Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
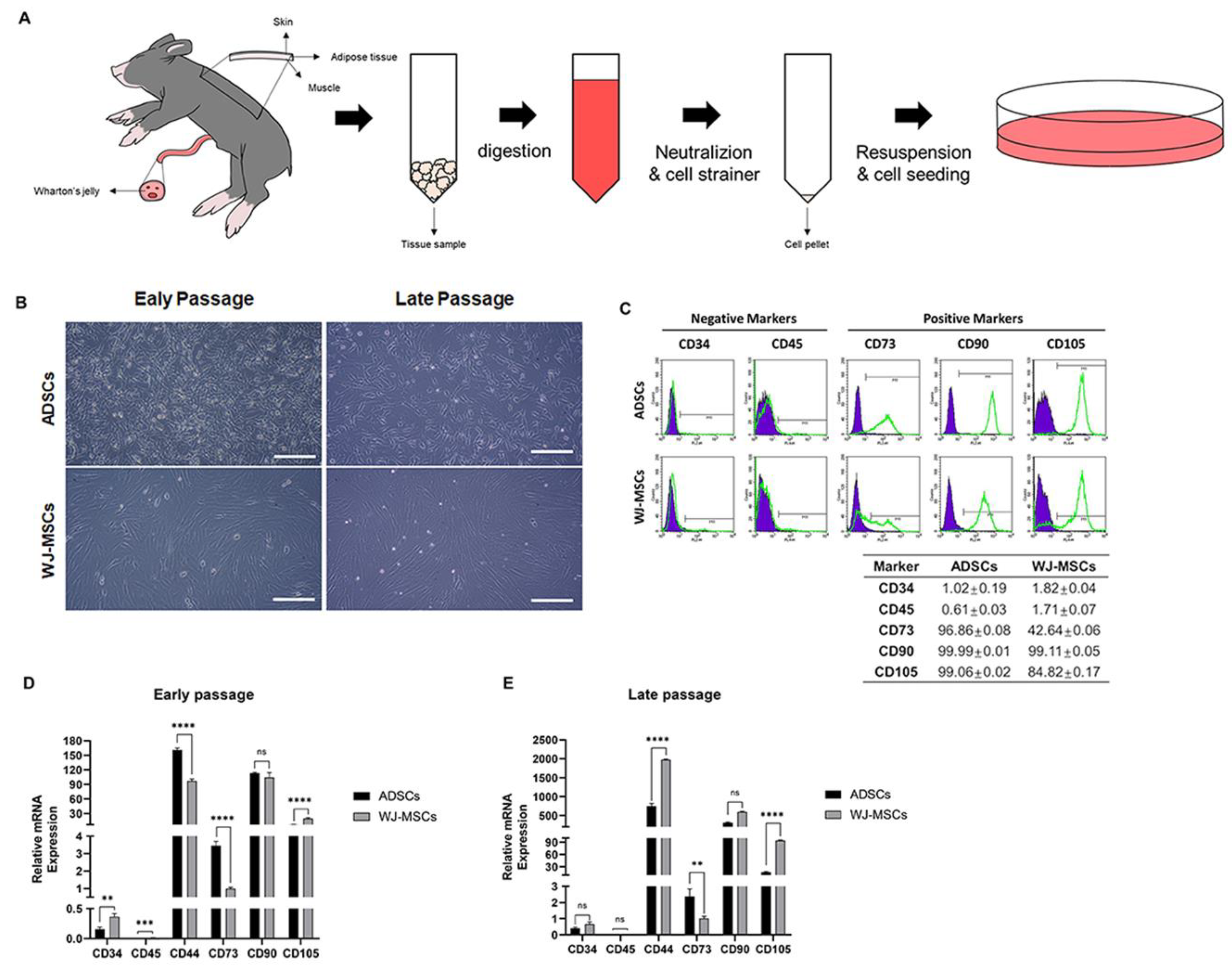
2.1. Isolation and Culture of Porcine Wharton’s Jelly MSCs (WJ-MSCs) and Adipose Derived-Stem Cells (ADSCs)
2.2. Flow Cytometry
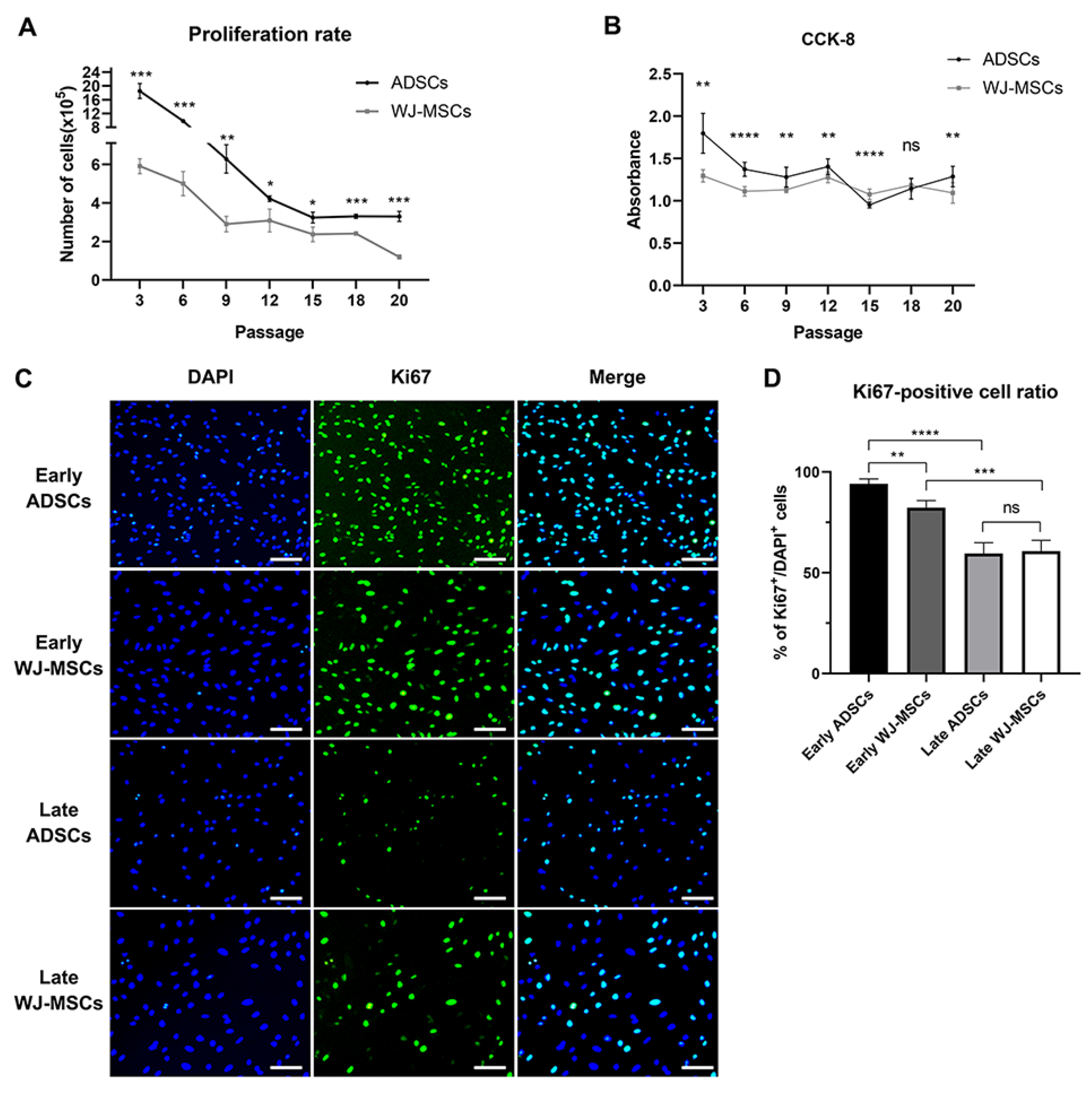
2.3. Cell Proliferation Analysis
2.4. Cell Counting Assay Using Kit-8 (CCK-8)
2.5. Immunocytochemistry
2.6. Adipogenic, Chondrogenic, and Osteogenic Differentiation and Staining
2.7. RNA Isolation and RT-qPCR
2.8. Oxygen Consumption Rate Analysis
2.9. Statistical Analysis
3. Results
3.1. Characterization of Porcine ADSCs and WJ-MSCs
3.2. Comparison of Proliferation Rate between ADSCs and WJ-MSCs
3.3. Characterization of Differentiation Potential of ADSCs and WJ-MSCs
3.4. Comparative Analysis of Oxidative Phosphorylation in ADSCs and WJ-MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elisa, M.; Massimo, B.; Aaron, M.; Shanna M., W.; Walter L., H.; Matthew B., W. Morphological and transcriptomic comparison of adipose and bone marrow derived porcine stem cells. Open Tissue Eng. Regen. Med. J. 2009, 2. [Google Scholar]
- Tomar, N.R.; Bhat, I.A.; Bharti, M.K.; John, J.K.; Sharma, V.; Chandra, V.; Sharma, G.T.; Saikumar, G. Isolation and propagation of classical swine fever virus in porcine Wharton’s Jelly mesenchymal stem cells. Anim. Biotechnol. 2022, 33, 629–637. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Dawson, H.D.; Lunney, J.K. Porcine cluster of differentiation (CD) markers 2018 update. Res. Vet. Sci. 2018, 118, 199–246. [Google Scholar] [CrossRef]
- Liu, R.; Jia, W.; Zou, H.; Wang, X.; Ren, Y.; Zhao, J.; Wang, L.; Li, M.; Qi, Y.; Shen, Y. Expression of CD44 and CD29 by PEComa cells suggests their possible origin of mesenchymal stem cells. Int. J. Clin. Exp. Pathol. 2015, 8, 13023. [Google Scholar]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar]
- Li, L.; Yuan, Y.; Dong, Y. Comparison of Stemness and Immunogenicity of Osteo-Differentiated Mesenchymal Stem Cells Derived from Rabbit Bone Marrow and Wharton’s Jelly. J. Biomater. Tissue Eng. 2017, 7, 1326–1335. [Google Scholar] [CrossRef]
- Noël, D.; Caton, D.; Roche, S.; Bony, C.; Lehmann, S.; Casteilla, L.; Jorgensen, C.; Cousin, B. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008, 314, 1575–1584. [Google Scholar] [CrossRef]
- Lv, F.; Lu, M.; MC Cheung, K.; YL Leung, V.; Zhou, G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr. Stem Cell Res. Ther. 2012, 7, 389–399. [Google Scholar] [CrossRef]
- Rochette, L.; Mazini, L.; Malka, G.; Zeller, M.; Cottin, Y.; Vergely, C. The crosstalk of adipose-derived stem cells (ADSC), oxidative stress, and inflammation in protective and adaptive responses. Int. J. Mol. Sci. 2020, 21, 9262. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Li, L.; Peng, Y.; Chen, P.; Huang, H.; Guo, Y.; Xia, X.; Wang, Y.; Wang, H. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PloS ONE 2013, 8, e59020. [Google Scholar] [CrossRef]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial dynamics in stem cells and differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Noda, S.; Kawashima, N.; Yamamoto, M.; Hashimoto, K.; Nara, K.; Sekiya, I.; Okiji, T. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 2019, 9, 5430. [Google Scholar] [CrossRef]
- Hendrijantini, N.; Hartono, P. Phenotype characteristics and osteogenic differentiation potential of human mesenchymal stem cells derived from amnion membrane (HAMSCs) and umbilical cord (HUC-MSCs). Acta Inform. Medica 2019, 27, 72. [Google Scholar] [CrossRef]
- Pham, H.; Tonai, R.; Wu, M.; Birtolo, C.; Chen, M. CD73, CD90, CD105 and Cadherin-11 RT-PCR screening for mesenchymal stem cells from cryopreserved human cord tissue. Int. J. Stem Cells 2018, 11, 26–38. [Google Scholar] [CrossRef]
- Wu, C.-L.; Diekman, B.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2013, 37, 1079–1087. [Google Scholar] [CrossRef]
- Gierloff, M.; Petersen, L.; Oberg, H.-H.; Quabius, E.; Wiltfang, J.; Açil, Y. Adipogenic differentiation potential of rat adipose tissue-derived subpopulations of stromal cells. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1427–1435. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chen, C.-H.; Wang, J.-Y.; Lin, Y.-S.; Chang, J.-K.; Ho, M.-L. Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater. 2018, 66, 224–237. [Google Scholar]
- Wu, S.-C.; Chen, C.-H.; Chang, J.-K.; Fu, Y.-C.; Wang, C.-K.; Eswaramoorthy, R.; Lin, Y.-S.; Wang, Y.-H.; Lin, S.-Y.; Wang, G.-J. Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J. Appl. Physiol. 2013, 114, 1610–1618. [Google Scholar] [PubMed]
- Srinivasan, A.; Chang, S.-Y.; Zhang, S.; Toh, W.S.; Toh, Y.-C. Substrate stiffness modulates the multipotency of human neural crest derived ectomesenchymal stem cells via CD44 mediated PDGFR signaling. Biomaterials 2018, 167, 153–167. [Google Scholar] [PubMed]
- Tan, K.; Zhu, H.; Zhang, J.; Ouyang, W.; Tang, J.; Zhang, Y.; Qiu, L.; Liu, X.; Ding, Z.; Deng, X. CD73 expression on mesenchymal stem cells dictates the reparative properties via its anti-inflammatory activity. Stem Cells Int. 2019, 2019, 12. [Google Scholar]
- Arufe, M.; De la Fuente, A.; Fuentes, I.; De Toro, F.; Blanco, F. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J. Cell. Biochem. 2010, 111, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, J.; Wang, Y.; Pan, J.; Li, S.; Zhu, L.; Guo, C.; Yan, Z. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem. Biophys. Res. Commun. 2016, 474, 338–344. [Google Scholar] [PubMed]
- Asai, S.; Otsuru, S.; Candela, M.E.; Cantley, L.; Uchibe, K.; Hofmann, T.J.; Zhang, K.; Wapner, K.L.; Soslowsky, L.J.; Horwitz, E.M. Tendon progenitor cells in injured tendons have strong chondrogenic potential: The CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells 2014, 32, 3266–3277. [Google Scholar]
- Jiang, T.; Liu, W.; Lv, X.; Sun, H.; Zhang, L.; Liu, Y.; Zhang, W.J.; Cao, Y.; Zhou, G. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials 2010, 31, 3564–3571. [Google Scholar]
- Cleary, M.; Narcisi, R.; Focke, K.; Van der Linden, R.; Brama, P.; van Osch, G. Expression of CD105 on expanded mesenchymal stem cells does not predict their chondrogenic potential. Osteoarthr. Cartil. 2016, 24, 868–872. [Google Scholar]
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016, 13, 5207–5215. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 1–14. [Google Scholar]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 1–7. [Google Scholar]
- Vacanti, V.; Kong, E.; Suzuki, G.; Sato, K.; Canty, J.M.; Lee, T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J. Cell. Physiol. 2005, 205, 194–201. [Google Scholar] [PubMed]
- Li, X.; Wang, X.; Zhang, C.; Wang, J.; Wang, S.; Hu, L. Dysfunction of metabolic activity of bone marrow mesenchymal stem cells in aged mice. Cell Prolif. 2022, 55, e13191. [Google Scholar] [CrossRef] [PubMed]
- Marquez, M.P.; Alencastro, F.; Madrigal, A.; Jimenez, J.L.; Blanco, G.; Gureghian, A.; Keagy, L.; Lee, C.; Liu, R.; Tan, L. The role of cellular proliferation in adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2017, 26, 1578–1595. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar]
- Xiao, L.; Sobue, T.; Esliger, A.; Kronenberg, M.S.; Coffin, J.D.; Doetschman, T.; Hurley, M.M. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010, 47, 360–370. [Google Scholar] [CrossRef]
- Weiss, S.; Hennig, T.; Bock, R.; Steck, E.; Richter, W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol. 2010, 223, 84–93. [Google Scholar]
- Li, L.; Dong, J.; He, Y.; Mao, W.; Tang, H.; Dong, Y.; Lyu, F. Comparative analysis of mesenchymal stromal cells derived from rabbit bone marrow and Wharton’s jelly for adipose tissue engineering. Connect. Tissue Res. 2020, 61, 537–545. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 2013, 15, 330–343. [Google Scholar] [CrossRef]
- Kuhajda, F.P. Fatty-acid synthase and human cancer: New perspectives on its role in tumor biology. Nutrition 2000, 16, 202–208. [Google Scholar] [CrossRef]
- Hu, E.; Tontonoz, P.; Spiegelman, B.M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 9856–9860. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Ishihara, S.; Kawashima, K.; Moriyama, N.; Suetsugu, H.; Kazumori, H.; Okuyama, T.; Rumi, M.; Fukuda, R.; Nagasue, N. Expression of peroxisome proliferator-activated receptor (PPAR) γ in gastric cancer and inhibitory effects of PPARγ agonists. Br. J. Cancer 2000, 83, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Reka, A.K.; Goswami, M.T.; Krishnapuram, R.; Standiford, T.J.; Keshamouni, V.G. Molecular cross-regulation between PPAR-γ and other signaling pathways: Implications for lung cancer therapy. Lung Cancer 2011, 72, 154–159. [Google Scholar] [CrossRef]
- Samulin, J.; Berget, I.; Lien, S.; Sundvold, H. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 147–152. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, S.H.; Jung, Y.; Lee, Y.; Yoon, J.H.; Choi, J.Y.; Hwang, C.Y.; Son, Y.H.; Park, S.S.; Hwang, G.-S. FABP3-mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat. Commun. 2020, 11, 5661. [Google Scholar] [CrossRef]
- Reilly, N.A.; Lutgens, E.; Kuiper, J.; Heijmans, B.T.; Wouter Jukema, J. Effects of fatty acids on T cell function: Role in atherosclerosis. Nat. Rev. Cardiol. 2021, 18, 824–837. [Google Scholar] [CrossRef]
- Angela, M.; Endo, Y.; Asou, H.K.; Yamamoto, T.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat. Commun. 2016, 7, 13683. [Google Scholar] [CrossRef]
- Sato, M.; Evans, B.A.; Sandstrom, A.L.; Chia, L.Y.; Mukaida, S.; Thai, B.S.; Nguyen, A.; Lim, L.; Tan, C.Y.R.; Baltos, J.A.; et al. alpha(1A)-Adrenoceptors activate mTOR signalling and glucose uptake in cardiomyocytes. Biochem. Pharmacol. 2018, 148, 27–40. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Ye, N.; Chen, Y.; Zhang, N.; Sun, Y. Advanced glycation end products-induced mitochondrial energy metabolism dysfunction alters proliferation of human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 15, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Yao, G. Modelling mammalian cellular quiescence. Interface Focus 2014, 4, 20130074. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Wang, R.; Wang, Y.; Kung, C.P.; Weber, J.D.; Patti, G.J. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.A.; Silachev, D.N.; Danilina, T.I.; Goryunov, K.V.; Pevzner, I.B.; Zorova, L.D.; Popkov, V.A.; Chernikov, V.P.; Plotnikov, E.Y.; Sukhikh, G.T.; et al. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells 2021, 10, 1273. [Google Scholar] [CrossRef] [PubMed]
- Nuschke, A.; Rodrigues, M.; Wells, A.W.; Sylakowski, K.; Wells, A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res. Ther. 2016, 7, 179. [Google Scholar] [CrossRef]
- Jitschin, R.; Bottcher, M.; Saul, D.; Lukassen, S.; Bruns, H.; Loschinski, R.; Ekici, A.B.; Reis, A.; Mackensen, A.; Mougiakakos, D. Inflammation-induced glycolytic switch controls suppressivity of mesenchymal stem cells via STAT1 glycosylation. Leukemia 2019, 33, 1783–1796. [Google Scholar] [CrossRef]
- Jeon, R.; Rho, G.-J. Porcine somatic cell nuclear transfer using telomerase reverse transcriptase-transfected mesenchymal stem cells reduces apoptosis induced by replicative senescence. J. Anim. Reprod. Biotechnol. 2020, 35, 215–222. [Google Scholar] [CrossRef]
- Zhu, R.; Yan, T.; Feng, Y.; Liu, Y.; Cao, H.; Peng, G.; Yang, Y.; Xu, Z.; Liu, J.; Hou, W. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021, 31, 1244–1262. [Google Scholar] [CrossRef]
- Song, K.; Dayem, A.A.; Lee, S.; Choi, Y.; Lim, K.M.; Kim, S.; An, J.; Shin, Y.; Park, H.; Jeon, T.-I. Superior therapeutic activity of TGF-β-induced extracellular vesicles against interstitial cystitis. J. Control. Release 2022, 348, 924–937. [Google Scholar] [CrossRef]
- Hong, T.K.; Shin, D.-M.; Choi, J.; Do, J.T.; Han, S.G. Current issues and technical advances in cultured meat production: A review. Food Sci. Anim. Resour. 2021, 41, 355. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence | Product Size (bp) | NCBI |
|---|---|---|---|
| GAPDH | F: ACCCAGAAGACTGTGGATGG | 79 | XM_021091114.1 |
| R: AAGCAGGGATGATGTTCTGG | |||
| β-actin | F: GCAAGAGAGGCATCCTGACC | 182 | XM_021086047.1 |
| R: GGTCATCTTCTCACGGTTGGC | |||
| CD34 | F: GAACCGTCGCAGTTGGAGC | 198 | NM_214086.1 |
| R: GGTTGCCTCGCTGAATGGC | |||
| CD44 | F: ATGGTCGCTACAGCATCTCG | 264 | XM_021085286.1 |
| R: CTTCAGGTGGAGCTGATGCA | |||
| CD45 | F: CTGAAGACCCTCACCTGCTC | 226 | XM_003130596.6 |
| R: GCC TCC ACC TGA ACC ATC AG | |||
| CD73 | F: GAGAACCTGGCTGCTGTGT | 411 | XM_001927095.4 |
| R: CCGACCTTCAACTGCTGGAT | |||
| CD90 | F: CAG AAG GTG ACC AGC CTG AC | 176 | XM_013979447.2 |
| R: GTT CGA GAG CGG TAG GAG TG | |||
| CD105 | F: GTAGCACCAACCACAGCATCG | 128 | NM_214031.1 |
| R: CTGCTCAGTCTCTCCTGCTG | |||
| PPARγ | F: CGACCACTCCCACTCCTTTGAC | 172 | XM_005669788.3 |
| R: CACAGGCTCCACTTTGATGGCA | |||
| C/EBPα | F: GCAGGCAAAGCCAAGAAGTCG | 143 | XM_003127015.4 |
| R: GTCAGCTCCAGCACCTTCTGT | |||
| FAS | F: GTCCTGCTGAAGCCTAACTC | 206 | NM_001099930.1 |
| R: TCCTTGGAACCGTCTGTG | |||
| FABP3 | F: ATGGAGGCAAACTTGTCCAC | 98 | NM_001099931.1 |
| R: ATGGGTGAGTGTCAGGATGAG | |||
| FABP4 | F: CTGGTACAGGTGCAGAAGTGG | 107 | NM_001002817.1 |
| R: CTGGTAGCCGTGACACCTT | |||
| GLUT4 | F: GCTGCCTCCTACGAGATGCT | 145 | NM_001128433.1 |
| R: TGGCCAGCTGGTTGAGTGT | |||
| COL1A1 | F: GTGTCTGCGACAACGGCAATG | 240 | XM_021067155.1 |
| R: GAAGTCCAGGTTGTCCAGGGA | |||
| COL2A1 | F: GCAACTGGGACCAAAGGGAC | 113 | XM_021092611.1 |
| R: CACCTCTGGGTCCTTGTTCAC | |||
| RUNX2 | F: CAGCCTCTTCAGCACAGTGAC | 119 | XM_005666074.3 |
| R: GGCTCACGTCGCTCATCTTG | |||
| DLX5 | F: CCGAGGTGAGAATGGTGAACGG | 165 | NM_001159660.1 |
| R: GTGTTTGCGTCAGTCCCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.Y.; Choi, G.T.; Park, J.; Lee, J.; Do, J.T. Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells. Animals 2023, 13, 2947. https://doi.org/10.3390/ani13182947
Kim GY, Choi GT, Park J, Lee J, Do JT. Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells. Animals. 2023; 13(18):2947. https://doi.org/10.3390/ani13182947
Chicago/Turabian StyleKim, Ga Yeon, Gyu Tae Choi, Jinryong Park, Jeongeun Lee, and Jeong Tae Do. 2023. "Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells" Animals 13, no. 18: 2947. https://doi.org/10.3390/ani13182947
APA StyleKim, G. Y., Choi, G. T., Park, J., Lee, J., & Do, J. T. (2023). Comparative Analysis of Porcine Adipose- and Wharton’s Jelly-Derived Mesenchymal Stem Cells. Animals, 13(18), 2947. https://doi.org/10.3390/ani13182947





