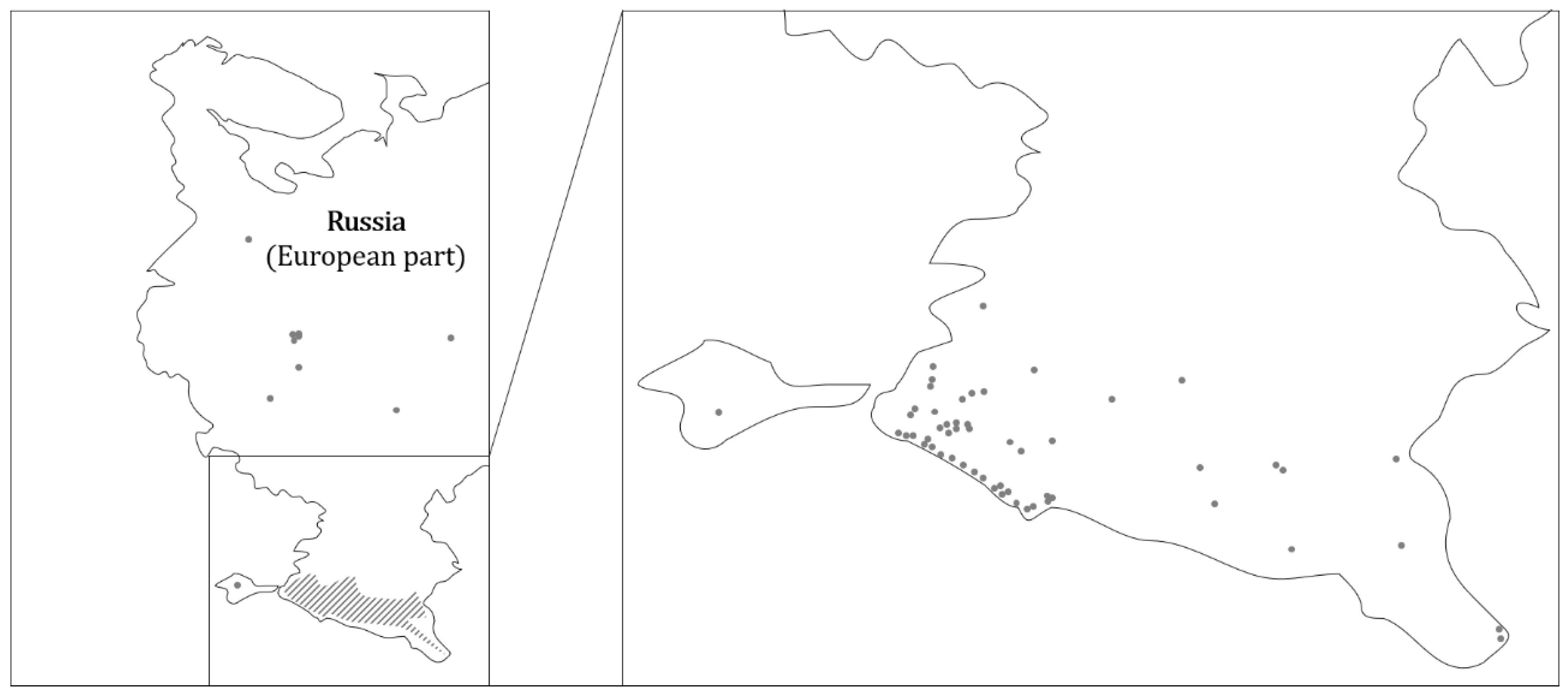
3. The Distribution of Raccoons in Europe
4. The Impact of Raccoons on European Habitats
5. Raccoons as Vectors of Zoonotic Diseases
6. Outlook
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikeda, T.; Asano, M.; Matoba, Y.; Abe, G. Present status of invasive alien raccoon and its impact in Japan. Glob. Environ. Res. 2004, 8, 125–131. [Google Scholar]
- Beltran-Beck, B.; Garcia, F.J.; Gortazar, C. Raccoons in Europe: Disease hazards due to the establishment of an invasive species. Eur. J. Wildl. Res. 2012, 58, 5–15. [Google Scholar] [CrossRef]
- García, J.T.; García, F.J.; Alda, F.; González, J.L.; Aramburu, M.J.; Cortés, Y.; Prieto, B.; Pliego, B.; Pérez, M.; Herrera, J.; et al. Recent invasion and status of the raccoon (Procyon lotor) in Spain. Biol. Invasions 2012, 14, 1305–1310. [Google Scholar] [CrossRef]
- Lotze, J.H.; Anderson, S. Procyon lotor. Mamm. Species 1979, 119, 1–8. [Google Scholar] [CrossRef]
- Kays, R. Raccoons and relatives. In Walker’s Mammals of the World, 6th ed.; Nowak, R.M., Walker, E.P., Eds.; The John Hopkins University Press: Baltimore, MD, USA; London, UK, 1999; pp. 694–700. [Google Scholar]
- Pedlar, J.H.; Fahrig, L.; Merriam, H.G. Raccoon habitat use at 2 spatial scales. J. Wildl. Manag. 1997, 61, 105–112. [Google Scholar] [CrossRef]
- Hohmann, U.; Gerhard, R.; Kasper, M. Home range size of adult raccoons (Procyon lotor) in Germany. Z. Saugetierkd. 2000, 65, 124–127. [Google Scholar]
- Rosatte, R.C. Management of raccoons (Procyon lotor) in Ontario, Canada: Do human intervention and disease have significant impact on raccoon populations? Mammalia 2000, 64, 369–390. [Google Scholar] [CrossRef]
- Engelmann, A.; Michler, B.A.; Michler, F.U. Eine Frage der Saison—Aktuelle Ergebnisse zur Nahrungsökologie des Waschbären (Procyon lotor) in der nordostdeutschen Tiefebene. Labus 2012, 36, 47–62. [Google Scholar]
- Bozek, C.K.; Prange, S.; Gehrt, S.D. The influence of anthropogenic resources on multiscale habitat selection by raccoons. Urban Ecosyst. 2007, 10, 413–425. [Google Scholar] [CrossRef]
- Prange, S.; Gehrt, S.D.; Wiggers, E.P. Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J. Mammal. 2004, 85, 483–490. [Google Scholar] [CrossRef]
- Gehrt, S.D. Ecology and Management of Striped Skunks, Raccoons and Coyotes in Urban Landscapes. In People and Predators: From Conflict to Coexistence, 2nd ed.; ND Press: Washington, DC, USA, 2004; pp. 81–94. [Google Scholar]
- Köhnemann, B.A.; Michler, F.U. Sumpf- und Moorlandschaften der nordostdeutschen Tiefebene—Idealhabitate für Waschbären (Procyon lotor L., 1758) in Mitteleuropa? Beitr. Jagd. Wildforsch. 2009, 34, 511–524. [Google Scholar]
- Riley, S.P.D.; Hadidad, J.; Mansli, D.A. Population density, survival, and rabies in raccoons in an urban national park. Can. J. Zool. 1998, 76, 1153–1164. [Google Scholar] [CrossRef]
- Fritzell, E.K. Reproduction of raccoons (Procyon lotor) in North Dakota. Am. Midl. Nat. 1978, 100, 253–256. [Google Scholar] [CrossRef]
- Johnson, A.S. Biology of the raccoon (Procyon lotor varius Nelson and Goldman) in Alabama. Auburn Univ. Ala. Agric. Exp. Stn. Bull. 1970, 402, 1–148. [Google Scholar]
- Grau, G.A.; Sanderson, G.C.; Rogers, J.P. Age determination of raccoons. J. Wildl. Manag. 1970, 34, 364–372. [Google Scholar] [CrossRef]
- Hohmann, U.; Bartussek, I. Der Waschbär, 2nd ed.; Oertel und Spörer: Reutlingen, Germany, 2005. [Google Scholar]
- Wiegmann, A.F.A. Ueber die Gattung. Procyon. Arch. Nat. 1837, 3, 353–372. [Google Scholar]
- Franz, J.M. Die Einbürgerung von Säugetieren und Vögeln in Europa. Ergebnisse und Aussichten. Entomophaga 1963, 8, 374. [Google Scholar] [CrossRef]
- Leicht, E. Waschbär—Kleiner Feldversuch mit großer Wirkung. AFZ-DerWald 2009, 11, 570–573. [Google Scholar]
- Pagel, T.; Spieß, W. Der Zoologische Garten in Cöln eröffnet am 22. Juli 1860—150 Jahre Wildtierhaltung und -zucht. Zool. Gart. NF 2011, 80, 117–202. [Google Scholar] [CrossRef]
- Müller-Using, D. Die Ausbreitung des Waschbären (Procyon lotor [L.]) in Westdeutschland. Z. Jagdwiss. 1959, 5, 108–109. [Google Scholar] [CrossRef]
- Lutz, W. Occurrence and morphometrics of the raccoon Procyon lotor L. in Germany. Ann. Zool. Fenn. 1995, 32, 15–20. [Google Scholar]
- Frantz, A.C.; Cyriacks, P.; Schley, L. Spatial behaviour of a female raccoon (Procyon lotor) at the edge of the species’ European distribution range. Eur. J. Wildl Res. 2005, 51, 126–130. [Google Scholar] [CrossRef]
- Stubbe, M. Der Waschbär Procyon lotor (L., 1758) in der DDR. Hercynia. NF 1975, 12, 80–91. [Google Scholar]
- Bartussek, I. Die Waschbären Kommen, 1st ed.; Cognitio: Niedenstein, Germany, 2004. [Google Scholar]
- Gehrt, S.D. Raccoon Social Organization in South Texas. Ph.D. Thesis, University of Missouri, Missouri, MO, USA, 1994. [Google Scholar]
- Pitt, J.A.; Lariviere, S.; Messier, F. Social organization and group formation of raccoons at the edge of their distribuition. J. Mamm. 2008, 89, 646–653. [Google Scholar] [CrossRef]
- Muschik, I.; Köhnemann, B.A.; Michler, F.U. Untersuchungen zur Entwicklung des Raum und Sozialverhaltens von Waschbär-Mutterfamilien (Procyon lotor L.). Beitr. Jagd. Wildforsch. 2011, 36, 573–585. [Google Scholar]
- Bartoszewicz, M.; Okarma, H.; Zalwski, A.; Szczesna, J. Ecology of the raccoon (Procyon lotor) from western Poland. Ann. Zool. Fenn. 2008, 45, 291–298. [Google Scholar] [CrossRef]
- Michler, F.U.; Hohmann, U.; Stubbe, M. Aktionsräume, Tagesschlafplätze und Sozialsystem des Waschbären (Procyon lotor Linné 1758) im urbanen Lebensraum der Großstadt Kassel (Nordhessen). Beitr. Jagd. Wildforsch. 2004, 29, 57–273. [Google Scholar]
- Fischer, M.L.; Hochkirch, A.; Heddergott, M.; Schulze, C.; Anheyer-Behmenburg, H.E.; Lang, J.; Michler, F.U.; Hohmann, U.; Ansorge, H.; Hoffmann, L.; et al. Historical invasion records can be misleading: Genetic evidence for multiple introductions of invasive raccoons (Procyon lotor) in Germany. PLoS ONE 2015, 10, e0125441. [Google Scholar] [CrossRef]
- Salgado, I. Is the raccoon (Procyon lotor) out of control in Europe? Biodivers. Conserv. 2018, 27, 2243–2256. [Google Scholar] [CrossRef]
- Aliev, F.F.; Sanderson, G.C. Distribution and status of the raccoon in the Soviet Union. J. Wildl. Manag. 1966, 30, 497–502. [Google Scholar] [CrossRef]
- Michler, F.U.; Köhnemann, B.A. Tierische Spitzenleistung—Abwanderungsverhalten von Waschbären (Procyon lotor L., 1758) in Norddeutschland. Labus 2010, 31, 52–59. [Google Scholar]
- Lynch, G.M. Long-range movement of a raccoon in Manitoba. J. Mammal. 1967, 48, 659–660. [Google Scholar] [CrossRef]
- Priewert, A.R. Record of an extensive movement by a raccoon. J. Mammal. 1961, 42, 113. [Google Scholar] [CrossRef]
- Helbig, D. Untersuchungen zum Waschbären (Procyon lotor LINNE, 1758) im Raum Bernburg. Nat. Land Sachs. Anhalt. 2011, 48, 3–19. [Google Scholar]
- Cerutti, H. Der Immigrant. Neue Zürcher Zeitung. 2006, 1, 4. [Google Scholar]
- Alda, F.; Ruiz-López, M.J.; García, F.J.; Gompper, M.E.; Eggert, L.S.; Garcia, J.T. Genetic evidence for multiple introduction events of raccoons (Procyon lotor) in Spain. Biol. Invasions 2013, 15, 687–698. [Google Scholar] [CrossRef]
- Mori, E.; Mazza, G.; Menchetti, M.; Panzeri, M.; Gager, Y.; Bertolino, S.; Di Febbraro, M. The masked invader strikes again: The conquest of Italy by the Northern raccoon. Hystrix 2015, 26, 47–51. [Google Scholar]
- Frantz, A.C.; Heddergott, M.; Lang, J.; Schulze, C.; Ansorge, H.; Runge, M.; Braune, S.; Michler, F.U.; Wittstatt, U.; Hoffmann, L.; et al. Limited mitochondrial DNA diversity is indicative of a small number of founders of the German raccoon (Procyon lotor) population. Eur. J. Wildl. Res. 2013, 59, 665–674. [Google Scholar] [CrossRef]
- Cullingham, C.I.; Kyle, C.J.; Pond, B.A.; White, B.N. Genetic structure of raccoons in eastern North America based on mtDNA: Implications for subspecies designation and rabies disease dynamics. Can. J. Zool. 2008, 86, 947–958. [Google Scholar] [CrossRef]
- Gramlich, S.; Schulz, H.; Köhnemann, B.A.; Michler, F.U. Mater semper certa?—Molekularbiologische Analyse einer Waschbärenpopulation (Procyon lotor Linné, 1758) im Müritz-Nationalpark. Beitr. Jagd. Wildforsch. 2011, 36, 521–530. [Google Scholar]
- Broekhuizen, S.; Müskens, G.J.D.M.; Niewold, F.J.J.; Thissen, J.B.M. Heimkehrer und Neubürger unter den Säugetieren der Niederlande im 19. und 20. Jahrhundert. Beitr. Jagd. Wildforsch. 2001, 26, 155–170. [Google Scholar]
- Schlei, L.; Schank, C.; Schaul, M.; Sinner, C. Neubürger und Heimkehrer unter den Wildtieren Luxenburgs. Beitr. Jagd. Wildforsch. 2001, 26, 141–154. [Google Scholar]
- Léger, F. Répartition en France de trois petits carnivores introduits. Beitr. Jagd. Wildforsch. 2001, 26, 137–139. [Google Scholar]
- Heltai, M.; Szemethy, L.; Lanszky, J.; Csanyi, S. Returning and new mammal predators in Hungary: The status and distribution of the golden jackal (Canis aureus), raccoon dog (Nyctereutes procyonides) and raccoon (Procyon lotor) in 1997-2000. Beitr. Jagd. Wildforsch. 2000, 26, 95–102. [Google Scholar]
- Spitenberger, F.; Bauer, K.; Sackl, P.; Sieber, J. Heimkehrer und Neubürger der österreichischen Säugertierfauna. Beitr. Jagd. Wildforsch. 2001, 26, 127–136. [Google Scholar]
- Canova, L.; Rossi, S. First records of the northern raccoon Procyon lotor in Italy. Hystrix. It. J. Mamm. 2008, 19, 179–182. [Google Scholar]
- Zimina, R.P. The main features of the Caucasian natural landscapes and their conservation, USSR. Artic. Alp. Res. 1978, 10, 479–488. [Google Scholar] [CrossRef]
- Stille, M.; Gasteratos, I.; Stille, B. Alien and invasive terrestrial vertebrate species on Corfu, Ionian Islands, Greece. J. Vertebr. Biol. 2021, 70, 20126. [Google Scholar] [CrossRef]
- Aliev, F.F. Acclimatization and the economic acclimatization of the raccoon in Azerbaijan. Acad. Sci. Azerbaijan SSR Rept. 1955, 8, 571–578. [Google Scholar]
- Redford, P. Raccoon in the U.S.S.R. J. Mammal. 1962, 43, 541–542. [Google Scholar] [CrossRef]
- Khlyap, L.A.; Bobrov, V.V.; Warshavsky, A.A. Biological invasions on Russian territory: Mammals. Russ. J. Biol. Invasions 2010, 1, 127–140. [Google Scholar] [CrossRef]
- Kauhala, K. Introduced carnivores in Europe with special reference to central and northern Europe. Wildl. Biol. 1996, 2, 197–204. [Google Scholar] [CrossRef]
- Kudaktin, A.N.; Romas, A.V. Raccon in the Russian Black Sea Coast. Biota Environ. 2019, 2, 88–103. [Google Scholar]
- Langgemach, T.; Bellebaum, J. Prädation und der Schutz bodenbrütender Vogelarten in Deutschland. Vogelwelt 2005, 126, 259–298. [Google Scholar]
- Gehrt, S.D.; Fritzell, E.K. Resource distribution, female home range dispersion and male spatial interactions: Group structure in a solitary carnivore. Anim. Behav. 1998, 55, 1211–1227. [Google Scholar] [CrossRef]
- Miller, C.A.; Campbell, L.K.; Yeagle, J.A. Attitudes of Homeowners in the Greater Chicago Metropolitan Region Toward Nuisance Wildlife; Program Report SR-00-02; Illinois Natural History Survey: Champaign, IL, USA, 2001. [Google Scholar]
- Beasly, J.C.; Rhodes, O.E. Relationship between raccoon abundance and crop damage. Hum. Wildl. Confl. 2006, 2, 248–259. [Google Scholar]
- Michler, F.U. Untersuchungen zur Raumnutzung des Waschbären (Procyon lotor, L. 1758) im urbanen Lebensraum am Beispiel der Stadt Kassel (Nordhessen). Diploma Thesis, Halle-Wittenberg University, Halle, Germany, 2003. [Google Scholar]
- Hohmann, U.; Voigt, S.; Andreas, U. Quo vadis raccoon? New visitors in our backyards—On the urbanization of an allochthone carnivore in Germany. Nat. Verhalt. 2001, 2, 143–148. [Google Scholar]
- Jernelöv, A. Raccoons in Europe (Germany). In The Long-Term Fate of Invasive Species: Aliens Forever or Integrated Immigrants with time? 1st ed.; Jernelöv, A., Ed.; Springer: Basel, Switzerland, 2017; pp. 217–230. [Google Scholar]
- Boscherini, A.; Mazza, G.; Menchetti, M.; Laurenzi, A.; Mori, E. Time is running out! Rapid range expansion of the invasive northern raccoon in central Italy. Mammalia 2020, 84, 98–101. [Google Scholar] [CrossRef]
- Lutz, W. The introduced raccoon Procyon lotor population in Germany. Wildl. Biol. 1996, 2, 228. [Google Scholar] [CrossRef]
- Michler, F.U.; Michler, B.A. Ökologische, ökonomische und epidemiologische Bedeutung des Waschbären (Procyon lotor) in Deutschland—Eine aktuelle Übersicht. Beitr. Jagd. Wildforsch. 2012, 37, 385–397. [Google Scholar]
- Mulder, J.L. A review of the ecology of the raccoon d.dog (Nyctereutes procyonoides) in Europe. Lutra 2012, 55, 101–127. [Google Scholar]
- Mulder, J.L. The raccoon dog (Nyctereutes procyonoides) in the Netherlands-its present status and a risk assessment. Lutra 2013, 56, 23–43. [Google Scholar]
- Davis, M.B.; Simons, T.R.; Groom, M.J.; Weaver, J.L.; Cordes, J.R. The breeding status of the American oystercatcher on the east coast of North America and breeding success in North Carolina. Waterbirds 2001, 24, 195–202. [Google Scholar] [CrossRef]
- Erwin, R.M.; Truitt, B.R.; Jiménez, J.E. Ground-nesting waterbirds and mammalian carnivores on the Virginia barrier island region: Running out of options. J. Coast. Res. 2001, 17, 292–296. [Google Scholar]
- Hadidian, J.; Prange, S.; Rosatte, R.; Riley, S.P.D.; Gehrt, S.D. Raccoons (Procyon lotor). In Urban Carnivores. Ecology, Conflict, and Conservation, 1st ed.; Clark, H.O., Ed.; The John Hopkins University Press: Baltimore, MD, USA; London, UK, 2010; pp. 35–47. [Google Scholar]
- Manchester, S.J.; Bullock, J.M. The impacts of non-native species on UK biodiversityand the effectiveness of control. J. Appl Ecol 2000, 37, 845–864. [Google Scholar] [CrossRef]
- Galton, M.M. The epidemiology of leptospirosis in the United States. Public Health Rep. 1896, 74, 141–148. [Google Scholar] [CrossRef]
- Orihel, T.C.; Eberhard, M.L. Zoonotic filariasis. Clin. Microbiol Rev. 1998, 11, 366–381. [Google Scholar] [CrossRef]
- Fried, B.; Abruzzi, A. Food-borne trematode infections of humans in the United States of America. Parasitol. Res. 2010, 106, 1263–1280. [Google Scholar] [CrossRef]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef]
- Weinstein, S.B. Baylisascaris procyonis demography and egg production in a California raccoon population. J. Parasitol. 2016, 102, 622–628. [Google Scholar] [CrossRef]
- Schwarz, S.; Sutor, A.; Mattis, R.; Conraths, F.J. Der Waschbärspulwurm (Baylisascaris procyonis)—Kein Zoonoserisiko für Brandenburg? Berl. Münchener Tierärztl. Wochenschr. 2015, 128, 34–38. [Google Scholar]
- Stope, M. Wild life raccoons in Germany as a reservoir for zoonotic agents. Eur. J. Wildl Res. 2019, 65, 94. [Google Scholar] [CrossRef]
- Sorvillo, F.; Ash, L.R.; Berlin, O.G.W.; Morse, S.A. Baylisascaris procyonis: An emerging helminthic zoonosis. Emerg. Infect. Dis. 2002, 8, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kazakos, K.R. Baylisascaris procyonis and related species. In Parasitic Diseases of Wild Mammals, 2nd ed.; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University: Ames, IA, USA, 2001; pp. 301–341. [Google Scholar]
- Küchle, M.; Knorr, H.L.; Medenblik-Frysch, S.; Weber, A.; Bauer, C.; Naumann, G.O. Diffuse unilateral subacute neuroretinitis syndrome in a German most likely caused by the raccoon roundworm, Baylisascaris procyonis. Graefes Arch. Clin. Exp. Ophthalmol. 1993, 231, 48–51. [Google Scholar] [CrossRef]
- Stolte, M.; Odening, K.; Walter, G.; Bockhardt, I. The raccoon as intermediate host of three Sarcocystis species in Europe. Comp. Parasitol. 1996, 63, 145–149. [Google Scholar]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Morse, E.V.; Midla, D.A.; Kazakos, K.R. Raccoons (Procyon lotor L.) as carriers of Salmonella. J. Environ. Sci. Health A 1983, 18, 541–560. [Google Scholar]
- Hildebrand, J.; Bunkowska-Gawlik, K.; Adamczyk, M.; Gajda, E.; Merta, D.; Popiolek, M.; Perec-Matysiak, A. The occurrence of Anaplasmataceae in European populations of invasive carnivores. Ticks Tick Borne Dis. 2018, 9, 934–937. [Google Scholar] [CrossRef]
- Cybulska, A.; Skopek, R.; Kornacka, A.; Popiolek, M.; Pirog, A.; Laskowski, Z.; Moskwa, B. First detection of Trichinella pseudospiralis infection in raccoon (Procyon lotor) in Central Europe. Vet. Parasitol. 2018, 254, 114–119. [Google Scholar] [CrossRef]
- Bourhy, H.; Reynes, J.M.; Dunham, E.J.; Dacheux, L.; Larrous, F.; Huong, V.T.Q.; Xu, G.; Yan, J.; Miranda, M.E.G.; Holmes, E.C. The origin and phylogeography of dog rabies virus. J. Gen. Virol. 2008, 89, 2673–2681. [Google Scholar] [CrossRef]
- Faber, M.; Dietzschold, B.; Li, J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health 2009, 56, 262–269. [Google Scholar] [CrossRef]
- Holmala, K.; Kauhala, K. Habitat use of medium-sized carnivores in southeast Finland-key habitats for rabies spread? Ann. Zool. Fenn. 2009, 46, 233–246. [Google Scholar] [CrossRef]
- Kuzmin, I.V.; Botvinkin, A.D.; McElhinney, L.M.; Smith, J.S.; Orciari, L.A.; Hughes, G.J.; Fooks, A.R.; Rupprecht, C.E. Molecular epidemiology of terrestrial rabies in the former Soviet Union. J. Wildl. Dis. 2004, 40, 617–631. [Google Scholar] [CrossRef]
- Nakano, H.; Kameo, Y.; Sato, H.; Mochizuki, M.; Yokoyama, M.; Uni, S.; Shibasaki, T.; Maeda, K. Detection of antibody to canine distemper virus in wild raccoons (Procyon lotor) in Japan. J. Vet. Med. Sci. 2009, 71, 1661–1663. [Google Scholar] [CrossRef]
- Roscoe, D.E. Epizootiology of canine distemper in New Jersey raccoons. J. Wildl. Dis. 1993, 29, 390–395. [Google Scholar] [CrossRef]
- Nikolin, V.M.; Wibbelt, G.; Michler, F.U.; Wolf, P.; East, M.L. Susceptibility of carnivore hosts to strains of canine distemper virus from distinct genetic lineages. Vet. Microbiol. 2012, 156, 45–53. [Google Scholar] [CrossRef]
- Benetka, V.; Leschnik, M.; Affenzeller, N.; Mostl, K. Phylogenetic analysis of Austrian canine distemper virus strains from clinical samples from dogs and wild carnivores. Vet. Rec. 2011, 168, 377. [Google Scholar] [CrossRef]
- Michler, F.U.; Köhnemann, B.A.; Roth, M.; Speck, S.; Fickel, J.; Wibbelt, G. Todesursache sendermarkierter Waschbären (Procyon lotor L., 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern). Beiträge Jagd. Wildforsch. 2009, 34, 339–355. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stope, M.B. The Raccoon (Procyon lotor) as a Neozoon in Europe. Animals 2023, 13, 273. https://doi.org/10.3390/ani13020273
Stope MB. The Raccoon (Procyon lotor) as a Neozoon in Europe. Animals. 2023; 13(2):273. https://doi.org/10.3390/ani13020273
Chicago/Turabian StyleStope, Matthias Bernhard. 2023. "The Raccoon (Procyon lotor) as a Neozoon in Europe" Animals 13, no. 2: 273. https://doi.org/10.3390/ani13020273
APA StyleStope, M. B. (2023). The Raccoon (Procyon lotor) as a Neozoon in Europe. Animals, 13(2), 273. https://doi.org/10.3390/ani13020273





