Physical Injuries and Hair Corticosterone Concentration in Rabbit Kits from Single- and Group-Housed Does Kept on a Commercial Farm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Handling
2.2. Lesion Scoring and Health Status
2.3. Hair Samples
2.4. Measurement of Hair Corticosterone Concentration
2.4.1. Corticosterone Extraction
2.4.2. Corticosterone Concentration by LC-MS/MS
2.5. Statistical Analysis
2.5.1. Lesions
2.5.2. Hair Corticosterone Concentration
3. Results
3.1. Body Weight, Lesions, and Health Status
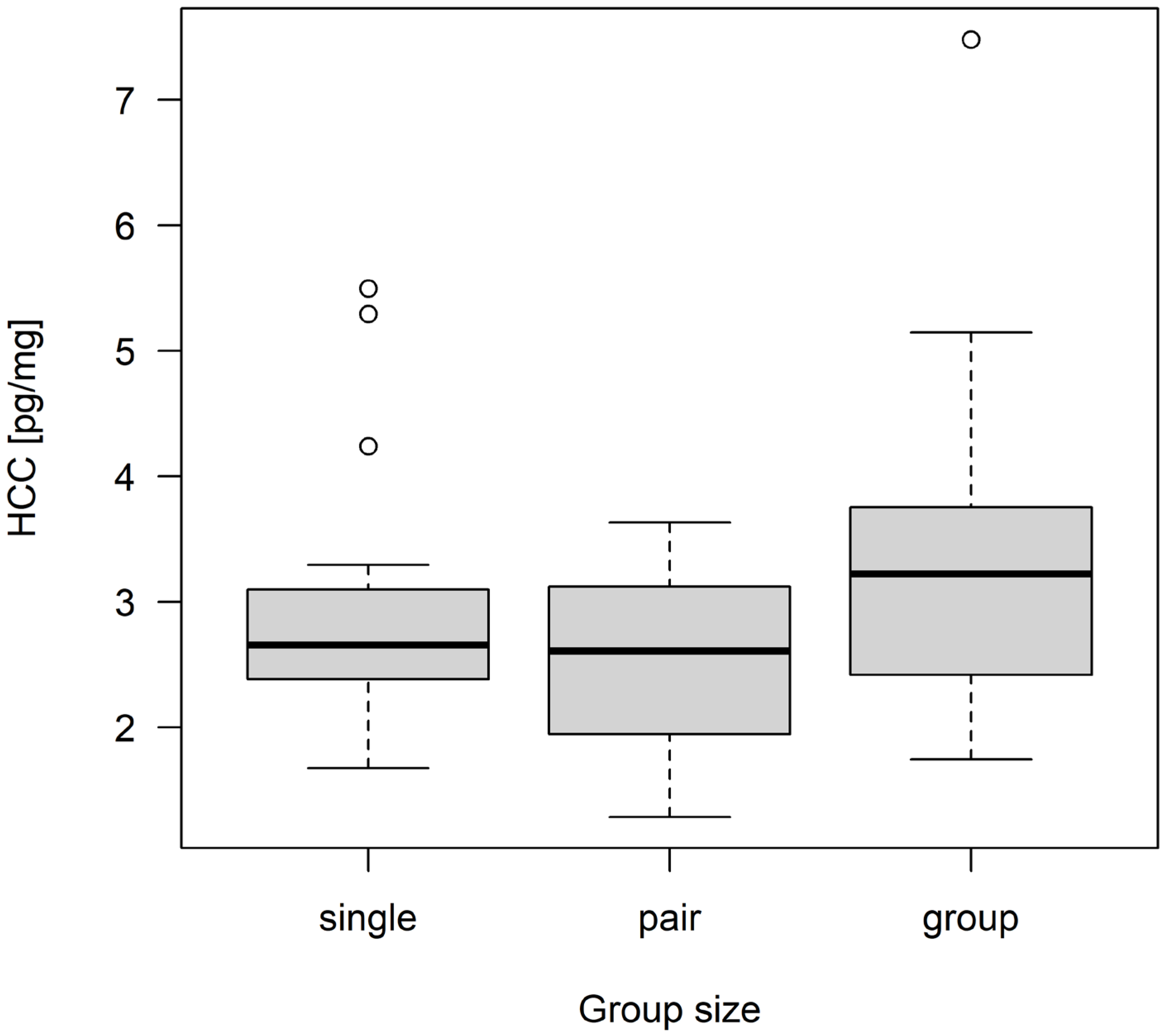
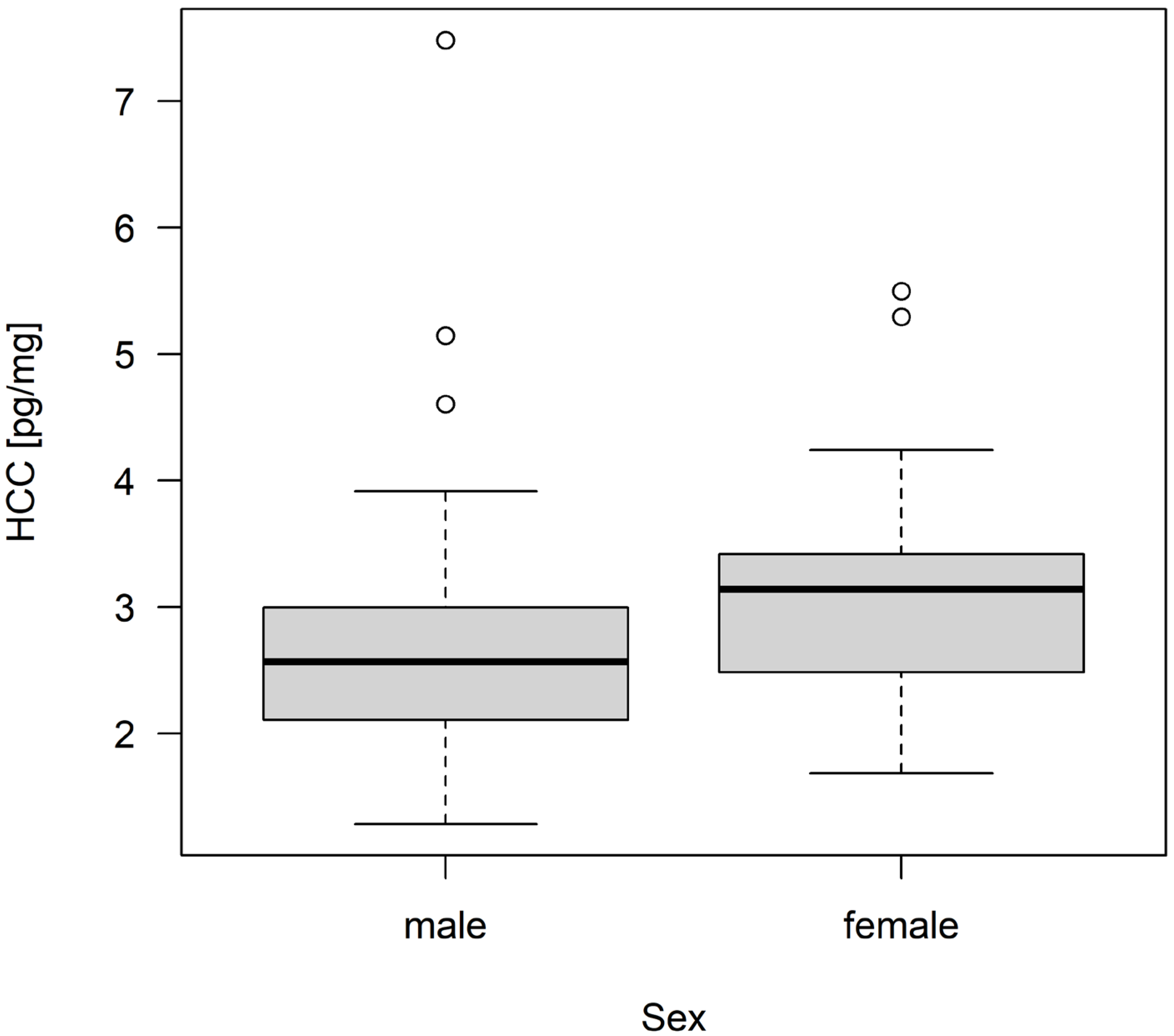
3.2. Hair Corticosterone Concentration
4. Discussion
4.1. Lesions
4.2. Hair Corticosterone Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, L.; Garner, J.P.; Mench, J.A. A Behavioral Comparison of New Zealand White Rabbits (Oryctolagus Cuniculus) Housed Individually or in Pairs in Conventional Laboratory Cages. Appl. Anim. Behav. Sci. 2004, 85, 121–139. [Google Scholar] [CrossRef]
- Podberscek, A.L.; Blackshaw, J.K.; Beattie, A.W. The Behaviour of Group Penned and Individually Caged Laboratory Rabbits. Appl. Anim. Behav. Sci. 1991, 28, 353–363. [Google Scholar] [CrossRef]
- Verga, M.; Luzi, F.; Carenzi, C. Effects of Husbandry and Management Systems on Physiology and Behaviour of Farmed and Laboratory Rabbits. Horm. Behav. 2007, 52, 122–129. [Google Scholar] [CrossRef]
- Szendrő, Z.; Mikó, A.; Odermatt, M.; Gerencsér, Z.; Radnai, I.; Dezséry, B.; Garai, É.; Nagy, I.; Szendrő, K.; Matics, Z. Comparison of Performance and Welfare of Single-Caged and Group-Housed Rabbit Does. Animal 2013, 7, 463–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DiVincenti, L.; Rehrig, A.N. The Social Nature of European Rabbits (Oryctolagus Cuniculus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 729–736. [Google Scholar] [PubMed]
- Andrist, C.A.; Bigler, L.; Würbel, H.; Roth, B.A. Masking Odour When Regrouping Rabbit Does: Effect on Aggression, Stress and Lesions. Livest. Sci. 2014, 170, 150–157. [Google Scholar] [CrossRef]
- Buijs, S.; Maertens, L.; Hermans, K.; Vangeyte, J.; Tuyttens, F.A.M. Behaviour, Wounds, Weight Loss and Adrenal Weight of Rabbit Does as Affected by Semi-Group Housing. Appl. Anim. Behav. Sci. 2015, 172, 44–51. [Google Scholar] [CrossRef]
- Mirabito, L.; Galliot, P.; Souchet, C.; Dumont, F.; Thomeret, F. Group Housing of Rabbit Does: Zootechnical Traits. In Proceedings of the 11émes Jorn. Rech. Cunicole, Paris, France, 22–23 November 2005; pp. 53–56. [Google Scholar]
- Pollesel, M.; Tassinari, M.; Frabetti, A.; Fornasini, D.; Cavallini, D. Effect of Does Parity Order on Litter Homogeneity Parameters. Ital. J. Anim. Sci. 2020, 19, 1188–1194. [Google Scholar] [CrossRef]
- Rommers, J.M.; Kemp, B.; Houwers, H.W.; Gunnink, H.; de Jong, I.C. Description of Nestbox Visits and Suckling Events in a Group Housing System for Rabbit Does as Compared to Individual Cages. World Rabbit Sci. 2012, 20, 231–240. [Google Scholar] [CrossRef]
- Zomeño, C.; Birolo, M.; Zuffellato, A.; Xiccato, G.; Trocino, A. Aggressiveness in Group-Housed Rabbit Does: Influence of Group Size and Pen Characteristics. Appl. Anim. Behav. Sci. 2017, 194, 79–85. [Google Scholar] [CrossRef]
- Mykytowycz, R.; Dudzinski, M.L. Aggressive and Protective Behaviour of Adult Rabbits Oryctolagus Cuniculus (L.) Towards Juveniles. Behaviour 1972, 43, 97–120. [Google Scholar] [CrossRef]
- Buijs, S.; Tuyttens, F.A.M. Evaluating the Effect of Semi-Group Housing of Rabbit Does on Their Offspring’s Fearfulness: Can We Use the Open-Field Test? Appl. Anim. Behav. Sci. 2015, 162, 58–66. [Google Scholar] [CrossRef]
- Buijs, S.; Vangeyte, J.; Tuyttens, F.A.M. Effects of Communal Rearing and Group Size on Breeding Rabbits’ Post-Grouping Behaviour and Its Relation to Ano-Genital Distance. Appl. Anim. Behav. Sci. 2016, 182, 53–60. [Google Scholar] [CrossRef][Green Version]
- Heimbürge, S.; Kanitz, E.; Otten, W. The Use of Hair Cortisol for the Assessment of Stress in Animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Wiechers, D.-H.; Brunner, S.; Herbrandt, S.; Kemper, N.; Fels, M. Analysis of Hair Cortisol as an Indicator of Chronic Stress in Pigs in Two Different Farrowing Systems. Front. Vet. Sci. 2021, 8, 605078. [Google Scholar] [CrossRef]
- Bechshøft, T.; Sonne, C.; Dietz, R.; Born, E.W.; Novak, M.A.; Henchey, E.; Meyer, J.S. Cortisol Levels in Hair of East Greenland Polar Bears. Sci. Total Environ. 2011, 409, 831–834. [Google Scholar] [CrossRef]
- Esposito, L.; Auletta, L.; Ciani, F.; Pelagalli, A.; Pasolini, M.P.; Lamagna, B.; Piscopo, N.; Amici, A. Hair Cortisol Levels in Captive Brown Hare (Lepus Europaeus): Potential Effect of Sex, Age, and Breeding Technology. Eur. J. Wildl. Res. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Burnard, C.; Ralph, C.; Hynd, P.; Hocking Edwards, J.; Tilbrook, A. Hair Cortisol and Its Potential Value as a Physiological Measure of Stress Response in Human and Non-Human Animals. Anim. Prod. Sci. 2017, 57, 401–414. [Google Scholar] [CrossRef]
- Meyer, J.S.; Novak, M.A. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef] [PubMed]
- Comin, A.; Zufferli, V.; Peric, T.; Canavese, F.; Barbetta, D.; Prandi, A. Hair Cortisol Levels Determined at Different Body Sites in the New Zealand White Rabbit. World Rabbit Sci. 2012, 20, 149–154. [Google Scholar] [CrossRef][Green Version]
- Peric, T.; Comin, A.; Corazzin, M.; Montillo, M.; Canavese, F.; Stebel, M.; Prandi, A. Relocation and Hair Cortisol Concentrations in New Zealand White Rabbits. J. Appl. Anim. Welf. Sci. 2017, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szeto, A.; Gonzales, J.A.; Spitzer, S.B.; Levine, J.E.; Zaias, J.; Saab, P.G.; Schneiderman, N.; McCabe, P.M. Circulating Levels of Glucocorticoid Hormones in WHHL and NZW Rabbits: Circadian Cycle and Response to Repeated Social Encounter. Psychoneuroendocrinology 2004, 29, 861–866. [Google Scholar] [CrossRef]
- Bill, J.; Kirschbaum, C.; Rauterberg, S.L.; Kemper, N.; Fels, M. Limits to the Usage of Hair Glucocorticoids to Detect Stress in Rabbits. In Proceedings of the UFAW International Symposium 2019, Brügge, Belgien, 3–4 July 2019; p. 55. [Google Scholar]
- Bill, J.; Rauterberg, S.L.; Herbrandt, S.; Ligges, U.; Kemper, N.; Fels, M. Agonistic Behavior and Social Hierarchy in Female Domestic Rabbits Kept in Semi-Groups. J. Vet. Behav. 2020, 38, 21–31. [Google Scholar] [CrossRef]
- Stalder, T.; Steudte, S.; Miller, R.; Skoluda, N.; Dettenborn, L.; Kirschbaum, C. Intraindividual Stability of Hair Cortisol Concentrations. Psychoneuroendocrinology 2012, 37, 602–610. [Google Scholar] [CrossRef]
- Gao, W.; Stalder, T.; Foley, P.; Rauh, M.; Deng, H.; Kirschbaum, C. Quantitative Analysis of Steroid Hormones in Human Hair Using a Column-Switching LC-APCI-MS/MS Assay. J. Chromatogr. B 2013, 928, 1–8. [Google Scholar] [CrossRef]
- Koren, L.; Whiteside, D.; Fahlman, Å.; Ruckstuhl, K.; Kutz, S.; Checkley, S.; Dumond, M.; Wynne-Edwards, K. Cortisol and Corticosterone Independence in Cortisol-Dominant Wildlife. Gen. Comp. Endocrinol. 2012, 177, 113–119. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Version 4.1.1. Available online: https://www.R-project.org/ (accessed on 17 January 2022).
- Fahrmeir, L.; Kneib, T.; Lang, S.; Marx, B. Regression—Models, Methods and Applications; Springer: Berlin, Heidelberg, 2013; pp. 148 & 270–271. [Google Scholar]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2021, R package version 1.7.0. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 17 January 2022).
- Whary, M.; Peper, R.; Borkowski, G.; Lawrence, W.; Ferguson, F. The Effects of Group Housing on the Research Use of the Laboratory Rabbit. Lab. Anim. 1993, 27, 330–341. [Google Scholar] [CrossRef]
- Nelson, E.W. The Rabbits of North America, 29th ed.; US Government Printing Office: Washington, DC, USA, 1909; ISBN 3663537137.
- Perdue, K.A.; Shaw, R.E.; Mage, R.G. Declawing of Neonatal Rabbits Destined for Use in Animal Biosafety Level 4 Containment Studies. J. Am. Assoc. Lab. Anim. Sci. 2000, 39, 13–18. [Google Scholar]
- Azevedo, A.; Wauters, J.; Kirschbaum, C.; Serra, R.; Rivas, A.; Jewgenow, K. Sex Steroids and Glucocorticoid Ratios in Iberian Lynx Hair. Conserv. Physiol. 2020, 8, coaa075. [Google Scholar] [CrossRef]
- Hein, A.; Baumgartner, K.; von Fersen, L.; Bechshoft, T.; Woelfing, B.; Kirschbaum, C.; Mastromonaco, G.; Greenwood, A.D.; Siebert, U. Analysis of Hair Steroid Hormones in Polar Bears (Ursus Maritimus) via Liquid Chromatography-Tandem Mass Spectrometry: Comparison with Two Immunoassays and Application for Longitudinal Monitoring in Zoos. Gen. Comp. Endocrinol. 2021, 310, 113837. [Google Scholar] [CrossRef]
- Koren, L.; Bryan, H.; Matas, D.; Tinman, S.; Fahlman, Å.; Whiteside, D.; Smits, J.; Wynne-Edwards, K. Towards the Validation of Endogenous Steroid Testing in Wildlife Hair. J. Appl. Ecol. 2019, 56, 547–561. [Google Scholar] [CrossRef]
- Bill, J.; Rauterberg, S.; Kemper, N.; Fels, M. Auswirkungen Einer Gruppenhaltung von Zuchthäsinnen Auf Die Gesundheit Und Stressbelastung Ihrer Jungtiere. Talk. In Proceedings of the Tagungsband Internationale DVG-Fachtagung zum Thema Tierschutz, München, Germany, 15–17 March 2018; pp. 88–98. [Google Scholar]
- Trocino, A.; Filiou, E.; Tazzoli, M.; Bertotto, D.; Negrato, E.; Xiccato, G. Behaviour and Welfare of Growing Rabbits Housed in Cages and Pens. Livest. Sci. 2014, 167, 305–314. [Google Scholar] [CrossRef]
- Michelena, P.; Pillot, M.H.; Henrion, C.; Toulet, S.; Boissy, A.; Bon, R. Group Size Elicits Specific Physiological Response in Herbivores. Biol. Lett. 2012, 8, 537–539. [Google Scholar] [CrossRef]
- Scott, K.; Heistermann, M.; Cant, M.A.; Vitikainen, E.I.K. Group Size and Visitor Numbers Predict Faecal Glucocorticoid Concentrations in Zoo Meerkats. R. Soc. Open Sci. 2017, 4, 161017. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, S.; Blas, J.; Marchant, T.A.; Moreno, S. Physiological Stress Levels Predict Survival Probabilities in Wild Rabbits. Horm. Behav. 2007, 51, 313–320. [Google Scholar] [CrossRef]
- Monclús, R.; Rödel, H.G.; Palme, R.; Von Holst, D.; de Miguel, J. Non-Invasive Measurement of the Physiological Stress Response of Wild Rabbits to the Odour of a Predator. Chemoecology 2006, 16, 25–29. [Google Scholar] [CrossRef]
- Macbeth, B.J.; Cattet, M.R.L.; Obbard, M.E.; Middel, K.; Janz, D.M. Evaluation of Hair Cortisol Concentration as a Biomarker of Long-Term Stress in Free-Ranging Polar Bears. Wildl. Soc. Bull. 2012, 36, 747–758. [Google Scholar] [CrossRef]
- Lafferty, D.J.R.; Laudenslager, M.L.; Mowat, G.; Heard, D.; Belant, J.L. Sex, Diet, and the Social Environment: Factors Influencing Hair Cortisol Concentration in Free-Ranging Black Bears (Ursus Americanus). PLoS ONE 2015, 10, e0141489. [Google Scholar] [CrossRef]
- Fourie, N.H.; Brown, J.L.; Jolly, C.J.; Phillips-Conroy, J.E.; Rogers, J.; Bernstein, R.M. Sources of Variation in Hair Cortisol in Wild and Captive Non-Human Primates. Zoology 2016, 119, 119–125. [Google Scholar] [CrossRef]
- Carlitz, E.H.D.; Runge, J.N.; König, B.; Winkler, L.; Kirschbaum, C.; Gao, W.; Lindholm, A.K. Steroid Hormones in Hair Reveal Sexual Maturity and Competition in Wild House Mice (Mus Musculus Domesticus). Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stewart, N.D.; Mastromonaco, G.F.; Burness, G. No Island-Effect on Glucocorticoid Levels for a Rodent from a near-Shore Archipelago. PeerJ 2020, 8, e8590. [Google Scholar] [CrossRef]
- Scorrano, F.; Carrasco, J.; Pastor-Ciurana, J.; Belda, X.; Rami-Bastante, A.; Bacci, M.L.; Armario, A. Validation of the Long-Term Assessment of Hypothalamic-Pituitary-Adrenal Activity in Rats Using Hair Corticosterone as a Biomarker. FASEB J. 2015, 29, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, B.J.; Cattet, M.R.L.; Stenhouse, G.B.; Gibeau, M.L.; Janz, D.M. Hair Cortisol Concentration as a Noninvasive Measure of Long-Term Stress in Free-Ranging Grizzly Bears (Ursus Arctos): Considerations with Implications for Other Wildlife. Can. J. Zool. 2010, 88, 935–949. [Google Scholar] [CrossRef]
- Waterhouse, M.D.; Sjodin, B.; Ray, C.; Erb, L.; Wilkening, J.; Russello, M.A. Individual-Based Analysis of Hair Corticosterone Reveals Factors Influencing Chronic Stress in the American Pika. Ecol. Evol. 2017, 7, 4099–4108. [Google Scholar] [CrossRef] [PubMed]
- Janicki, Z.; Konjević, D.; Pintur, K.; Severin, K.; Slavica, A.; Mašek, T. Non-Invasive Monitoring of Cortisol Metabolites Level in Farmed Brown Hare (Lepus Europaeus). Vet. Arh. 2006, 76, 251–257. [Google Scholar]
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Krebs, C.J.; Boonstra, R. The Sensitive Hare: Sublethal Effects of Predator Stress on Reproduction in Snowshoe Hares. J. Anim. Ecol. 2009, 78, 1249–1258. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Krebs, C.J.; Boonstra, R. The Ghosts of Predators Past: Population Cycles and the Role of Maternal Programming under Fluctuating Predation Risk. Ecology 2010, 91, 2983–2994. [Google Scholar] [CrossRef]
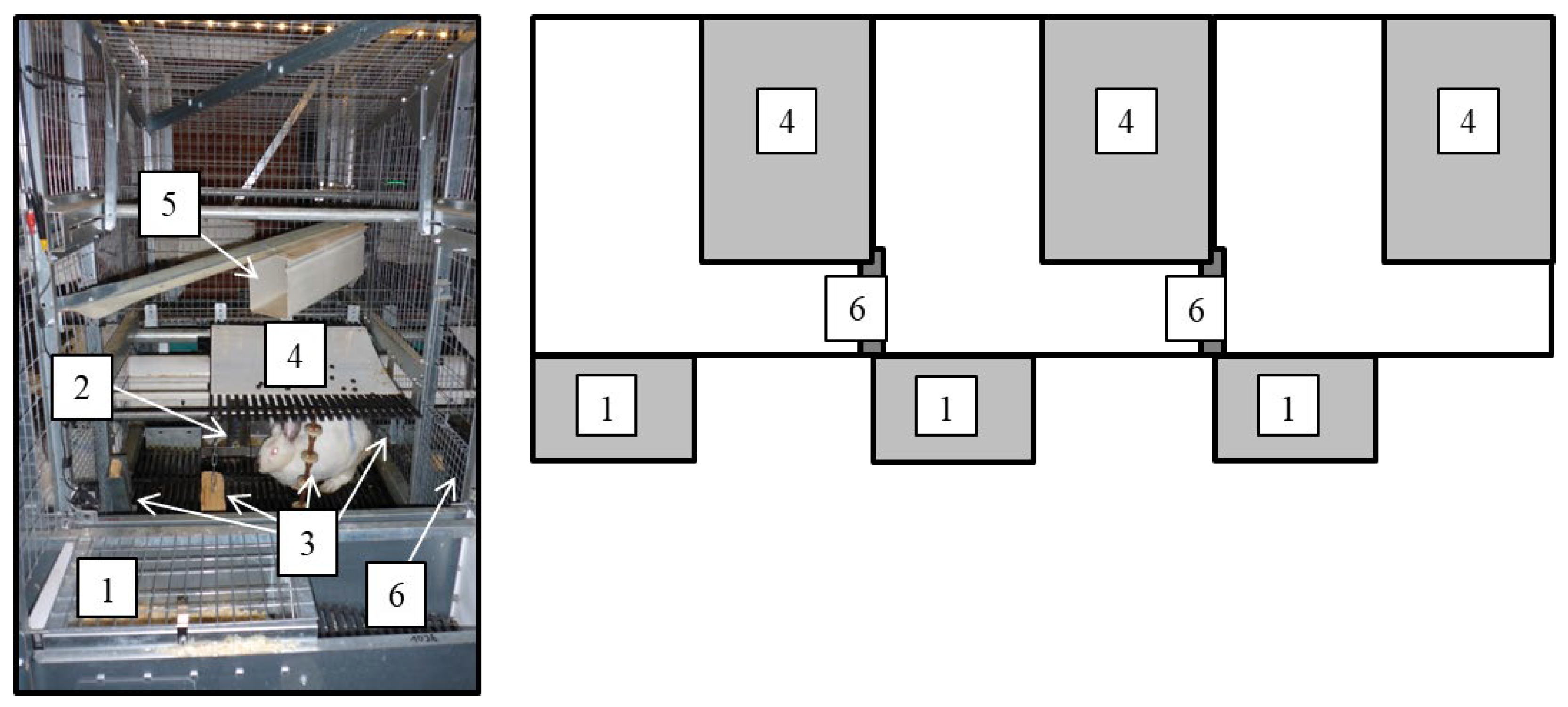
| Housing Treatment | Pen No. | No. of Male Kits Used for the Study | No. of Female Kits Used for the Study | Total No. of Kits per Pen |
|---|---|---|---|---|
| single | 1 | 4 | 4 | 8 |
| single | 2 | 6 | 0 | 9 |
| single | 3 | 0 | 6 | 9 |
| pair | 4/5 | 5 | 5 | 15 |
| pair | 6/7 | 5 | 5 | 14 |
| group of three | 8/9/10 | 10 | 10 | 26 |
| Estimate | Odds Ratio | Std. Error | |
|---|---|---|---|
| Intercept | 1.8855 | 6.5896 | 2.0312 |
| weight | −0.0042 | 0.9958 | 0.0028 |
| pair (single) | 1.8827 | 6.5715 | 0.8197 |
| group of three (single) | 1.7323 | 5.6538 | 0.7164 |
| group of three (pair) | −0.1504 | 0.8603 | 0.7563 |
| Model Term | df1 | df2 | F-Ratio | p-Value |
|---|---|---|---|---|
| group size | 2 | inf | 3.659 | 0.0258 * |
| weight | 1 | inf | 2.232 | 0.1352 |
| Contrast | Estimate | Odds Ratio | SE | z-Ratio | p-Value |
|---|---|---|---|---|---|
| pair—single | 1.88 | 6.57 | 0.820 | 2.297 | 0.0562 |
| group of three—single | 1.73 | 5.65 | 0.716 | 2.418 | 0.0413 * |
| group of three—pair | −0.15 | 0.86 | 0.756 | −0.199 | 0.9784 |
| Estimate | Std. Error | |
|---|---|---|
| Intercept | 0.9460 | 0.1120 |
| female (male) | 0.1265 | 0.0882 |
| with symptoms (healthy) | −0.0257 | 0.0963 |
| lesions (no lesions) | −0.0221 | 0.0900 |
| pair (single) | −0.1033 | 0.1071 |
| group of three (single) | 0.1312 | 0.1077 |
| group of three (pair) | 0.2345 | 0.1024 |
| Model Term | df1 | df2 | F-Ratio | p-Value |
|---|---|---|---|---|
| group size | 2 | 54 | 2.629 | 0.0814 |
| sex | 1 | 54 | 2.060 | 0.1570 |
| health status | 1 | 54 | 0.071 | 0.7908 |
| lesion | 1 | 54 | 0.060 | 0.8072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hube, D.; Bill, J.; Knop, E.S.; Herbrandt, S.; Kemper, N.; Fels, M. Physical Injuries and Hair Corticosterone Concentration in Rabbit Kits from Single- and Group-Housed Does Kept on a Commercial Farm. Animals 2023, 13, 196. https://doi.org/10.3390/ani13020196
Hube D, Bill J, Knop ES, Herbrandt S, Kemper N, Fels M. Physical Injuries and Hair Corticosterone Concentration in Rabbit Kits from Single- and Group-Housed Does Kept on a Commercial Farm. Animals. 2023; 13(2):196. https://doi.org/10.3390/ani13020196
Chicago/Turabian StyleHube, Dana, Joana Bill, Eric Samuel Knop, Swetlana Herbrandt, Nicole Kemper, and Michaela Fels. 2023. "Physical Injuries and Hair Corticosterone Concentration in Rabbit Kits from Single- and Group-Housed Does Kept on a Commercial Farm" Animals 13, no. 2: 196. https://doi.org/10.3390/ani13020196
APA StyleHube, D., Bill, J., Knop, E. S., Herbrandt, S., Kemper, N., & Fels, M. (2023). Physical Injuries and Hair Corticosterone Concentration in Rabbit Kits from Single- and Group-Housed Does Kept on a Commercial Farm. Animals, 13(2), 196. https://doi.org/10.3390/ani13020196






