Assessment of Luteal Function Using Rectal Palpation, B-Mode Ultrasonography, and Progesterone Determination to Improve Recipient Selection in Embryo Transfer Programs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
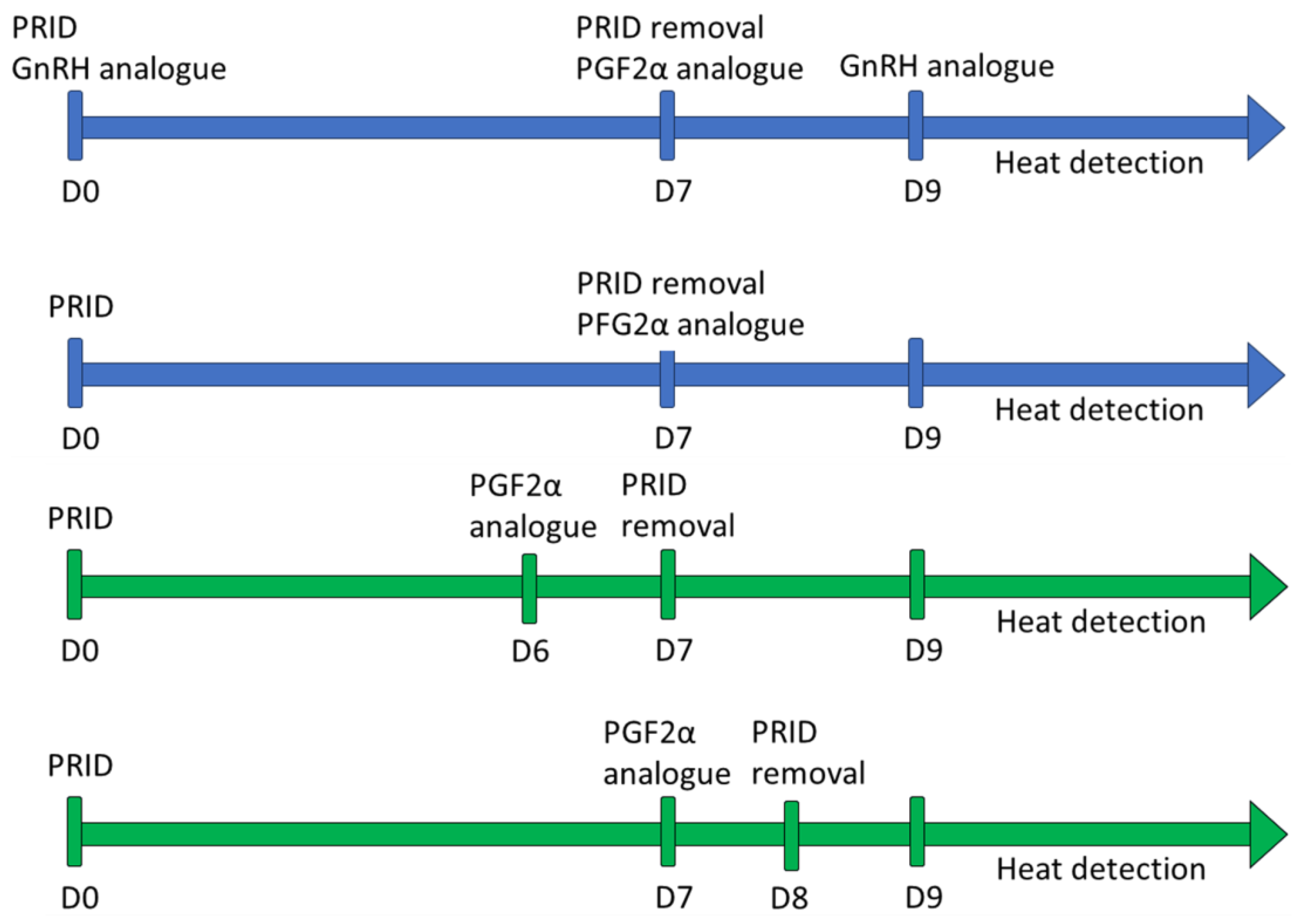
2.2. Synchronization Protocol
2.3. Recipient Selection
2.4. Blood Sample Collection and Analysis
2.5. Embryo Transfer Procedure
2.6. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberio, R.H. Manejo de Donantes y Receptoras. In Biotecnología de la Reproducción; Palma, G.A., Ed.; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2001; pp. 21–26. [Google Scholar]
- Hasler, J.F. Forty Years of Embryo Transfer in Cattle: A Review Focusing on the Journal Theriogenology, the Growth of the Industry in North America, and Personal Reminisces. Theriogenology 2014, 81, 152–169. [Google Scholar] [CrossRef]
- Roper, D.A.; Schrick, F.N.; Edwards, J.L.; Hopkins, F.M.; Prado, T.M.; Wilkerson, J.B.; Saxton, A.M.; Young, C.D.; Smith, W.B. Factors in Cattle Affecting Embryo Transfer Pregnancies in Recipient Animals. Anim. Reprod. Sci. 2018, 199, 79–83. [Google Scholar] [CrossRef]
- Hansen, P.J. The Incompletely Fulfilled Promise of Embryo Transfer in Cattle—Why Aren’t Pregnancy Rates Greater and What Can We Do about It? J. Anim. Sci. 2020, 98, skaa288. [Google Scholar] [CrossRef] [PubMed]
- Pugliesi, G.; Dalmaso de Melo, G.; Silva, J.B.; Carvalhêdo, A.S.; Lopes, E.; de Siqueira Filho, E.; Silva, L.A.; Binelli, M. Use of Color-Doppler Ultrasonography for Selection of Recipients in Timed-Embryo Transfer Programs in Beef Cattle. Theriogenology 2019, 135, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Stroud, B.; Hasler, J.F. Dissecting Why Superovulation and Embryo Transfer Usually Work on Some Farms but Not on Others. Theriogenology 2006, 65, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.L.M.; Demétrio, D.G.B.; Santos, R.M.; Chiari, J.R.; Rodrigues, C.A.; Filho, O.G.S. Factors Potentially Affecting Fertility of Lactating Dairy Cow Recipients. Theriogenology 2006, 65, 192–200. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Torres, C.A.A.; Souza, E.D.; Monteiro, P.L.J.; Arashiro, E.K.N.; Camargo, L.S.A.; Fernandes, C.A.C.; Viana, J.H.M. Pregnancy Rates and Corpus Luteum–Related Factors Affecting Pregnancy Establishment in Bovine Recipients Synchronized for Fixed-Time Embryo Transfer. Theriogenology 2009, 72, 949–958. [Google Scholar] [CrossRef]
- Mann, G.; Lamming, G. Relationships between Maternal Endocrine Environement, Early Embryo Development and Inhibition of the Luteolytic Mechanism in Cows. Reproduction 2001, 121, 175–180. [Google Scholar] [CrossRef]
- Wathes, D.; Taylor, V.; Cheng, Z.; Mann, G. Follicle Growth, Corpus Luteum Function and Their Effects on Embryo Development in Postpartum Dairy Cows. Reproduction 2003, 61, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Jaśkowski, B.M.; Herudzińska, M.; Gehrke, M.; Niżański, W. The Impact of the Cavitary Corpus Luteum on the Blood Progesterone Concentration and Pregnancy Rate of Embryo Recipient Heifers. Theriogenology 2022, 178, 73–76. [Google Scholar] [CrossRef]
- Say, E.; Özmen, M.F.; Sağirkaya, H. The Influence of Corpus Luteum Size on the Conception in Embryo Transfer Recipient Cows. Livest. Stud. 2021, 61, 77–81. [Google Scholar] [CrossRef]
- Yoshida, T.; Seki, M.; Watanabe, N.; Furuta, H.; Yoshimura, I.; Osada, M.; Chiba, K.; Okada, K.; Kawasumi, K.; Ushijima, H. Relation of Reproductive Performances and Rectal Palpation for Luteum Function of Heifers 7° Days after Estrus. Anim. Sci. J. 2012, 83, 207–212. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Torres, C.A.A.; Amorim, L.S.; Souza, E.D.; Camargo, L.S.A.; Fernandes, C.A.C.; Viana, J.H.M. Interrelationships among Morphology, Echotexture, and Function of the Bovine Corpus Luteum during the Estrous Cycle. Anim. Reprod. Sci. 2009, 115, 18–28. [Google Scholar] [CrossRef]
- Utt, M.D.; Johnson, G.L.; Beal, W.E. The Evaluation of Corpus Luteum Blood Flow Using Color-Flow Doppler Ultrasound for Early Pregnancy Diagnosis in Bovine Embryo Recipients. Theriogenology 2009, 71, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, U.; Murillo, A.V.; Becerra, J.J.; Herradón, P.G.; Peña, A.I.; Quintela, L.A. Comparison between Transrectal Palpation, B-Mode and Doppler Ultrasonography to Assess Luteal Function in Holstein Cattle. Front. Vet. Sci. 2023, 10, 1162589. [Google Scholar] [CrossRef]
- Daly, J.; Smith, H.; McGrice, H.A.; Kind, K.L.; van Wettere, W.H.E.J. Towards Improving the Outcomes of Assisted Reproductive Technologies of Cattle and Sheep, with Particular Focus on Recipient Management. Animals 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Jaśkowski, B.M.; Bostedt, H.; Gehrke, M.; Jaśkowski, J.M. Ultrasound Characteristics of the Cavitary Corpus Luteum after Oestrus Synchronization in Heifers in Relation to the Results of Embryo Transfer. Animals 2021, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Wenzinger, B.; Bleul, U. Effect of a Prostaglandin F2α Analogue on the Cyclic Corpus Luteum during Its Refractory Period in Cows. BMC Vet. Res. 2012, 8, 220. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Pulley, S.L.; Mellieon, H.I. Prostaglandin F2α and Gonadotropin-Releasing Hormone Administration Improve Progesterone Status, Luteal Number, and Proportion of Ovular and Anovular Dairy Cows with Corpora Lutea before a Timed Artificial Insemination Program. J. Dairy Sci. 2012, 95, 1831–1844. [Google Scholar] [CrossRef]
- Szelényi, Z.; Győri, D.; Boldizsár, S.; Kovács, L.; Répási, A.; Molnár, L.; Szenci, O. Pregnancy and Stillbirth Losses in Dairy Cows with Singleton and Twin Pregnancies. Acta Vet. Hung. 2019, 67, 115–126. [Google Scholar] [CrossRef]
- Bo, G.A.; Mapletoft, R.J. Evaluation and Classification of Bovine Embryos. Anim. Reprod. 2013, 10, 344–348. [Google Scholar]
- Zemjanis, R. Diagnostic and Therapeutic Tecniques in Animal Reproduction; The Williams and Wilkins Company: Baltimore, MA, USA, 1970. [Google Scholar]
- Hanzen, C.H.; Pieterse, M.; Scenczi, O.; Drost, M. Relative Accuracy of the Identification of Ovarian Structures in the Cow by Ultrasonography and Palpation Per Rectum. Vet. J. 2000, 159, 161–170. [Google Scholar] [CrossRef]
- Rocha, C.C.; Martins, T.; Cardoso, B.O.; Silva, L.A.; Binelli, M.; Pugliesi, G. Ultrasonography-Accessed Luteal Size Endpoint That Most Closely Associates with Circulating Progesterone during the Estrous Cycle and Early Pregnancy in Beef Cows. Anim. Reprod. Sci. 2019, 201, 12–21. [Google Scholar] [CrossRef]
- Acosta, T.J.; Yoshizawa, N.; Ohtani, M.; Miyamoto, A. Local Changes in Blood Flow Within the Early and Midcycle Corpus Luteum after Prostaglandin F 2 Injection in the Cow 1. Biol. Reprod. 2002, 66, 651–658. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Bollwein, H. Evaluation of Bovine Luteal Blood Flow by Using Color Doppler Ultrasonography. Reprod. Biol. 2014, 14, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J. Ultrasonic Imaging and Animal Reproduction; Equiservices Pub: Cross Plains, WI, USA, 1995; ISBN 0964007282. [Google Scholar]
- Yáñez, U.; Becerra, J.J.; Herradón, P.G.; Peña, A.I.; Quintela, L.A. Ecografía Doppler y Su Aplicación En Reproducción Bovina: Revisión. Inf. Tec. Econ. Agrar. 2021, 118, 82–100. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Areas, V.S.; Ghetti, A.M.; Fonseca, J.F.; Palhao, M.P.; Fernandes, C.A.C.; Viana, J.H.M. Color Doppler Flow Imaging for the Early Detection of Nonpregnant Cattle at 20 Days after Timed Artificial Insemination. J. Dairy Sci. 2013, 96, 6461–6472. [Google Scholar] [CrossRef]
- Dubuc, J.; Houle, J.; Rousseau, M.; Roy, J.P.; Buczinski, S. Short Communication: Accuracy of Corpus Luteum Color Flow Doppler Ultrasonography to Diagnose Nonpregnancy in Dairy Cows on Day 21 after Insemination. J. Dairy Sci. 2020, 103, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.G.; Arashiro, E.K.; Ghetti, A.M.; Souza, E.D.; Feres, L.F.; Pfeifer, L.F.; Fonseca, J.F.; Viana, J.H. Vascular and Morphological Features of the Corpus Luteum 12 to 20 Days after Timed Artificial Insemination in Dairy Cattle. J. Dairy Sci. 2019, 102, 5612–5622. [Google Scholar] [CrossRef]
- Herzog, K.; Brockhan-Lüdemann, M.; Kaske, M.; Beindorff, N.; Paul, V.; Niemann, H.; Bollwein, H. Luteal Blood Flow Is a More Appropriate Indicator for Luteal Function during the Bovine Estrous Cycle than Luteal Size. Theriogenology 2010, 73, 691–697. [Google Scholar] [CrossRef]
- Pugliesi, G.; de Melo, G.D.; Ataíde, G.A.; Pellegrino, C.A.G.; Silva, J.B.; Rocha, C.C.; Motta, I.G.; Vasconcelos, J.L.M.; Binelli, M. Use of Doppler Ultrasonography in Embryo Transfer Programs: Feasibility and Field Results. Anim. Reprod. 2018, 15, 239–246. [Google Scholar] [CrossRef]
- Ferraz, P.A.; Burnley, C.; Karanja, J.; Viera-Neto, A.; Santos, J.E.P.; Chebel, R.C.; Galvão, K.N. Factors Affecting the Success of a Large Embryo Transfer Program in Holstein Cattle in a Commercial Herd in the Southeast Region of the United States. Theriogenology 2016, 86, 1834–1841. [Google Scholar] [CrossRef]
- Herzog, K.; Kiossis, E.; Bollwein, H. Examination of Cyclic Changes in Bovine Luteal Echotexture Using Computer-Assisted Statistical Pattern Recognition Techniques. Anim. Reprod. Sci. 2008, 106, 289–297. [Google Scholar] [CrossRef]
- Scully, S.; Evans, A.C.O.; Carter, F.; Duffy, P.; Lonergan, P.; Crowe, M.A. Ultrasound Monitoring of Blood Flow and Echotexture of the Corpus Luteum and Uterus during Early Pregnancy of Beef Heifers. Theriogenology 2015, 83, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Frade, M.C.; Frade, C.; Cordeiro, M.B.; Sá Filho, M.F.d.; Mesquita, F.S.; Nogueira, G.d.P.; Binelli, M.; Membrive, C.M.B. Manifestation of Estrous Behavior and Subsequent Progesterone Concentration at Timed-Embryo Transfer in Cattle Are Positively Associated with Pregnancy Success of Recipients. Anim. Reprod. Sci. 2014, 151, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sá Filho, M.F.; Santos, J.E.P.; Ferreira, R.M.; Sales, J.N.S.; Baruselli, P.S. Importance of Estrus on Pregnancy per Insemination in Suckled Bos Indicus Cows Submitted to Estradiol/Progesterone-Based Timed Insemination Protocols. Theriogenology 2011, 76, 455–463. [Google Scholar] [CrossRef]
- Perez-Marin, C. Formation of Corpora Lutea and Central Luteal Cavities and Their Relationship with Plasma Progesterone Levels and Other Metabolic Parameters in Dairy Cattle. Reprod. Domest. Anim. 2009, 44, 384–389. [Google Scholar] [CrossRef]
- Martinez, M.F.; Adams, G.P.; Kastelic, J.P.; Bergfelt, D.R.; Mapletoft, R.J. Induction of Follicular Wave Emergence for Estrus Synchronization and Artificial Insemination in Heifers. Theriogenology 2000, 54, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Scenna, F.N.; Hockett, M.E.; Towns, T.M.; Saxton, A.M.; Rohrbach, N.R.; Wehrman, M.E.; Schrick, F.N. Influence of a Prostaglandin Synthesis Inhibitor Administered at Embryo Transfer on Pregnancy Rates of Recipient Cows. Prostaglandins Other Lipid Mediat. 2005, 78, 38–45. [Google Scholar] [CrossRef]
- Purcell, S.H.; Beal, W.E.; Gray, K.R. Effect of a CIDR Insert and Flunixin Meglumine, Administered at the Time of Embryo Transfer, on Pregnancy Rate and Resynchronization of Estrus in Beef Cattle. Theriogenology 2005, 64, 867–878. [Google Scholar] [CrossRef]
- Barnes, M.; Kasimanickam, R.; Kasimanickam, V. Effect of Subclinical Endometritis and Flunixin Meglumine Administration on Pregnancy in Embryo Recipient Beef Cows. Theriogenology 2023, 201, 76–82. [Google Scholar] [CrossRef] [PubMed]
| CL Classification | Days of Cycle | Description |
|---|---|---|
| Absence of corpus luteum | 1–2 | Collapsed follicle |
| Hemorrhagic body 1 (HB1) | 2–3 | Soft, friable, developing CL, <1 cm |
| Hemorrhagic body 2 (HB2) | 3–5 | Soft CL, 1–2 cm |
| Hemorrhagic body 3 (HB3) | 6–7 | Consistent CL, >2 cm, still mobile, with palpable crown |
| Corpus luteum 3 (CL3) | 8–17 | Big and firm CL, crown integrated into the ovary |
| Corpus luteum 2 (CL2) | 18–20 | Hard CL, under regression |
| Corpus luteum 1 (CL1) | 20–21 | Very hard CL, <1 cm |
| Serum P4 | CLA | LADCL | MIDCL | |
|---|---|---|---|---|
| Serum P4 | 1 | 0.335 ** | 0.189 | 0.368 ** |
| CLA | - | 1 | 0.758 ** | 0.799 ** |
| LADCL | - | - | 1 | 0.511 ** |
| MIDCL | - | - | - | 1 |
| n | Serum P4 (ng/mL) | CLA (cm2) | LADCL (cm) | MIDCL (cm) | |
|---|---|---|---|---|---|
| Absent CL | 7 | 2.17 ± 1.99 a | - | - | - |
| HB2 | 14 | 7.67 ± 5.70 ab | 3.04 ± 1.10 a | 2.39 ± 0.46 a | 1.53 ± 0.29 a |
| HB3 | 51 | 7.81 ± 4.37 b | 4.16 ± 1.02 b | 2.81 ± 0.43 b | 1.90 ± 0.32 b |
| CL3 | 15 | 7.52 ± 6.14 ab | 3.49 ± 1.01 ab | 2.66 ± 0.67 ab | 1.72 ± 0.36 ab |
| CL2 | 7 | 3.84 ± 2.98 ab | 2.54 ± 1.03 a | 2.27 ± 0.51 ab | 1.35 ± 0.29 a |
| Pregnancy | n | Serum P4 (ng/mL) | CLA (cm2) | LADCL (cm) | MIDCL (cm) |
|---|---|---|---|---|---|
| NO | 27 | 9.02 ± 8.31 | 3.92 ± 1.19 | 2.74 ± 0.42 | 1.87 ± 0.35 |
| YES | 22 | 9.54 ± 7.16 | 4.36 ± 1.00 | 2.93 ± 0.48 | 1.92 ± 0.33 |
| Variable | Pregnant (n = 67) | Not Pregnant (n = 27) |
|---|---|---|
| LADCL (mm) | 28.55 ± 3.73 | 27.86 ± 3.95 |
| MIDCL (mm) | 18.96 ± 3.93 | 19.60 ± 3.99 |
| VOLCL (mm3) | 7148.52 ± 3064.74 | 7165.99 ± 2876.72 |
| %CLOV | 48.11 ± 15.71 | 45.62 ± 11.13 |
| Serum P4 (ng/mL) | 16.30 ± 11.89 | 17.45 ± 13.28 |
| BCS | 2.94 ± 0.27 | 3.03 ± 0.31 |
| Glucose (mg/dL) | 76.85 ± 28.32 | 76.13 ± 16.35 |
| Total cholesterol (mg/dL) | 118.29 ± 43.12 | 112.22 ± 31.00 |
| Albumin (g/L) | 31.82 ± 7.85 | 30.75 ± 7.34 |
| Total proteins (g/L) | 62.12 ± 4.27 | 63.00 ± 7.29 |
| Triglycerides (mg/dL) | 54.39 ± 76.03 | 62.48 ± 87.56 |
| AST (U/L) | 116.99 ± 63.54 | 119.48 ± 60.99 |
| ALT (U/L) | 51.92 ± 38.13 | 49.77 ± 53.97 |
| GGT (U/L) | 25.73 ± 7.39 | 27.21 ± 11.48 |
| Urea (mg/dL) | 31.61 ± 10.41 | 30.21 ± 9.36 |
| BHB (mmol/L) | 0.36 ± 0.32 | 0.32 ± 0.21 |
| NEFA (mmol/L) | 0.58 ± 0.81 | 0.79 ± 1.13 |
| Variable | n | Pregnant Heifers | |
|---|---|---|---|
| CL with cavity | YES | 33 | 22/33 (66.7%) |
| NO | 61 | 45/61 (73.4%) | |
| Flunixin meglumine | YES | 9 | 5/9 (55.6%) |
| NO | 85 | 62/85 (72.9%) | |
| Embryo preservation | Ethylene glycol | 64 | 44/64 (68.7%) |
| Glycerol | 17 | 12/17 (70.6%) | |
| Fresh | 13 | 11/13 (84.6%) | |
| Veterinarian * | 1 | 48 | 39/48 (81.2%) |
| 2 | 45 | 27/45 (61.7%) | |
| Synchronization protocol | PRID7PG6 | 76 | 55/76 (72.2%) |
| PRID8PG7 | 18 | 12/18 (66.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yáñez, U.; Barrio, M.; Fernández, I.; Becerra, J.J.; Herradón, P.G.; Peña, A.I.; Quintela, L.A. Assessment of Luteal Function Using Rectal Palpation, B-Mode Ultrasonography, and Progesterone Determination to Improve Recipient Selection in Embryo Transfer Programs. Animals 2023, 13, 2865. https://doi.org/10.3390/ani13182865
Yáñez U, Barrio M, Fernández I, Becerra JJ, Herradón PG, Peña AI, Quintela LA. Assessment of Luteal Function Using Rectal Palpation, B-Mode Ultrasonography, and Progesterone Determination to Improve Recipient Selection in Embryo Transfer Programs. Animals. 2023; 13(18):2865. https://doi.org/10.3390/ani13182865
Chicago/Turabian StyleYáñez, Uxía, Mónica Barrio, Ismael Fernández, Juan J. Becerra, Pedro G. Herradón, Ana I. Peña, and Luis A. Quintela. 2023. "Assessment of Luteal Function Using Rectal Palpation, B-Mode Ultrasonography, and Progesterone Determination to Improve Recipient Selection in Embryo Transfer Programs" Animals 13, no. 18: 2865. https://doi.org/10.3390/ani13182865
APA StyleYáñez, U., Barrio, M., Fernández, I., Becerra, J. J., Herradón, P. G., Peña, A. I., & Quintela, L. A. (2023). Assessment of Luteal Function Using Rectal Palpation, B-Mode Ultrasonography, and Progesterone Determination to Improve Recipient Selection in Embryo Transfer Programs. Animals, 13(18), 2865. https://doi.org/10.3390/ani13182865







