Effects of Different-Sized Cages on the Production Performance, Serum Parameters, and Caecal Microbiota Composition of Laying Hens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Production Performance
2.3. Serum Biochemical Parameters and Antibody Titres
2.4. Sample Collection
2.5. Bacterial DNA Extraction and PCR Amplification of 16S rRNA
2.6. 16S rRNA Sequencing and Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
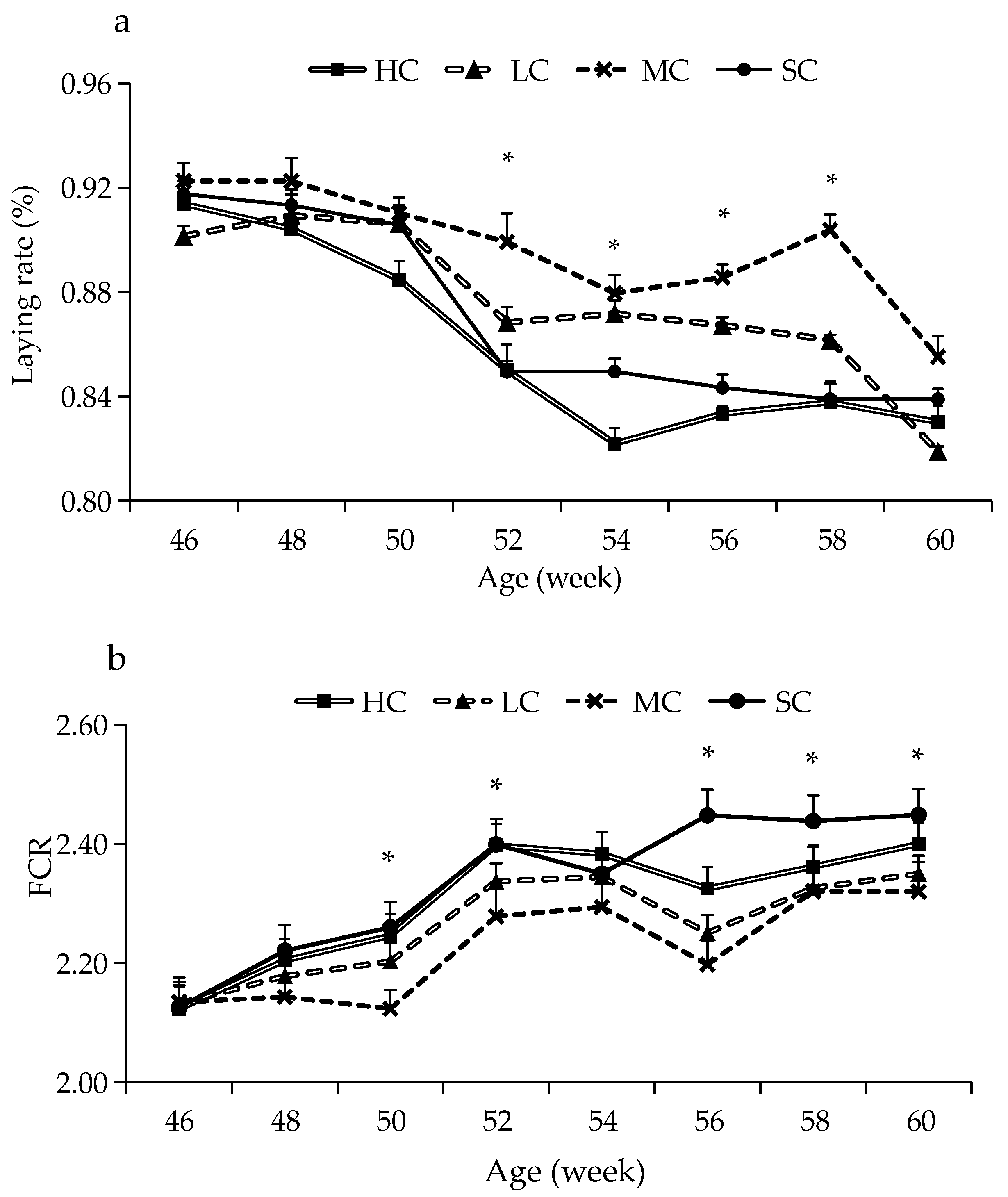
3.1. Production Performance
3.2. Immune Response
3.3. Serum Biochemical Parameters
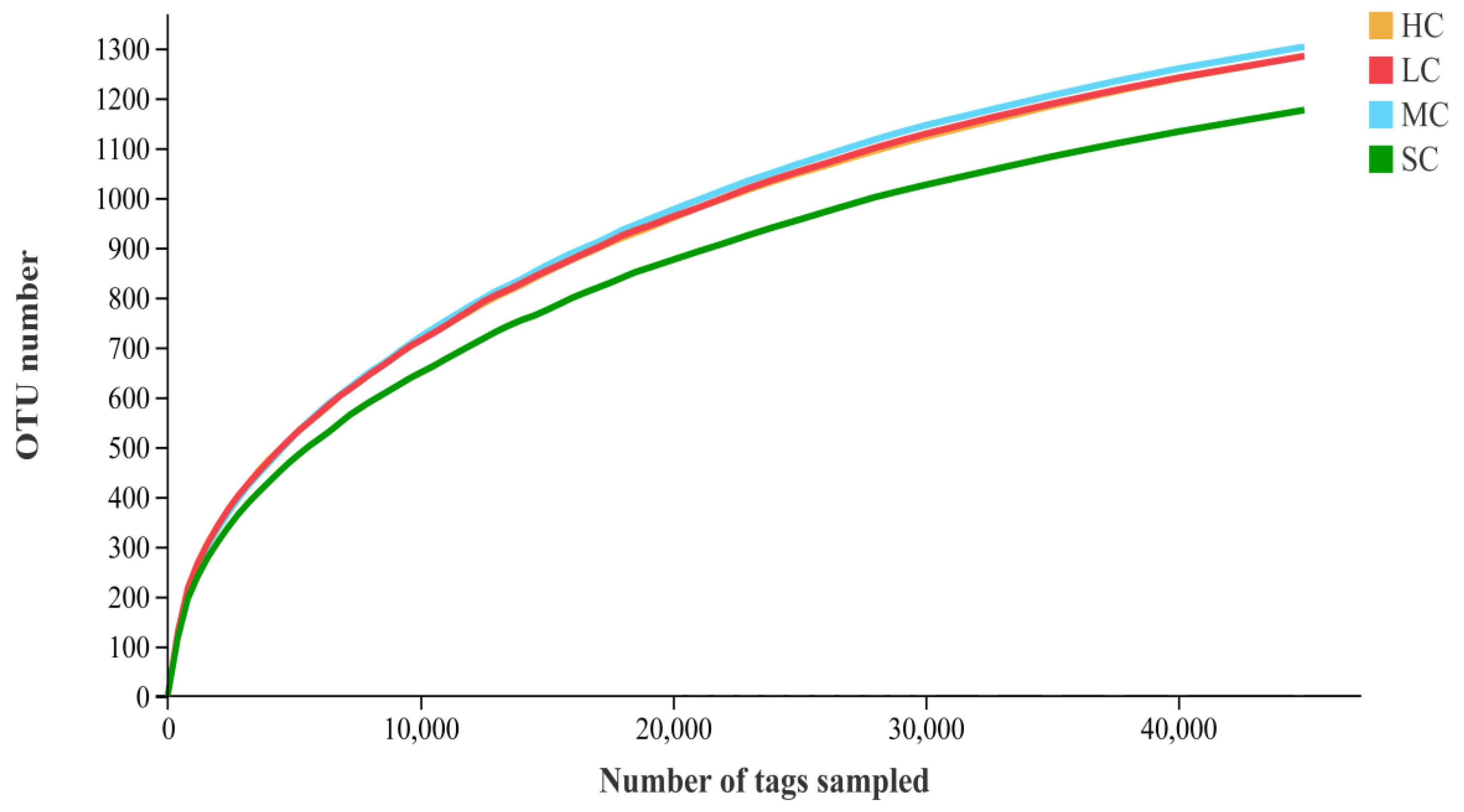
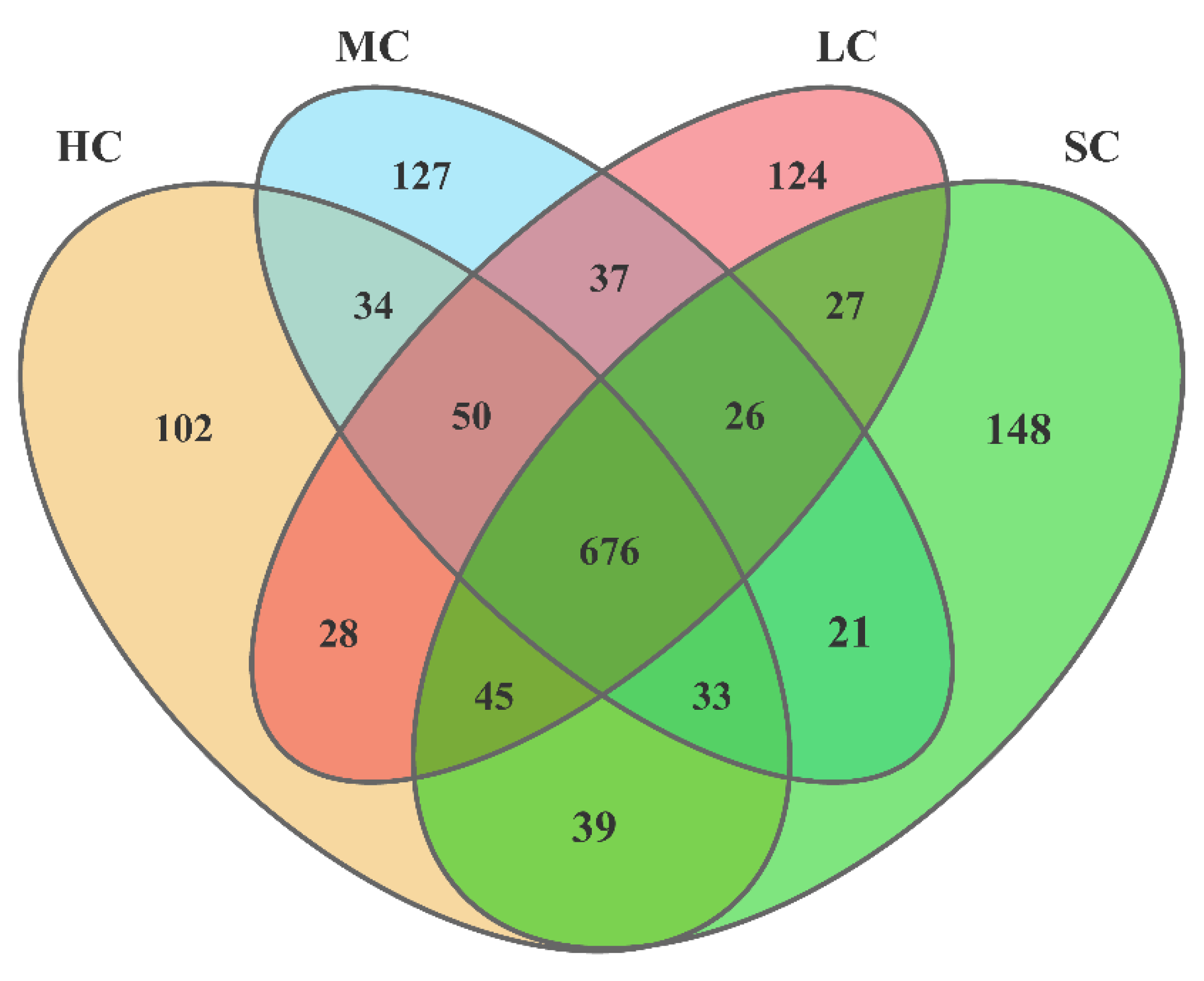
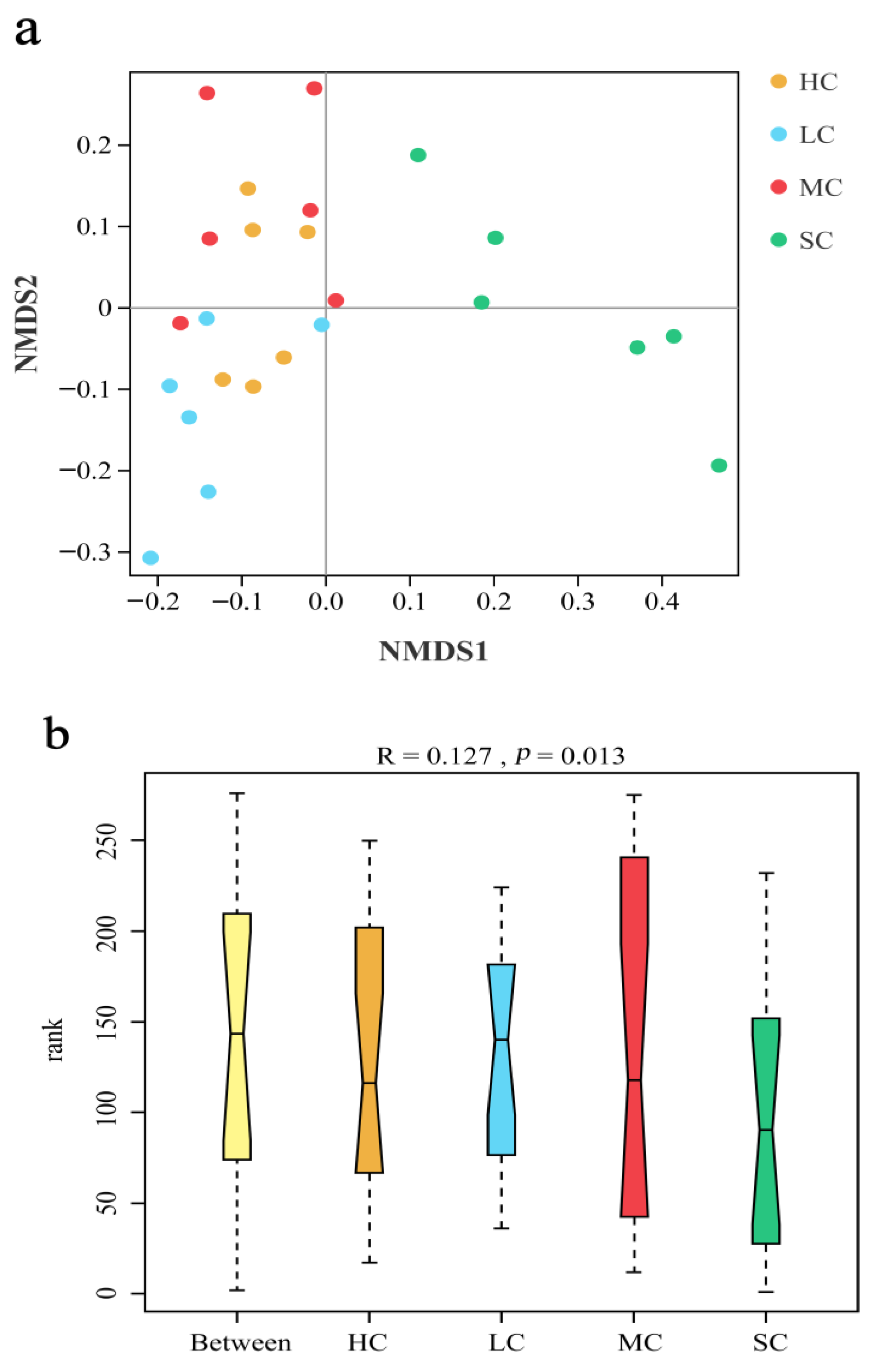
3.4. Diversity Analysis of the Microbiota in the Caecum
3.5. Composition Analysis of the Microbiota in the Caecum
3.6. Correlation Analysis of Differentially Detected Bacterial Genera with Productive Performance and Serum Biochemical Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elston, J.J.; Beck, M.M.; Kachman, S.D.; Scheideler, S.E. Laying hen behavior. 1. Effects of cage type and startle stimuli. Poult. Sci. 2000, 79, 471–476. [Google Scholar] [CrossRef]
- Baker, P.E.; Stokes, J.E.; Weeks, C.A. Enabling behaviour change in laying hen farmers using motivational interviewing. Proceedings 2021, 73, 16. [Google Scholar] [CrossRef]
- Weeks, C.A.; Lambton, S.L.; William, A.G. Implications for welfare, productivity and sustainability of the variation in reported levels of mortality for laying hen flocks kept in different housing systems: A meta-analysis of ten studies. PLoS ONE 2016, 11, e0146394. [Google Scholar] [CrossRef] [PubMed]
- Widowski, T.M.; Caston, L.J.; Casey-Trott, T.M.; Hunniford, M.E. The effect of space allowance and cage size on laying hens housed in furnished cages, Part II: Behavior at the feeder. Poult. Sci. 2017, 96, 3816–3823. [Google Scholar] [CrossRef] [PubMed]
- Hartcher, K.M.; Jones, B. The welfare of layer hens in cage and cage-free housing systems. World’s Poult. Sci. J. 2019, 73, 767–782. [Google Scholar] [CrossRef]
- Meng, F.; Chen, D.; Li, X.; Li, J.; Bao, J. Effects of large or small furnished cages on performance, welfare and egg quality of laying hens. Anim. Prod. Sci. 2015, 55, 793. [Google Scholar] [CrossRef]
- Ducan, I.J.H. The pros and cons of cages. World’s Poult. Sci. J. 2001, 57, 381–390. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of stocking density on broiler growth performance, meat characteristics, behavioural components and indicators of physiological and oxidative stress. Br. Poult. Sci. 2012, 53, 721–730. [Google Scholar] [CrossRef]
- Simsek, U.G.; Dalkilic, B.; Ciftci, M.; Yuce, A. The influences of different stocking densities on some welfare indicators, lipid peroxidation (MDA) and antioxidant enzyme activities (GSH, GSH-Px, CAT) in broiler chickens. J. Anim. Vet. Adv. 2009, 8, 1568–1572. [Google Scholar] [CrossRef]
- Widowski, T.M.; Caston, L.J.; Hunniford, M.E.; Cooley, L.; Torrey, S. Effect of space allowance and cage size on laying hens housed in furnished cages, Part I: Performance and well-being. Poult. Sci. 2017, 96, 3805–3815. [Google Scholar] [CrossRef]
- Aguado, E.; Pascaretti-Grizon, F.; Goyenvalle, E.; Audran, M.; Chappard, D. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoS ONE 2015, 10, e0116763. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Bedecarrats, G.Y.; Widowski, T.M.; Kiarie, E.G. Rearing cage type and dietary limestone particle size: II, effects on egg production, eggshell, and bone quality in Lohmann selected Leghorn-Lite hens. Poult. Sci. 2020, 99, 5763–5770. [Google Scholar] [CrossRef] [PubMed]
- Onbaşılar, E.E.; Ünal, N.; Erde, E.; Kocakaya, A.; Yaranoğlu, B. Production performance, use of nest box, and external appearance of two strains of laying hens kept in conventional and enriched cages. Poult. Sci. 2015, 94, 559–564. [Google Scholar] [CrossRef]
- Nordentoft, S.; Mølbak, L.; Bjerrum, L.; Vylde, J.D.; Immerseel, F.V.; Pedersen, K. The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T-RFLP and 454 pyrosequencing. BMC Microbiol. 2011, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, W.; Ma, R.Y.; Li, Y.; Liu, Y.; Qi, R.R.; Zhan, K. Effects of cage size on growth performance, blood biochemistry, and antibody response in layer breeder males during rearing stage. Poult. Sci. 2019, 98, 3571–3577. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Ma, R.Y.; Li, Y.; Liu, W.; Li, J.Y.; Zhan, K. Effect of a large-sized cage with a low metabolizable energy and low crude protein diet on growth performance, feed cost, and blood parameters of growing layers. J. Poult. Sci. 2021, 58, 70–77. [Google Scholar] [CrossRef]
- Shimmura, T.; Hirahara, S.; Eguchi, Y.; Uetake, K.; Tanaka, T. Behavior, physiology, performance and physical condition of layers in conventional and large furnished cages in a hot environment. Anim. Sci. J. 2007, 78, 314–322. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Curtin, F.; Schulz, P. Multiple Correlations and Bonferroni’s Correction. Biol. Psychiat. 1998, 44, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Widowski, T.M.; Hemsworth, P.H.; Barnett, J.L.; Rault, J.L. Laying hen welfare I. Social environment and space. World’s Poult. Sci. J. 2019, 72, 333–342. [Google Scholar] [CrossRef]
- Zhao, X.L.; Ren, W.S.; Siegel, P.B.; Li, J.; Yin, H.D.; Liu, Y.P.; Wang, Y.; Zhang, Y.; Honaker, C.F.; Zhu, Q. Housing systems interacting with sex and genetic line affect broiler growth and carcass traits. Poult. Sci. 2015, 94, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Pohle, K.; Cheng, H.W. Comparative effects of furnished and battery cages on egg production and physiological parameters in White Leghorn hens. Poult. Sci. 2009, 88, 2042–2051. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.; Grashorn, M.; Stefanski, V. Short- and long-term consequences of stocking density during rearing on the immune system and welfare of laying hens. Poult. Sci. 2021, 100, 101243. [Google Scholar] [CrossRef]
- Altan, O.; Pabuccuoglu, A.; Altan, A.; Konyalioglu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550. [Google Scholar] [CrossRef]
- Oscai, L.B.; Patterson, J.A.; Bogard, D.L.; Beck, R.J.; Rothermel, B.L. Normalization of serum triglycerides and lipoprotein electrophoretic patterns by exercise. Am. J. Cardiol. 1972, 30, 775–780. [Google Scholar] [CrossRef]
- Arbona, D.V.; Anderson, K.E.; Hoffman, J.B. A comparison of humoral immune function in response to a killed newcastle’s vaccine challenge in caged vs. Free-range hy-line brown layers. Int. J. Poult. Sci. 2011, 10, 315–319. [Google Scholar] [CrossRef]
- Onbaşılar, E.E.; Aksoy, F.T. Stress parameters and immune response of layers under different cage floor and density conditions. Livest. Prod. Sci. 2005, 95, 255–263. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef] [PubMed]
- Torok, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacAlpine, R.; Percy, N.J.; Ophel-Keller, K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal gut microbiota and metabolites might contribute to the severity of acute myocardial ischemia by impacting the intestinal permeability, oxidative stress, and energy metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, H.; Zhang, L.; Su, Y.; Shi, D.; Xiao, H.; Tian, Y. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Kumar, N.; Khanna, S. Lactobacillus: A promising genus for production of industrially important metabolite. Res. J. Biotechnol. 2019, 14, 167–177. [Google Scholar]
- Wan, Y.; Ma, R.Y.; Zhang, H.Y.; Li, L.; Chai, L.L.; Qi, R.R.; Liu, W.; Li, J.Y.; Li, Y.; Zhan, K. Different non-cage housing systems alter duodenal and cecal microbiota composition in shendan chickens. Front. Vet. Sci. 2021, 8, 728538. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Min, Z.Z.; Yuan, J.M.; Zhang, B.K.; Guo, Y.M. Effects of dietary tryptophan and stocking density on the performance, meat quality, and metabolic status of broilers. J. Anim. Sci. Biotechnol. 2014, 5, 125–131. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, Z.Q.; Tian, W.X.; Yang, Y.; Wang, J.D.; Yang, Y. Effects of different rearing systems on growth, small intestinal morphology and selected indices of fermentation status in broilers. Anim. Sci. J. 2016, 88, 900–908. [Google Scholar] [CrossRef]
- Guardia, S.; Konsak, B.; Combes, S.; Levenez, F.; Cauquil, L.; Guillot, J.F.; Moreau-Vauzelle, C.; Lessire, M.; Juin, H.; Gabriel, I. Effects of stocking density on the growth perfor mance and digestive microbiota of broiler chickens. Poult. Sci. 2011, 90, 1878–1889. [Google Scholar] [CrossRef]
- Sauter, E.A.; Petersen, C.F.; Steele, E.E.; Parkinson, J.F.; Dixon, J.E.; Stroh, R.C. The airborne microflora of poultry houses. Poult. Sci. 1981, 60, 569–574. [Google Scholar] [CrossRef]
- Cengiz, O.; Köksal, B.H.; Tatlı, O.; Sevim, O.; Ahsan, U.; Uner, A.G.; Ulutas, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; et al. Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poult. Sci. 2015, 94, 2395–2403. [Google Scholar] [CrossRef]
- Shi, S.; Qi, Z.; Gu, B.; Cheng, B.; Tu, J.; Song, X.; Shao, Y.; Liu, H.; Qi, K.; Li, S. Analysis of high-throughput sequencing for cecal microbiota diversity and function in hens under different rearing systems. 3 Biotech. 2019, 9, 438. [Google Scholar] [CrossRef]
- Wen, C.; Yan, W.; Sun, C.; Ji, C.; Zhou, Q.; Zhang, D.; Zheng, J.; Yang, N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019, 13, 1422–1436. [Google Scholar] [CrossRef]

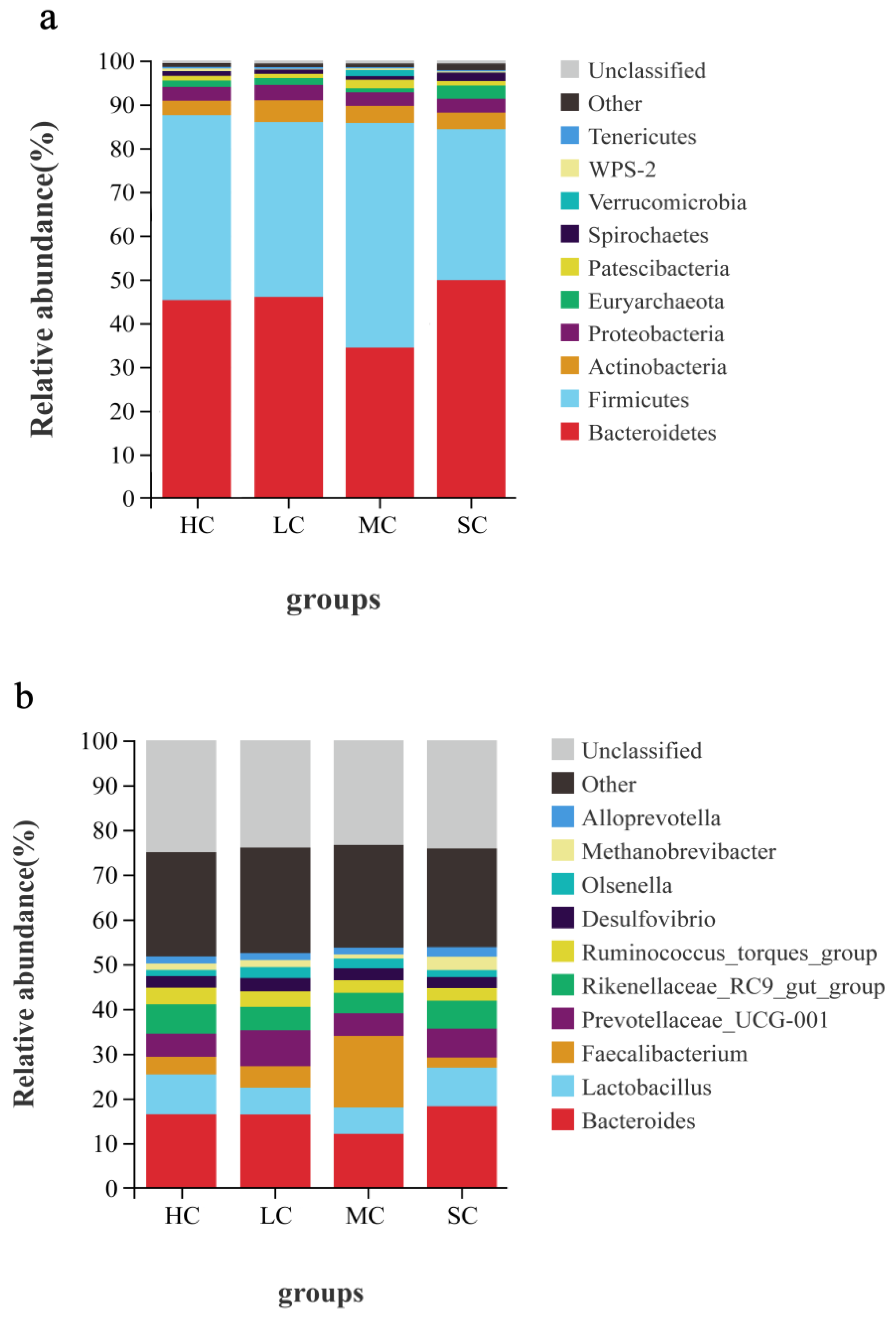
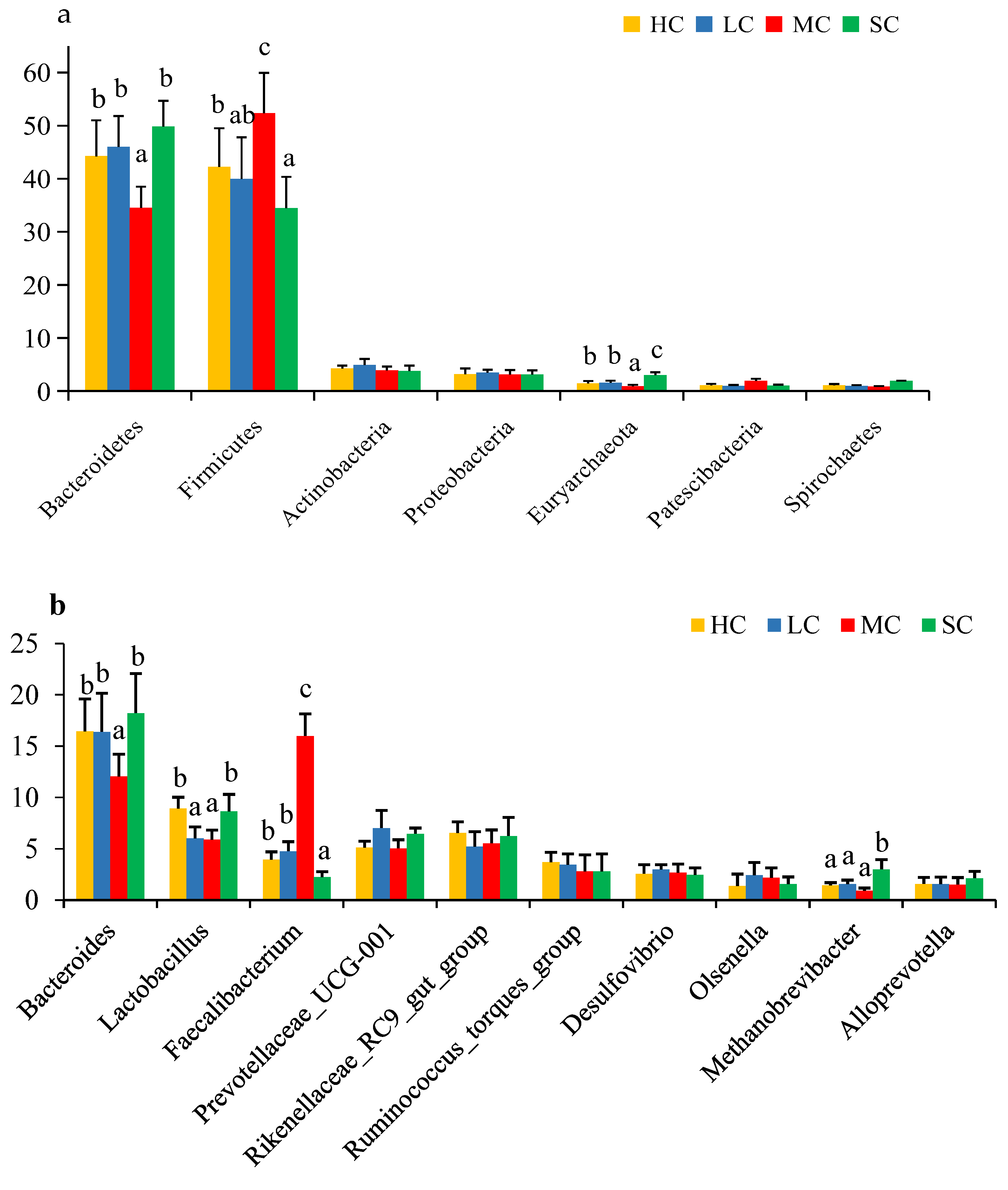
| Item | HC | LC | MC | SC |
|---|---|---|---|---|
| Cage dimension (length × width × height, m) | 0.6 × 2.25 × 0.4 | 0.6 × 1.35 × 0.4 | 0.6 × 0.9 × 0.4 | 0.6 × 0.45 × 0.4 |
| Base area of cage (m2) | 1.35 | 0.81 | 0.54 | 0.27 |
| Birds/cage | 25 | 15 | 10 | 5 |
| Number of cages (replicates) | 6 | 6 | 6 | 6 × 5 |
| Total number of birds | 150 | 90 | 60 | 150 |
| Stocking density (m2 per bird) | 0.054 | |||
| Group | BWG (g) | Laying Rate (%) | ADFI (g) | Egg Weight (g) | FCR |
|---|---|---|---|---|---|
| HC | 285.65 ± 10.78 a | 85.95 ± 1.08 a | 119.29 ± 0.86 ab | 61.95 ± 1.05 | 2.31 ± 0.05 b |
| LC | 227.73 ± 7.12 a | 87.56 ± 0.87 bc | 118.91 ± 0.98 a | 61.75 ± 0.97 | 2.27 ± 0.04 ab |
| MC | 202.24 ± 8.28 a | 89.73 ± 0.96 c | 117.55 ± 1.01 a | 61.62 ± 0.99 | 2.23 ± 0.04 a |
| SC | 454.99 ± 17.12 b | 86.96 ± 1.03 ab | 122.62 ± 0.81 b | 61.66 ± 1.41 | 2.33 ± 0.05 b |
| Item | HC (n = 12) | LC (n = 12) | MC (n = 12) | SC (n = 12) |
|---|---|---|---|---|
| TG (μmol/mL) | 337.06 ± 5.12 a | 359.08 ± 5.61 b | 362.68 ± 4.91 b | 382.82 ± 4.99 c |
| T-CH (μmol/L) | 900.01 ± 13.13 a | 920.65 ± 13.82 ab | 955.35 ± 10.41 b | 998.09 ± 9.78 c |
| MDA (nmol/mL) | 15.72 ± 0.44 | 15.51 ± 0.58 | 15.21 ± 0.69 | 14.38 ± 0.51 |
| GSH-Px (IU/L) | 349.67 ± 4.38 c | 343.65 ± 3.58 c | 322.95 ± 3.22 b | 298.61 ± 3.17 a |
| SOD (U/mL) | 16.51 ± 0.51 | 16.19 ± 0.26 | 16.30 ± 0.31 | 15.78 ± 0.28 |
| CORT (μg/L) | 189.78 ± 4.24 | 193.30 ± 4.08 | 174.16 ± 3.31 | 181.95 ± 4.35 |
| IgG (μg/mL) | 1036.48 ± 21.33 | 1056.51 ± 24.67 | 1077.61 ± 27.68 | 1033.24 ± 22.26 |
| Group | Effective Reads | Average Length | OTUs | Simpson | Shannon | ACE | Chao | Good’s Coverage |
|---|---|---|---|---|---|---|---|---|
| HC (n = 6) | 106337.50 ± 4038.45 | 454.63 ± 1.37 | 1375.33 ± 121.12 | 0.98 ± 0.00 | 7.23 ± 0.17 | 1576.16 ± 106.65 | 1494.55 ± 99.64 | 0.99 ± 0.01 |
| LC (n = 6) | 106424.83 ± 4757.15 | 453.92 ± 2.03 | 1380.33 ± 107.79 | 0.98 ± 0.00 | 7.24 ± 0.15 | 1587.90 ± 110.42 | 1504.42 ± 98.98 | 0.99 ± 0.00 |
| MC (n = 6) | 108906.50 ± 2699.38 | 451.70 ± 3.21 | 1385.33 ± 121.63 | 0.98 ± 0.01 | 7.13 ± 0.19 | 1587.68 ± 139.33 | 1505.09 ± 134.92 | 0.99 ± 0.01 |
| SC (n = 6) | 104360.83 ± 4051.66 | 455.45 ± 1.35 | 1289.33 ± 90.83 | 0.97 ± 0.02 | 6.72 ± 0.42 | 1493.62 ± 102.51 | 1415.16 ± 93.99 | 0.99 ± 0.00 |
| Genus | Body Weight | Laying Rate | FCR | Serum TG | Serum T-CH | Serum GSH-Px |
|---|---|---|---|---|---|---|
| Bacteroides | 0.013 | 0.150 | 0.032 | 0.026 | −0.211 | 0.131 |
| Lactobacillus | 0.350 * | 0.138 | 0.178 | −0.068 | −0.138 | 0.043 |
| Faecalibacterium | 0.070 | 0.282 | 0.269 | −0.091 | −0.130 | 0.214 * |
| Methanobrevibacter | 0.022 | −0.245 | −0.226 | 0.072 | 0.069 | −0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Du, Q.; Wang, D.; Ma, R.; Qi, R.; Yang, R.; Li, X.; Li, J.; Liu, W.; Li, Y.; et al. Effects of Different-Sized Cages on the Production Performance, Serum Parameters, and Caecal Microbiota Composition of Laying Hens. Animals 2023, 13, 266. https://doi.org/10.3390/ani13020266
Wan Y, Du Q, Wang D, Ma R, Qi R, Yang R, Li X, Li J, Liu W, Li Y, et al. Effects of Different-Sized Cages on the Production Performance, Serum Parameters, and Caecal Microbiota Composition of Laying Hens. Animals. 2023; 13(2):266. https://doi.org/10.3390/ani13020266
Chicago/Turabian StyleWan, Yi, Qiang Du, Duobiao Wang, Ruiyu Ma, Renrong Qi, Rongbin Yang, Xin Li, Junying Li, Wei Liu, Yan Li, and et al. 2023. "Effects of Different-Sized Cages on the Production Performance, Serum Parameters, and Caecal Microbiota Composition of Laying Hens" Animals 13, no. 2: 266. https://doi.org/10.3390/ani13020266
APA StyleWan, Y., Du, Q., Wang, D., Ma, R., Qi, R., Yang, R., Li, X., Li, J., Liu, W., Li, Y., & Zhan, K. (2023). Effects of Different-Sized Cages on the Production Performance, Serum Parameters, and Caecal Microbiota Composition of Laying Hens. Animals, 13(2), 266. https://doi.org/10.3390/ani13020266







