Comparison of Growth Performance and Biochemical Components between Low-Salinity-Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus vannamei)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
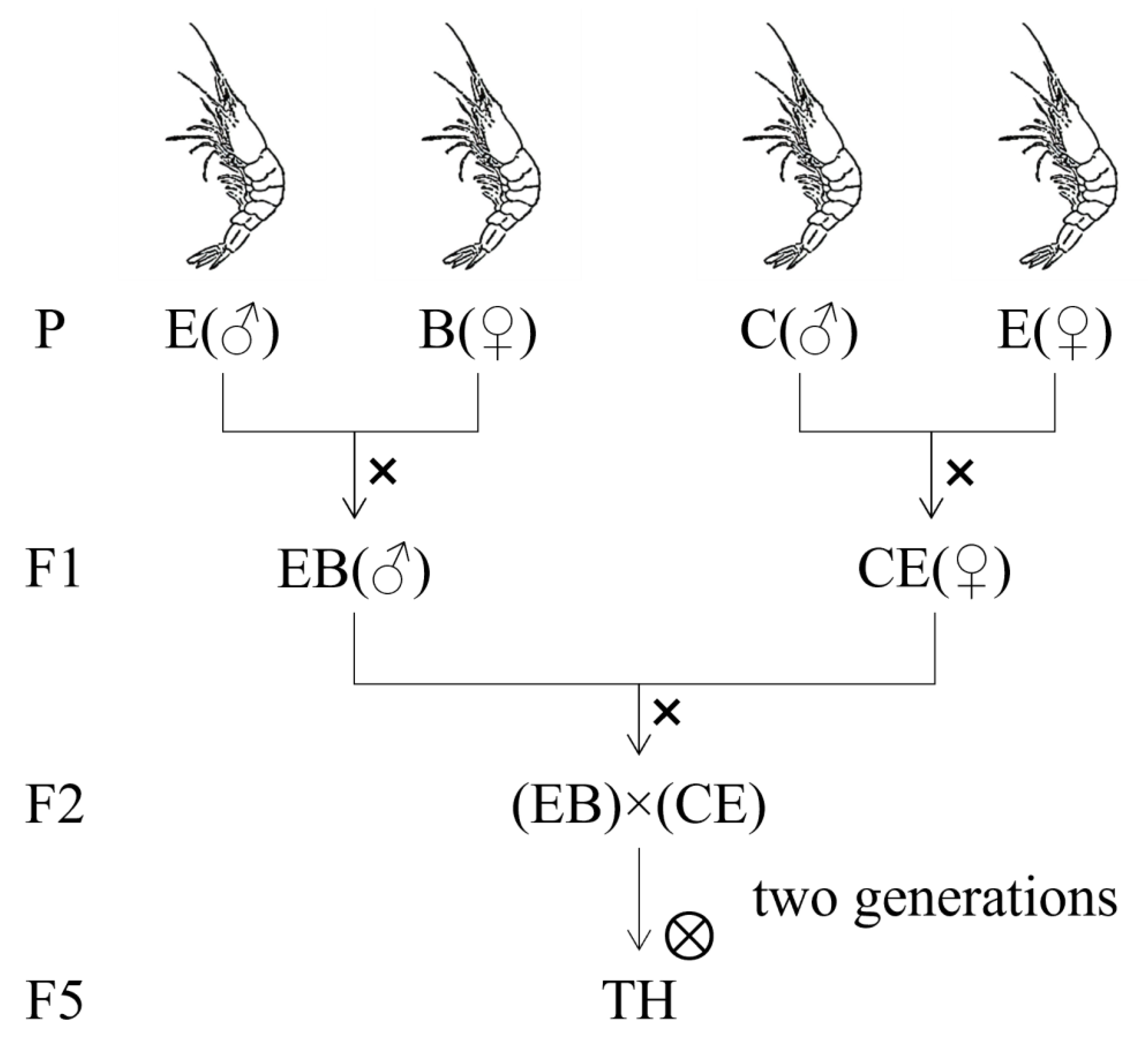
2.1. Populations and Experimental Families
2.2. Experimental Animal Culture
2.3. Sample Collection and Evaluation of Growth
2.4. Biochemical Parameters Analysis
2.5. Digestive Enzyme Activity Analysis
2.6. Amino Acid and Fatty Acid Analysis
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
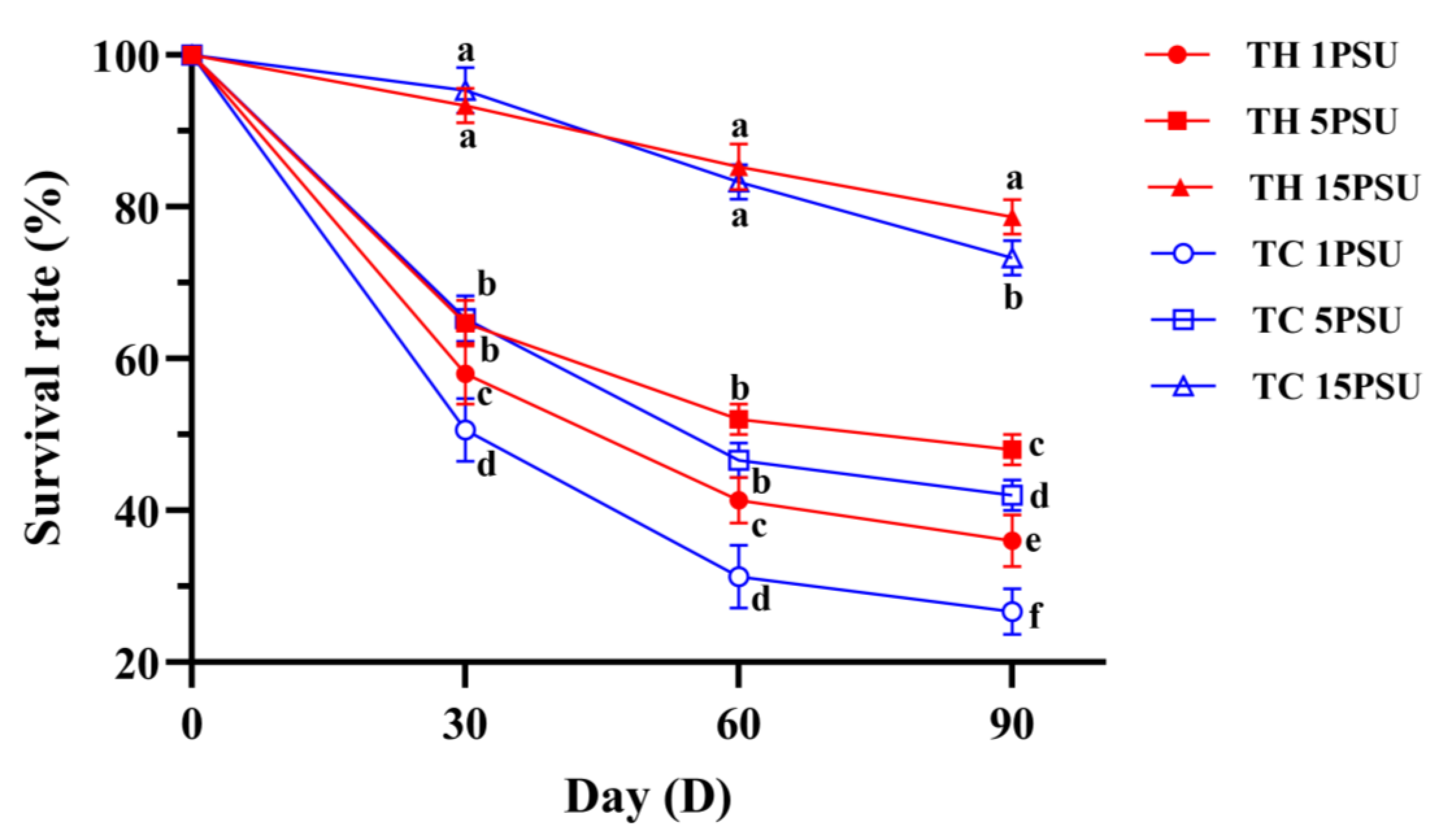
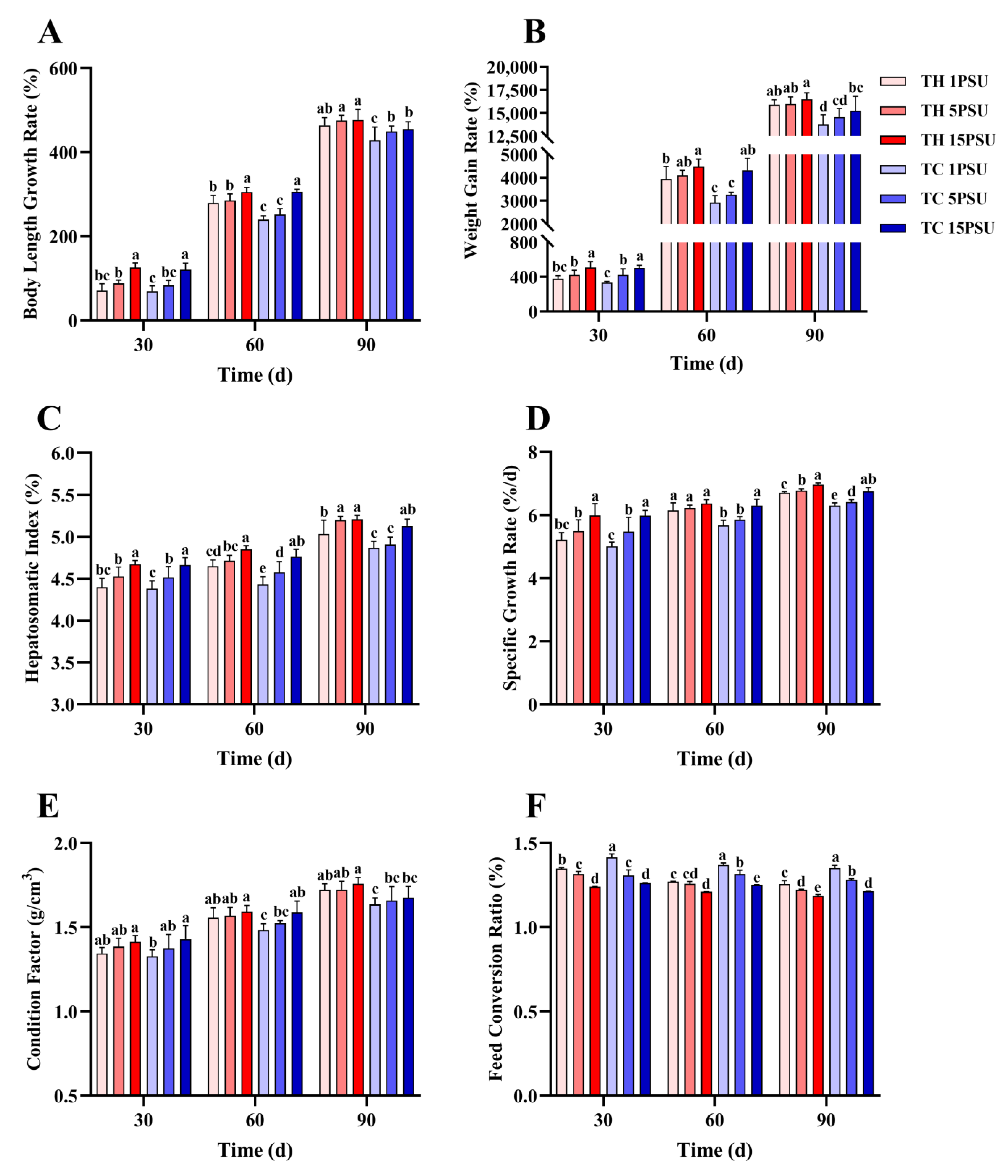
3.1. Growth and Survival
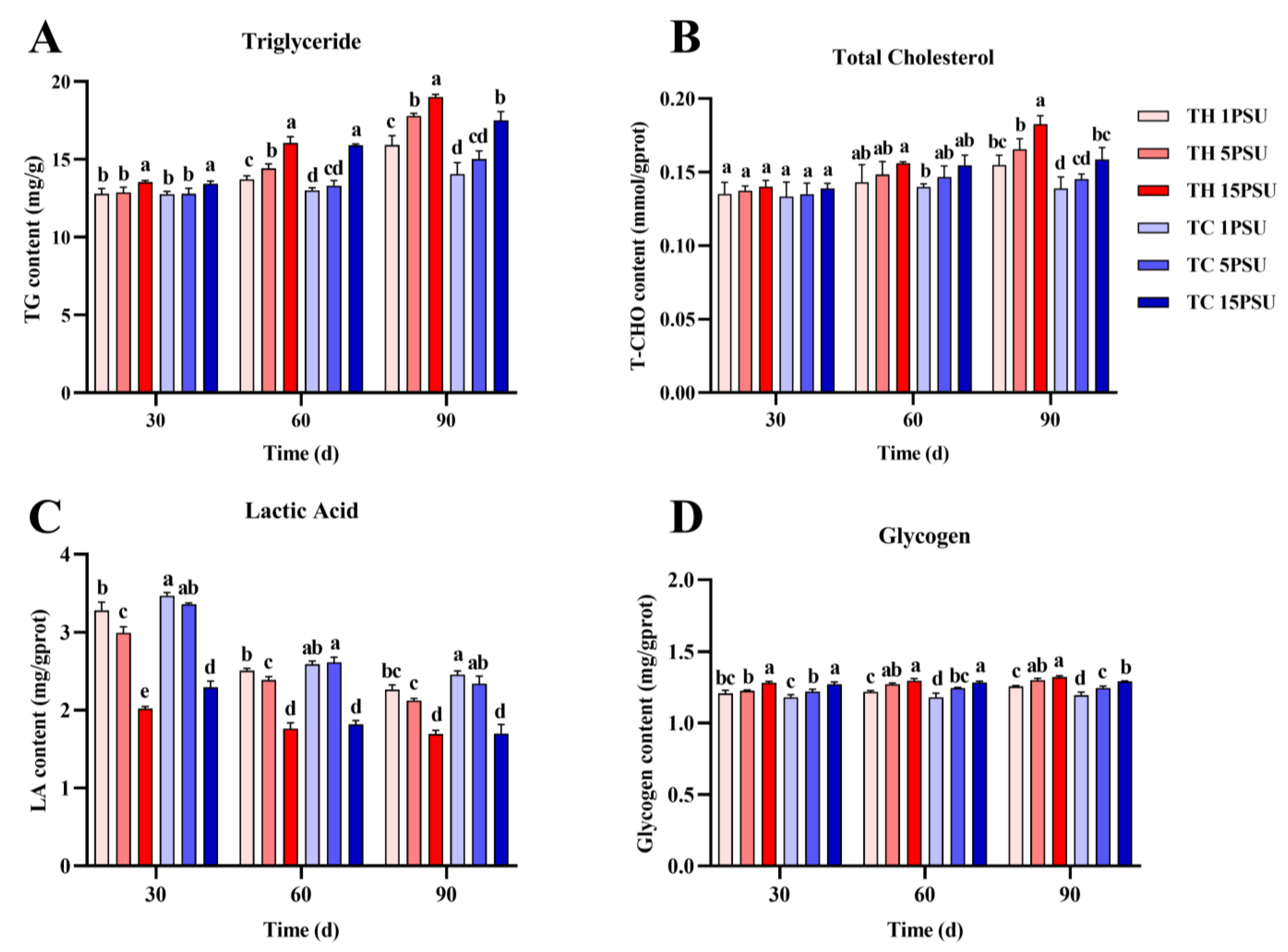
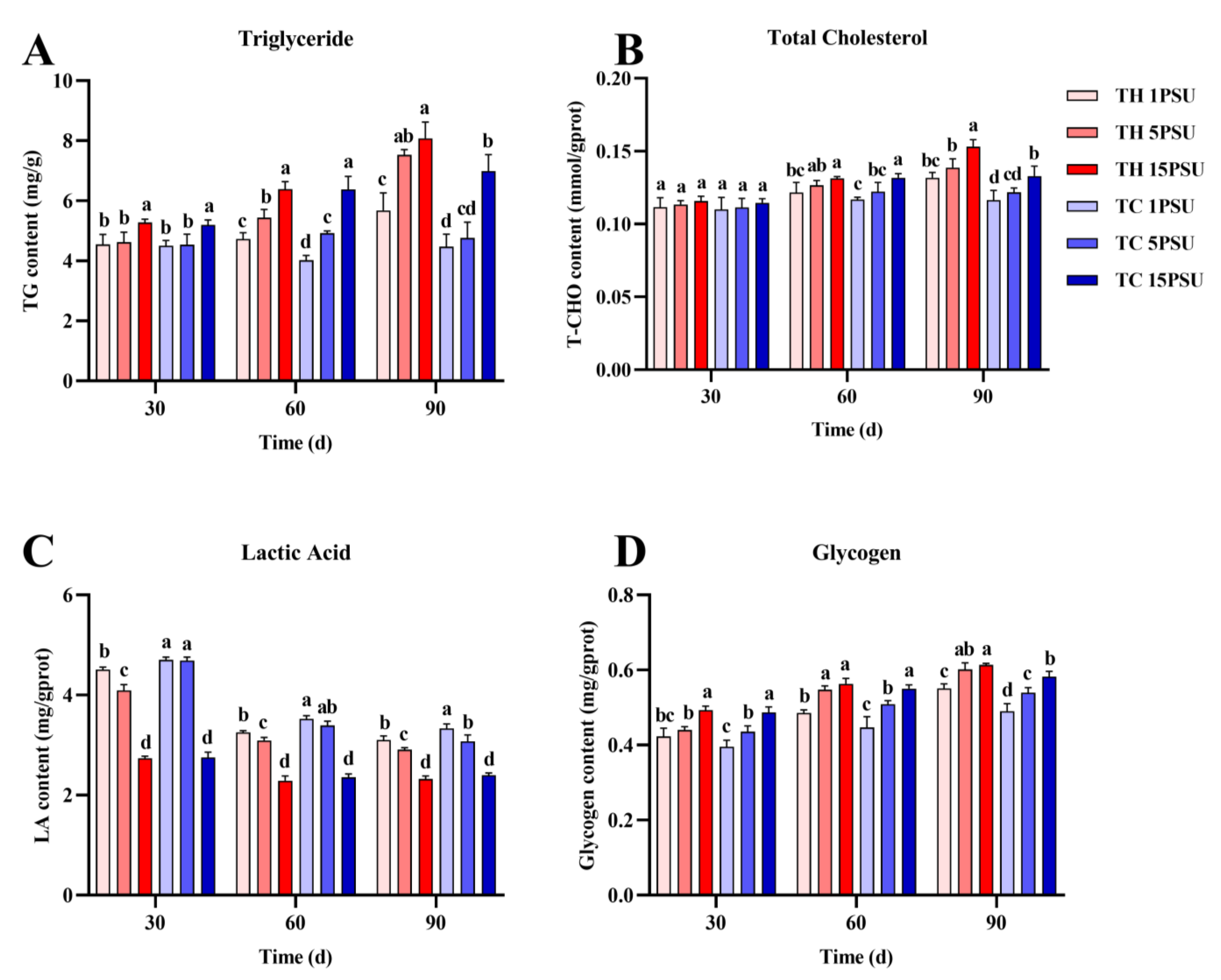
3.2. Biochemical Parameters of the Hepatopancreas and Muscle
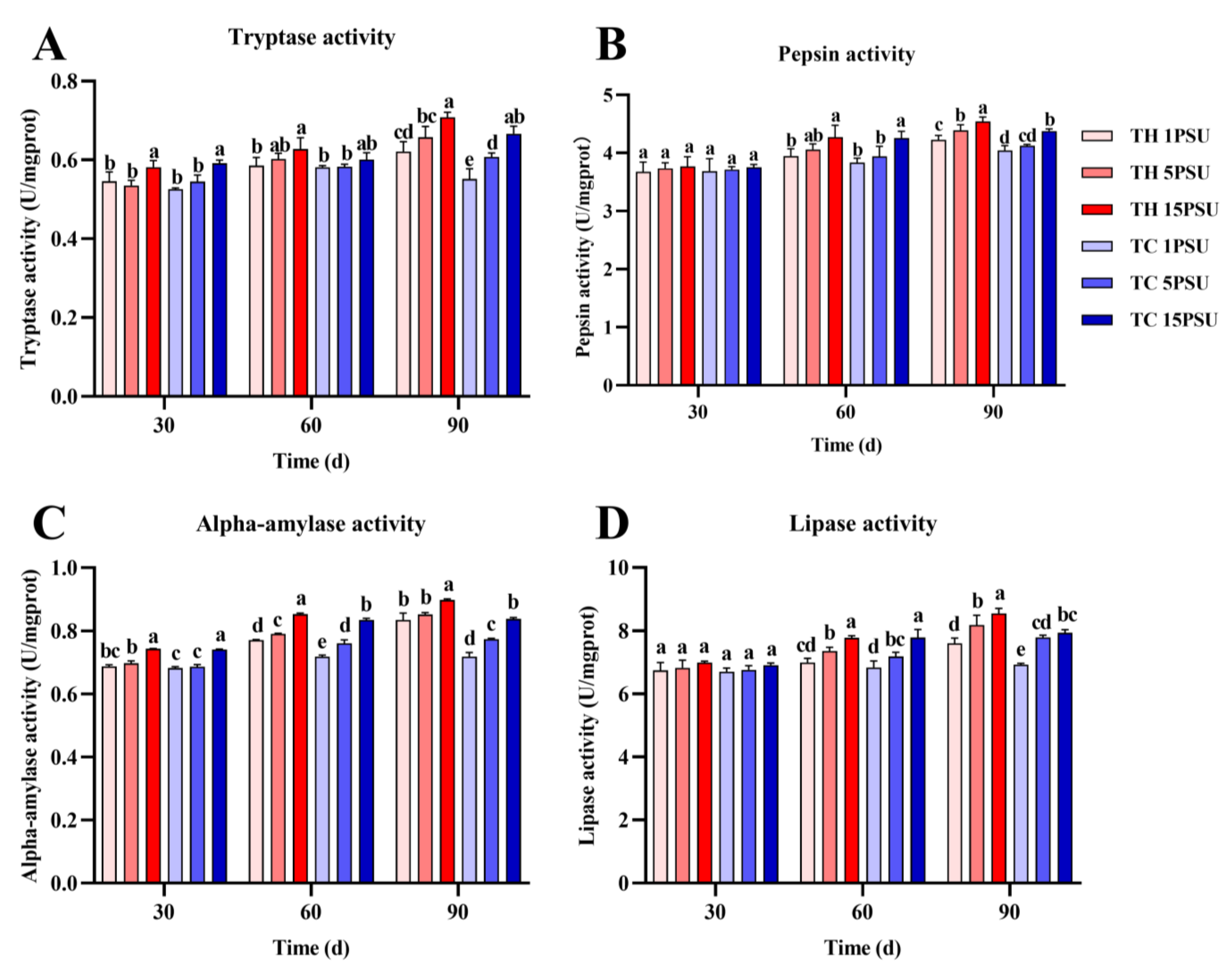
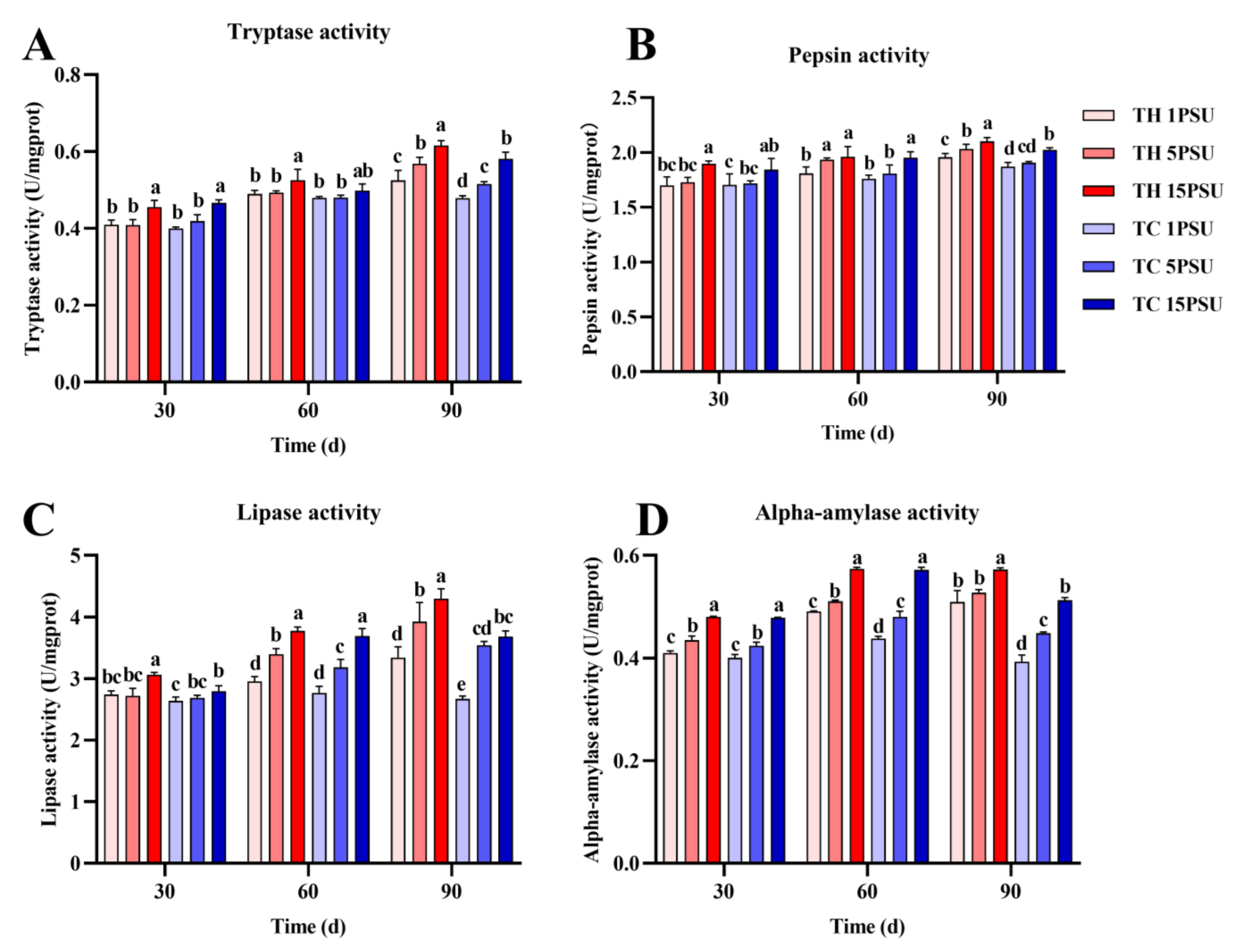
3.3. Digestive Enzymes in the Hepatopancreas and Muscle
3.4. Amino Acids and Fatty Acids Composition
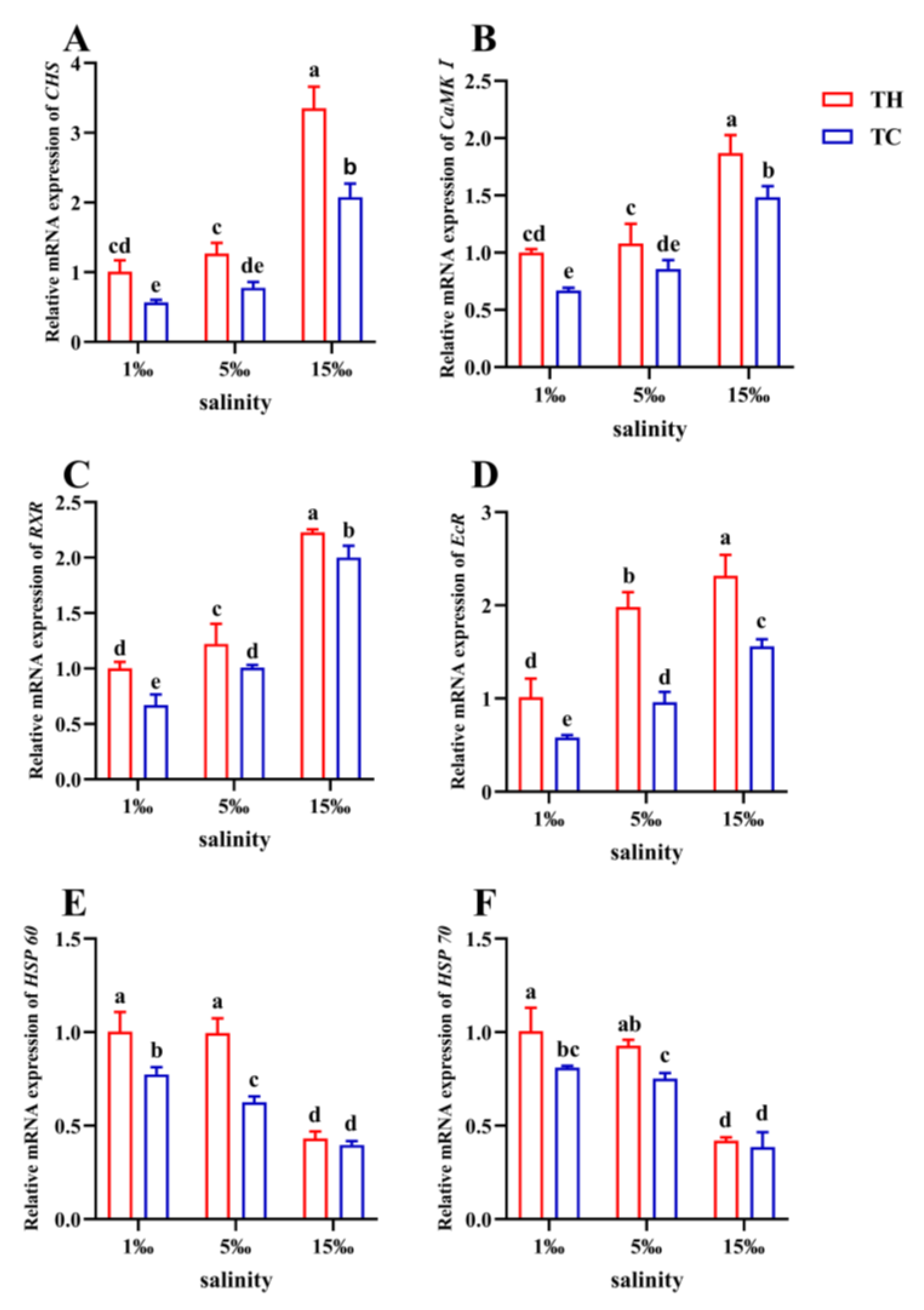
3.5. Growth-Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Wu, Q.; Liao, G.; Fan, L. New insights into the regulation mechanism of Litopenaeus vannamei hepatopancreas after lipopolysaccharide challenge using transcriptome analyses. Fish Shellfish Immunol. 2022, 128, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Guu, Y.-K.; Liu, C.-H.; Pan, T.-M.; Cheng, W. Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol. 2007, 23, 364–377. [Google Scholar] [CrossRef]
- Amoah, K.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z.; Zhang, H.T. Administration of probiotic Bacillus licheniformis induces growth, immune and antioxidant enzyme activities, gut microbiota assembly and resistance to Vibrio parahaemolyticus in Litopenaeus vannamei. Aquac. Nutr. 2020, 26, 1604–1622. [Google Scholar] [CrossRef]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus Vannamei). Aquaculture 2022, 556, 738276. [Google Scholar] [CrossRef]
- Ruan, X.; Luo, K.; Luan, S.; Kong, J.; Xu, S.; Chen, R.; Chen, G. Evaluation of growth performance in Litopenaeus vannamei populations introduced from other nations. J. Fish. China 2013, 37, 34–42. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, C.; Nie, P.; Ye, M. Rapid detection of Penaeus vannamei diseases via an improved LeNet. Aquac. Eng. 2023, 100, 102296. [Google Scholar] [CrossRef]
- Davis, A.D.; Saoud, I.P.; McGraw, W.J.; Rouse, D.B. Considerations for Litopenaeus vannamei Reared in Inland Low Salinity Waters. In Proceedings of the Memorias del VI Simposium Internacional de Nutrición Acuícola, Cancún, Quintana Roo, Mexico, 3–6 September 2002; Avances en Nutrición Acuicola: San Nicolás de los Garza, Mexico, 2002. [Google Scholar]
- Menz, A.; Blake, B.F. Experiments on the growth of Penaeus vannamei Boone. J. Exp. Mar. Biol. Ecol. 1980, 48, 99–111. [Google Scholar] [CrossRef]
- Diaz, F.; Farfan, C.; Sierra, E.; Re, A.D. Effects of temperature and salinity fluctuation on the ammonium excretion and osmoregulation of juveniles of Penaeus vannamei, Boone. Mar. Freshw. Behav. Physiol. 2001, 34, 93–104. [Google Scholar] [CrossRef]
- Bray, W.A.; Lawrence, A.L.; Leung-Trujillo, J.R. The effect of salinity on growth and survival of Penaeus vannamei, with observations on the interaction of IHHN virus and salinity. Aquaculture 1994, 122, 133–146. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.C.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2020, 518, 734563. [Google Scholar] [CrossRef]
- Zhu, M.; Long, X.; Wu, S. Effects of dietary trehalose on the growth performance and nonspecific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 78, 127–130. [Google Scholar] [CrossRef]
- Doyle, R.W. Inbreeding and disease in tropical shrimp aquaculture: A reappraisal and caution. Aquac. Res. 2016, 47, 21–35. [Google Scholar] [CrossRef]
- De los Rios-Perez, L.; Campos-Montes, G.R.; Martinez-Ortega, A.; Castillo-Juarez, H.; Montaldo, H.H. Inbreeding effects on reproductive traits in a breeding population of Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture 2017, 479, 442–446. [Google Scholar] [CrossRef]
- Yan, M.L.; Wang, W.L.; Huang, X.X.; Wang, X.L.; Wang, Y. Interactive effects of dietary cholesterol and phospholipids on the growth performance, expression of immune-related genes and resistance against Vibrio alginolyticus in white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2020, 97, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Luan, S.; Luo, K.; Meng, X.; Li, W.; Sui, J.; Cao, B.; Kong, J. Genetic analysis of the Pacific white shrimp (Litopenaeus vannamei): Heterosis and heritability for harvest body weight. Aquac. Res. 2016, 47, 3365–3375. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.C.; Tao, M.; Qin, Q.B.; Zhang, C.; Luo, K.K.; Zhao, R.R.; Wang, J.; Ren, L.; Xiao, J.; et al. Establishment and application of distant hybridization technology in fish. Sci. China-Life Sci. 2019, 62, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Hoffmann, A.A.; van Oppen, M.J.H. Hybridization as a conservation management tool. Conserv. Lett. 2019, 12, e12652. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Geng, C.; Liao, A.M.; Zhao, R.; Tan, H.; Yao, J.; Wang, S.; Luo, K.; Qin, Q.; et al. Production of a diploid hybrid with fast growth performance derived from the distant hybridization of Hypophthalmichthys nobilis (female) × Megalobrama amblycephala (male). Reprod. Breed. 2022, 2, 56–64. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, J.; Zhan, Y.; Liu, L.; Song, J.; Zhang, W.; Chang, Y. Comparative metabolic analysis between distant sea urchin hybrids (Heliocidaris crassispina ♀ × Strongylocentrotus intermedius ♂) and their parental purebred offspring. Aquaculture 2021, 541, 736796. [Google Scholar] [CrossRef]
- Ma, H.; Lv, W.; Qin, Y.; Li, J.; Li, X.; Liao, Q.; Li, Y.; Shi, G.; Yang, Y.; Guo, S.; et al. Aquaculture potential of two Kumamoto oyster (Crassostrea sikamea) populations and their reciprocal hybrids in southern China. Aquaculture 2022, 546, 737301. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.; Chen, Q.; Liu, Z.; Huang, Y.; Tian, J.; Huang, Y.; Zhao, Y. Comparison of growth performance and biochemical components between parent and hybrid offspring in the oriental river prawn, Macrobrachium nipponense. Anim. Genet. 2021, 52, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Manan, H.; Zhong, J.M.H.; Othman, F.; Ikhwanuddin, M. Histopathology of the hepatopancreas of pacific white shrimp, Penaeus vannamei from none early mortality syndrome (EMS) shrimp ponds. J. Fish. Aquatic. Sci. 2015, 10, 562. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Zheng, X.; Wang, Y.; Li, H.; Liu, Q.; Zhang, J. Effect of dietary poly-β-hydroxybutyrate (PHB) on growth performance, intestinal health status and body composition of Pacific white shrimp Litopenaeus vannamei (Boone, 1931). Fish Shellfish Immunol. 2017, 60, 520–528. [Google Scholar] [CrossRef]
- Long, J.; Cui, Y.; Wang, R.; Chen, Y.; Zhao, N.; Wang, C.; Wang, Z.; Li, Y. Combined effects of high salinity and ammonia-N exposure on the energy metabolism, immune response, oxidative resistance and ammonia metabolism of the Pacific white shrimp Litopenaeus vannamei. Aquac. Rep. 2021, 20, 100648. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Yu, N.; Xiong, Z.; Chen, X.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- An, W.; He, H.; Dong, X.; Tan, B.; Yang, Q.; Chi, S.; Zhang, S.; Liu, H.; Yang, Y. Regulation of growth, fatty acid profiles, hematological characteristics and hepatopancreatic histology by different dietary n-3 highly unsaturated fatty acids levels in the first stages of juvenile Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 17, 100321. [Google Scholar] [CrossRef]
- Rao, N.P.; Vaishnavi, C.M.; Kumar, M.S.; Vishnu, S.; Mukherjee, B.; Karthik, N.; Dutta, G.; Das, A.K. A fast survey on recent developments in designing colorimetric and fluorescent sensors for the selective detection of essential amino acids. Anal. Methods 2023, 15, 2546–2577. [Google Scholar] [CrossRef]
- Wei, Z.; Zhuang, Y.; Liu, X.; Zou, D.; Mai, K.; Sun, Z.; Ye, C. Leucine promotes protein synthesis of juvenile white shrimp Litopenaeus vannamei through TOR signaling pathway. Aquaculture 2023, 564, 739060. [Google Scholar] [CrossRef]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Silvia, G.-J.; Abel Antonio, U.-R.; Francisco, V.-O.; Georgina, H.-W. Ammonia efflux rates and free amino acid levels in Litopenaeus vannamei postlarvae during sudden salinity changes. Aquaculture 2004, 233, 573–581. [Google Scholar] [CrossRef]
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.G.; Chen, L. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Wu, Q.; Waiho, K.; Huang, Z.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Lin, F.; Ma, H. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture 2020, 515, 734560. [Google Scholar] [CrossRef]
- Zhang, Q.; Wong, M.K.S.; Li, Y.; Li, Y.; Takei, Y. Changes in Plasma and Tissue Long-Chain Polyunsaturated Fatty Acid (LC-PUFA) Content in the Eel Anguilla japonica after External and Internal Osmotic Stress. Zool. Sci. 2017, 34, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.S.; Mykles, D.L. Regulation of crustacean molting: A review and our perspectives. Gen. Comp. Endocrinol. 2011, 172, 323–330. [Google Scholar] [CrossRef]
- Spencer, E.L.; Fitzgibbon, Q.P.; Day, R.D.; Trotter, A.J.; Smith, G.G. Effects of acute salinity stress on the survival and haemolymph biochemistry of juvenile tropical rock lobster, Panulirus ornatus, at different moult stages. Aquaculture 2023, 573, 739597. [Google Scholar] [CrossRef]
- Ranasinghe, N.; Lin, C.-H.; Lee, T.-H. Cholesterol Accumulation in Livers of Indian Medaka, Oryzias dancena, Acclimated to Fresh Water and Seawater. Front. Mar. Sci. 2022, 9, 891706. [Google Scholar] [CrossRef]
- Rocha, J.; Garcia-Carreño, F.L.; Muhlia-Almazán, A.; Peregrino-Uriarte, A.B.; Yépiz-Plascencia, G.; Córdova-Murueta, J.H. Cuticular chitin synthase and chitinase mRNA of whiteleg shrimp Litopenaeus vannamei during the molting cycle. Aquaculture 2012, 330–333, 111–115. [Google Scholar] [CrossRef]
- Laramore, S.; Laramore, C.R.; Scarpa, J. Effect of Low Salinity on Growth and Survival of Postlarvae and Juvenile Litopenaeus vannamei. J. World Aquac. Soc. 2001, 32, 385–392. [Google Scholar] [CrossRef]
- Rosas, C.; Cuzon, G.; Gaxiola, G.; Arena, L.; Lemaire, P.; Soyez, C.; Van Wormhoudt, A. Influence of dietary carbohydrate on the metabolism of juvenile Litopenaeus stylirostris. J. Exp. Mar. Biol. Ecol. 2000, 249, 181–198. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Araújo, B.C.; Flores-Galvez, K.; Honji, R.M.; Barbosa, V.M.; Viana, M.T.; Tinajero, A.; Mata-Sotres, J.A. Arachidonic acid effects on the overall performance, fatty acid profile, hepatopancreas morphology and lipid-relevant genes in Litopenaeus vannamei juveniles. Aquaculture 2020, 523, 735207. [Google Scholar] [CrossRef]
- Li, Y.; Fan, W.; Huang, Y.; Huang, Y.; Du, X.; Liu, Z.; Huang, Y.; Zhao, Y. Comparison of morphology and genetic diversity between broodstock and hybrid offspring of oriental river prawn, Macrobrachium nipponense based on morphological analysis and SNP markers. Anim. Genet. 2021, 52, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Cody, J.J.; Conquest, L.D.; Divakaran, S.; Forster, I.P.; Decamp, O.E. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Guo, J.; Gan, C.; Cheng, B.; Cui, B.; Yi, F. Exploration of binding mechanism of apigenin to pepsin: Spectroscopic analysis, molecular docking, enzyme activity and antioxidant assays. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122281. [Google Scholar] [CrossRef]
- Miłek, J.; Tatarchuk, T. Modified magnetite nanoparticles synthesized using cetyltrimethylammonium bromide and their application to immobilize trypsin. Biocatal. Agric. Biotechnol. 2023, 47, 102586. [Google Scholar] [CrossRef]
- Huang, Y.; Condict, L.; Richardson, S.J.; Brennan, C.S.; Kasapis, S. Exploring the inhibitory mechanism of p-coumaric acid on α-amylase via multi-spectroscopic analysis, enzymatic inhibition assay and molecular docking. Food Hydrocoll. 2023, 139, 108524. [Google Scholar] [CrossRef]
- Rivera-Pérez, C.; del Toro, M.d.l.Á.N.; García-Carreño, F. Purification and characterization of an intracellular lipase from pleopods of whiteleg shrimp (Litopenaeus vannamei). Comp. Biochem. Phys. Part B Biochem. Mol. Biol. 2011, 158, 99–105. [Google Scholar] [CrossRef]
- Akbary, P.; Adeshina, I.; Jahanbakhshi, A. Growth performance, digestive enzymes, antioxidant activity and immune responses of Litopenaeus vannamei fed with Jania adhaerens J.V. Supplemented diet against Photobacterium damselae infection. Anim. Feed Sci. Technol. 2020, 270, 114696. [Google Scholar] [CrossRef]
- Macedo, C.F.; Pinto-Coelho, R.M. Nutritional status response of Daphnia laevis and Moina micrura from a tropical reservoir to different algal diets: Scenedesmus quadricauda and Ankistrodesmus gracilis. Braz. J. Biol. 2001, 61, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Racotta, I.S.; Hernández-Herrera, R. Metabolic responses of the white shrimp, Penaeus vannamei, to ambient ammonia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 125, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.W.; McKinney, R.J.; Hird, F.J.; Macmillan, D.L. Lactic acid formation in crustaceans and the liver function of the midgut gland questioned. Comp. Biochem. Physiol. B 1977, 56, 427–433. [Google Scholar] [CrossRef]
- Cota-Ruiz, K.; Peregrino-Uriarte, A.B.; Felix-Portillo, M.; Martínez-Quintana, J.A.; Yepiz-Plascencia, G. Expression of fructose 1,6-bisphosphatase and phosphofructokinase is induced in hepatopancreas of the white shrimp Litopenaeus vannamei by hypoxia. Mar. Environ. Res. 2015, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abdullah; Zhang, C.; Li, Y.; Zhang, H.; Wang, J.; Feng, F. Effects of dietary glycerol monolaurate on the growth performance, digestive enzymes, body composition and non-specific immune response of white shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 18, 100535. [Google Scholar] [CrossRef]
- Xie, F.; Zeng, W.; Zhou, Q.; Wang, H.; Wang, T.; Zheng, C.; Wang, Y. Dietary lysine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2012, 358–359, 116–121. [Google Scholar] [CrossRef]
- Gilles, R. “Compensatory” Organic Osmolytes in High Osmolarity and Dehydration Stresses: History and Perspectives. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 279–290. [Google Scholar] [CrossRef]
- Glencross, B.; Rutherford, N. A determination of the quantitative requirements for docosahexaenoic acid for juvenile barramundi (Lates calcarifer). Aquac. Nutr. 2011, 17, e536–e548. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Araújo, B.C.; Mata-Sotres, J.A.; Viana, M.T.; Tinajero, A.; Braga, A. Fish oil-free diets for Pacific white shrimp Litopenaeus vannamei: The effects of DHA-EPA supplementation on juvenile growth performance and muscle fatty acid profile. Aquaculture 2019, 511, 734276. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, M.Z.; Filer, K.; Xue, Y.; Ai, Q.H.; Mai, K.S. Evaluation of Schizochytrium meal in microdiets of Pacific white shrimp (Litopenaeus vannamei) larvae. Aquac. Res. 2017, 48, 2328–2336. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Sun, Y.; Li, S.; Gao, Y.; Yu, Y.; Liu, C.; Wang, Q.; Lv, X.; Zhang, X. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019, 10, 356. [Google Scholar] [PubMed]
- Shen, H.; Hu, Y.; Zhang, Y.; Zhou, X.; Xu, Z. Calcium-calmodulin dependent protein kinase I from Macrobrachium nipponense: cDNA cloning and involvement in molting. Gene 2014, 538, 235–243. [Google Scholar] [CrossRef]
- He, X.; Sun, Y.; Yang, F.; Zheng, G.; Li, R.; Liu, M.; Li, W.; Zhou, D.H.; Zheng, Y. Heat shock protein 60 in parasitic helminths: A role in immune responses and therapeutic applications. Mol. Biochem. Parasitol. 2023, 253, 111544. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequences (5′−3′) | GenBank No. |
|---|---|---|
| CaMKI-F | CATCATAGAATGGAGGGTA | KU601407.1 |
| CaMKI-R | AGAAGTCTTGGCACAGAA | |
| EcR-F | TGTAATCTGGTCCTCCCT | KF234770.1 |
| EcR-R | AATAACTGACGACGACTCTG | |
| CHS-F | CGCGACGAGTTACTTTAGCAGT | AF315689.1 |
| CHS-R | CGGCGGTTACAACGAGAA | |
| RXR-F | CTGTTGGGTCTGAGTTGAG | KC347569.1 |
| RXR-R | GGACAAAGGGAGATAAAGAA | |
| HSP60-F | ATAACTCCACGCCTGATC | FJ710169.2 |
| HSP60-R | GCCAACAACACCAACGAA | |
| HSP70-F | ACTCAGCTCGAACTTACCC | AY645906.1 |
| HSP70-R | ACCACCTACTCTGACAACCA | |
| β-actin-F | TCCATGCCCAGGAATGAG | AF300705.2 |
| β-actin-R | GAGCAGGAGATGACCACCG |
| Aminon Acid (g/kg) | TH 1 PSU | TH 5 PSU | TH 15 PSU | TC 1 PSU | TC 5 PSU | TC 15 PSU |
|---|---|---|---|---|---|---|
| Met 1 | 2.44 ± 0.02 a | 2.4 ± 0.15 ab | 2.14 ± 0.04 c | 2.36 ± 0.07 ab | 2.35 ± 0.07 ab | 2.23 ± 0.07 bc |
| Lys 1 | 7.6 ± 0.14 a | 7.52 ± 0.5 ab | 6.49 ± 0.13 d | 7.17 ± 0.22 bc | 6.92 ± 0.18 cd | 6.73 ± 0.28 cd |
| Val 1 | 3.65 ± 0.06 a | 3.63 ± 0.25 a | 3.1 ± 0.07 c | 3.51 ± 0.08 ab | 3.35 ± 0.09 bc | 3.27 ± 0.21 bc |
| Ile 1 | 3.6 ± 0.04 a | 3.52 ± 0.2 a | 3.06 ± 0.06 c | 3.33 ± 0.11 bc | 3.23 ± 0.07 bc | 3.12 ± 0.17 c |
| Phe 1 | 3.67 ± 0.04 a | 3.6 ± 0.26 a | 3.16 ± 0.05 b | 3.49 ± 0.07 ab | 3.48 ± 0.09 ab | 3.38 ± 0.19 ab |
| Leu 1 | 6.79 ± 0.08 a | 6.58 ± 0.38 ab | 5.84 ± 0.1 c | 6.34 ± 0.25 bc | 6.05 ± 0.14 bc | 5.94 ± 0.3 c |
| Thr 1 | 3.36 ± 0.04 a | 3.3 ± 0.22 a | 2.91 ± 0.02 c | 3.23 ± 0.07 ab | 3.04 ± 0.08 bc | 2.94 ± 0.12 c |
| His | 1.74 ± 0.08 a | 1.78 ± 0.18 a | 1.46 ± 0.04 b | 1.72 ± 0.05 ab | 1.66 ± 0.07 ab | 1.63 ± 0.14 ab |
| Arg | 8.34 ± 0.34 a | 7.8 ± 0.54 ab | 7.36 ± 0.2 b | 8.28 ± 0.36 a | 7.85 ± 0.37 ab | 7.14 ± 0.51 ab |
| Asp 2 | 9.4 ± 0.13 a | 9.28 ± 0.43 a | 8.09 ± 0.11 c | 8.81 ± 0.28 bc | 8.53 ± 0.22 bc | 8.3 ± 0.36 bc |
| Ser 2 | 3.22 ± 0.04 a | 3.1 ± 0.27 ab | 2.84 ± 0.04 c | 3.17 ± 0.07 ab | 2.96 ± 0.08 bc | 2.87 ± 0.09 bc |
| Glu 2 | 14.96 ± 0.37 a | 14.46 ± 1.17 a | 12.68 ± 0.09 c | 14.47 ± 0.42 a | 13.69 ± 0.26 bc | 13.41 ± 1.03 bc |
| Gly 2 | 7.83 ± 0.55 b | 7.33 ± 0.2 bc | 9.72 ± 0.72 a | 7.16 ± 0.71 c | 8.02 ± 0.9 b | 7.31 ± 0.09 bc |
| Ala 2 | 5.81 ± 0.25 a | 5.68 ± 0.29 ab | 5.11 ± 0.12 c | 5.31 ± 0.08 bc | 5.14 ± 0.09 c | 5.05 ± 0.18 c |
| Tyr 2 | 3.5 ± 0.04 a | 3.38 ± 0.22 a | 3.03 ± 0.05 b | 3.29 ± 0.13 ab | 3.21 ± 0.02 ab | 3.25 ± 0.19 ab |
| Pro 2 | 6.48 ± 0.82 a | 6.53 ± 1.46 a | 3.38 ± 0.3 b | 6.18 ± 0.8 a | 5.57 ± 0.21 a | 6.3 ± 0.33 a |
| WTAA | 92.3 ± 1.37 a | 89.88 ± 5.75 ab | 80.33 ± 0.94 c | 87.85 ± 2.43 bc | 85.02 ± 2.51 bc | 82.75 ± 3.84 bc |
| WEAA | 31.11 ± 0.36 a | 30.54 ± 1.92 ab | 26.69 ± 0.44 d | 29.42 ± 0.84 bc | 28.42 ± 0.71 cd | 27.61 ± 1.33 cd |
| WSEAA | 10.08 ± 0.42 a | 9.58 ± 0.72 ab | 8.82 ± 0.22 b | 9.99 ± 0.34 ab | 9.51 ± 0.43 ab | 8.77 ± 0.65 ab |
| WNEAA | 51.2 ± 0.72 a | 49.77 ± 3.06 ab | 44.84 ± 0.47 c | 48.39 ± 1.27 bc | 47.12 ± 1.36 bc | 46.49 ± 1.95 bc |
| WEAA/WTAA | 0.34 | 0.34 | 0.33 | 0.33 | 0.33 | 0.33 |
| WEAA/WNEAA | 0.61 | 0.61 | 0.60 | 0.61 | 0.60 | 0.59 |
| TH 1 PSU | TH 5 PSU | TH 15 PSU | TC 1 PSU | TC 5 PSU | TC 15 PSU | |
|---|---|---|---|---|---|---|
| C14:0 | 0.83 ± 0.056 a | 0.83 ± 0.032 a | 0.82 ± 0.058 ab | 0.88 ± 0.094 a | 0.77 ± 0.063 ab | 0.67 ± 0.051 b |
| C15:0 | 0.76 ± 0.042 b | 1.10 ± 0.105 a | 0.36 ± 0.013 c | 1.16 ± 0.151 a | 1.20 ± 0.111 a | 0.49 ± 0.010 c |
| C16:0 | 19.26 ± 0.329 a | 19.01 ± 0.995 ab | 17.65 ± 0.318 bc | 20.04 ± 0.522 a | 17.79 ± 0.606 bc | 16.53 ± 0.301 c |
| C17:0 | 0.77 ± 0.020 b | 0.74 ± 0.024 b | 0.50 ± 0.016 b | 1.58 ± 0.355 a | 1.42 ± 0.123 a | 0.71 ± 0.077 b |
| C18:0 | 4.54 ± 0.077 d | 7.31 ± 0.355 a | 7.02 ± 0.514 ab | 5.26 ± 0.486 cd | 7.00 ± 0.44 ab | 6.01 ± 0.699 bc |
| C22:0 | 0.18 ± 0.009 c | 0.21 ± 0.024 cd | 0.21 ± 0.015 cd | 0.26 ± 0.017 a | 0.26 ± 0.004 a | 0.23 ± 0.023 ab |
| C16:1 | 2.50 ± 0.083 c | 2.24 ± 0.046 c | 1.79 ± 0.218 d | 3.25 ± 0.238 b | 3.72 ± 0.140 a | 2.29 ± 0.175 c |
| C17:1 | 0.20 ± 0.012 a | 0.20 ± 0.026 a | 0.21 ± 0.011 a | 0.18 ± 0.012 a | 0.18 ± 0.019 a | 0.17 ± 0.017 a |
| C18:1n-9 | 25.05 ± 0.329 ab | 23.83 ± 0.415 bc | 22.85 ± 0.898 c | 24.61 ± 1.617 abc | 24.63 ± 0.091 abc | 25.95 ± 0.506 a |
| C20:1n-9 | 1.69 ± 0.044 abc | 1.53 ± 0.028 c | 1.63 ± 0.094 bc | 1.57 ± 0.101 bc | 1.73 ± 0.087 ab | 1.87 ± 0.112 a |
| C22:1n-9 | 0.38 ± 0.04 d | 0.49 ± 0.047 cd | 0.56 ± 0.07 abc | 0.51 ± 0.031 bcd | 0.68 ± 0.067 a | 0.67 ± 0.112 ab |
| C18:2n-6 | 26.52 ± 0.328 a | 23.55 ± 0.811 c | 26.00 ± 0.923 ab | 25.61 ± 0.196 ab | 24.56 ± 0.222 bc | 25.62 ± 0.608 ab |
| C18:3n-3 | 2.06 ± 0.111 ab | 1.14 ± 0.044 d | 1.82 ± 0.047 c | 1.94 ± 0.052 bc | 1.24 ± 0.138 d | 2.27 ± 0.090 a |
| C20:2n-6 | 1.63 ± 0.127 b | 1.07 ± 0.139 e | 1.51 ± 0.091 bc | 1.34 ± 0.125 cd | 1.19 ± 0.048 de | 2.10 ± 0.111 a |
| C20:3n-6 | 0.11 ± 0.007 b | 0.15 ± 0.004 a | 0.12 ± 0.005 b | 0.11 ± 0.008 b | 0.16 ± 0.005 a | 0.15 ± 0.012 a |
| C20:4n-6 | 1.07 ± 0.104 d | 2.88 ± 0.037 a | 2.33 ± 0.268 b | 1.42 ± 0.049 d | 1.96 ± 0.109 bc | 1.89 ± 0.217 c |
| C22:5n-3 | 6.27 ± 0.206 bc | 7.28 ± 0.223 a | 7.26 ± 0.318 a | 4.85 ± 0.057 d | 5.87 ± 0.390 c | 6.97 ± 0.503 ab |
| C22:6n-3 | 6.2 ± 0.134 bc | 6.44 ± 0.183 b | 7.37 ± 0.399 a | 5.43 ± 0.278 d | 5.63 ± 0.152 cd | 5.42 ± 0.246 d |
| SFAs | 26.33 ± 0.282 bc | 29.21 ± 1.448 a | 26.56 ± 0.775 bc | 29.19 ± 1.459 a | 28.44 ± 0.664 ab | 24.64 ± 1.022 c |
| MUFAs | 29.81 ± 0.308 ab | 28.29 ± 0.381 bc | 27.03 ± 1.215 c | 30.12 ± 1.524 ab | 30.95 ± 0.289 a | 30.94 ± 0.605 a |
| PUFAs | 28.58 ± 0.428 a | 24.69 ± 0.836 b | 27.81 ± 0.935 a | 27.54 ± 0.179 a | 25.80 ± 0.318 b | 27.89 ± 0.544 a |
| HUFAs | 15.28 ± 0.281 c | 17.82 ± 0.234 a | 18.59 ± 0.518 a | 13.15 ± 0.364 d | 14.81 ± 0.398 c | 16.53 ± 0.654 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Zhu, B.; Zhang, J.; Yang, Y.; Tian, J.; Xu, W.; Du, X.; Huang, Y.; Li, Y.; Zhao, Y. Comparison of Growth Performance and Biochemical Components between Low-Salinity-Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus vannamei). Animals 2023, 13, 2837. https://doi.org/10.3390/ani13182837
Ye Y, Zhu B, Zhang J, Yang Y, Tian J, Xu W, Du X, Huang Y, Li Y, Zhao Y. Comparison of Growth Performance and Biochemical Components between Low-Salinity-Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus vannamei). Animals. 2023; 13(18):2837. https://doi.org/10.3390/ani13182837
Chicago/Turabian StyleYe, Yucong, Bihong Zhu, Junya Zhang, Ying Yang, Jiangtao Tian, Wenyue Xu, Xinglin Du, Yizhou Huang, Yiming Li, and Yunlong Zhao. 2023. "Comparison of Growth Performance and Biochemical Components between Low-Salinity-Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus vannamei)" Animals 13, no. 18: 2837. https://doi.org/10.3390/ani13182837
APA StyleYe, Y., Zhu, B., Zhang, J., Yang, Y., Tian, J., Xu, W., Du, X., Huang, Y., Li, Y., & Zhao, Y. (2023). Comparison of Growth Performance and Biochemical Components between Low-Salinity-Tolerant Hybrid and Normal Variety of Pacific White Shrimp (Penaeus vannamei). Animals, 13(18), 2837. https://doi.org/10.3390/ani13182837





