Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diets
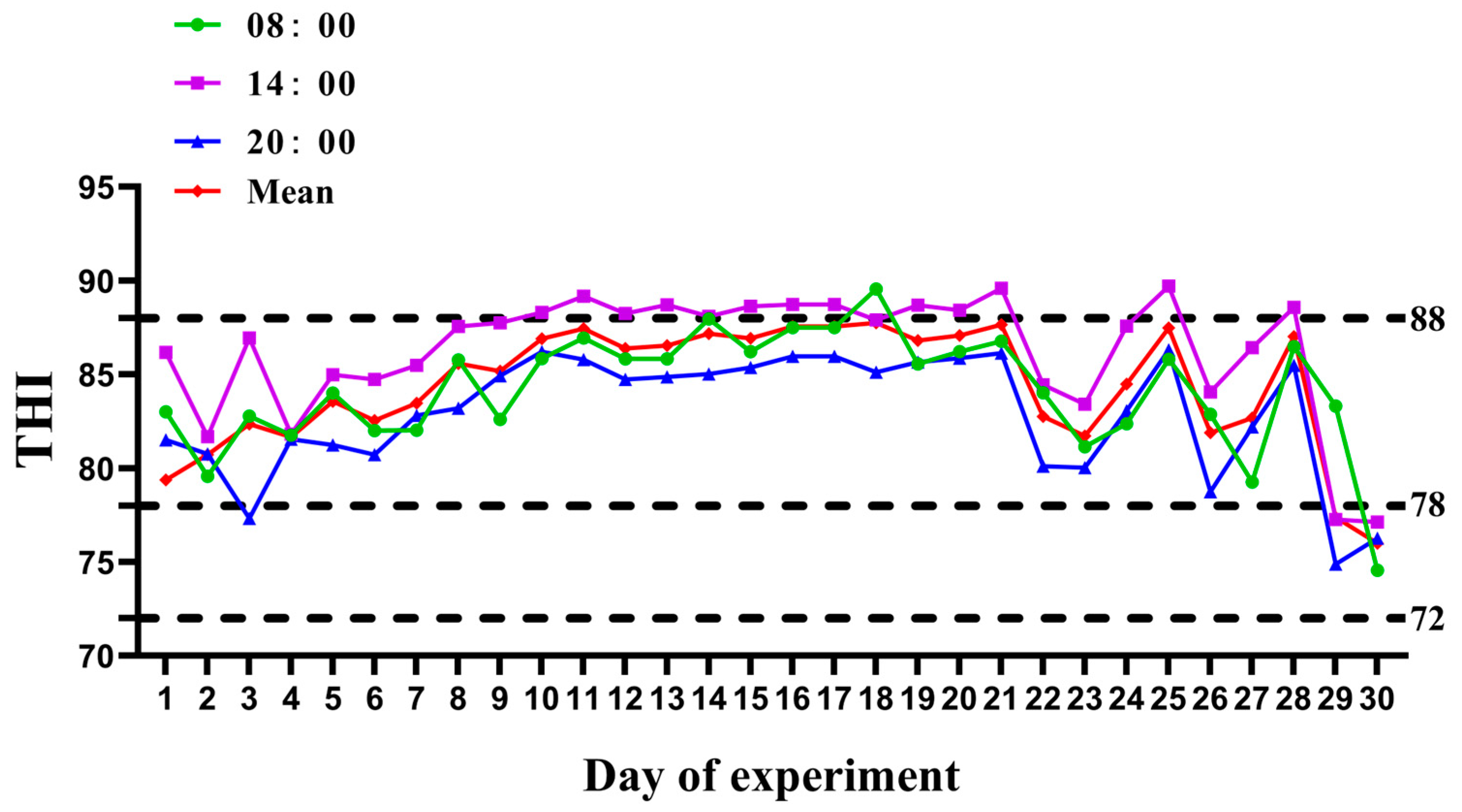
2.2. Environmental Temperature and Humidity
2.3. Rectal Temperature and Respiratory Rate
2.4. Sample Collection and Analyses
2.5. Environmental Impact: Predicted Enteric Methane Production
- CH4 = enteric methane production;
- DMI = dry matter intake (kg/head/day).
2.6. Statistical Analysis
3. Results
3.1. Measurement of THI and Heat Stress
3.2. Rectal Temperature and Respiratory Rate
3.3. Lactation Performance
3.4. Antioxidant Status and Heat Shock Signaling Molecules
3.5. Ruminal Fermentation Characteristics
3.6. Environmental Impact: Predicted Methane (CH4) Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Tang, L.; Bai, X.; Du, K.; Wang, H.; Jia, X.; Lai, S. Heat Stress Altered the Vaginal Microbiome and Metabolome in Rabbits. Front. Microbiol. 2022, 13, 813622. [Google Scholar] [CrossRef] [PubMed]
- Norskov, N.P.; Bruhn, A.; Cole, A.; Nielsen, M.O. Targeted and Untargeted Metabolic Profiling to Discover Bioactive Compounds in Seaweeds and Hemp Using Gas and Liquid Chromatography-Mass Spectrometry. Metabolites 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Cheng, J.B.; Shi, B.L.; Yang, H.J.; Zheng, N.; Wang, J.Q. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J. Zhejiang Univ. Sci. B 2015, 16, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Wang, Y.; Xia, S.W.; Zhao, F.; Zhong, J.F.; Wang, H.L.; Chen, K.L. SIRT4 Expression Ameliorates the Detrimental Effect of Heat Stress via AMPK/mTOR Signaling Pathway in BMECs. Int. J. Mol. Sci. 2022, 23, 13307. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shao, J.; Li, Y.; Zhao, F.Q.; Liu, J.X.; Liu, H. Protective Effects of Inorganic and Organic Selenium on Heat Stress in Bovine Mammary Epithelial Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1503478. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle. Antioxidants 2019, 9, 29. [Google Scholar] [CrossRef]
- Hu, L.; Sammad, A.; Zhang, C.; Brito, L.F.; Xu, Q.; Wang, Y. Transcriptome Analyses Reveal Essential Roles of Alternative Splicing Regulation in Heat-Stressed Holstein Cows. Int. J. Mol. Sci. 2022, 23, 10664. [Google Scholar] [CrossRef]
- Oh, S.; Hosseindoust, A.; Ha, S.; Moturi, J.; Mun, J.; Tajudeen, H.; Kim, J. Metabolic Responses of Dietary Fiber during Heat Stress: Effects on Reproductive Performance and Stress Level of Gestating Sows. Metabolites 2022, 12, 280. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Li, L.; Sun, X.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Effects of Herbal Tea Residue on Growth Performance, Meat Quality, Muscle Metabolome, and Rumen Microbiota Characteristics in Finishing Steers. Front. Microbiol. 2021, 12, 821293. [Google Scholar] [CrossRef]
- Poscic, N.; Montanari, T.; D’Andrea, M.; Licastro, D.; Pilla, F.; Ajmone-Marsan, P.; Minuti, A.; Sgorlon, S. Breed and adaptive response modulate bovine peripheral blood cells’ transcriptome. J. Anim. Sci. Biotechnol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Khan, M.Z.; Xiao, J.; Alugongo, G.M.; Liu, S.; Wang, J.; Cao, Z. Effect of the Combining Corn Steep Liquor and Urea Pre-treatment on Biodegradation and Hydrolysis of Rice Straw. Front. Microbiol. 2022, 13, 916195. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Zhang, P.; Zhou, Y.; Huang, X.; Yan, Q.; Tan, Z.; Tang, S.; Wan, F. Alterations in nutrient digestibility and performance of heat-stressed dairy cows by dietary L-theanine supplementation. Anim. Nutr. 2022, 11, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.C.; Datsomor, O.; Cheng, Z.Q.; Meng, Z.T.; Zhan, K.; Yang, T.Y.; Huang, Y.H.; Yan, Q.; Zhao, G.Q. Partial Substitution of Alfalfa Hay by Stevia (Stevia rebaudiana) Hay Can Improve Lactation Performance, Rumen Fermentation, and Nitrogen Utilization of Dairy Cows. Front. Vet. Sci. 2022, 9, 899148. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. Alfalfa pellet-induced subacute ruminal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation. J. Dairy Sci. 2009, 92, 1712–1724. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Peng, W.C.; Liu, J.X.; Xu, G.Z.; Wang, D.M. Effect of chromium methionine supplementation on lactation performance, hepatic respiratory rate and anti-oxidative capacity in early-lactating dairy cows. Animal 2021, 15, 100326. [Google Scholar] [CrossRef] [PubMed]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Vanhatalo, A.; Jaakkola, S. The effect of partial substitution of rapeseed meal and faba beans by Spirulina platensis microalgae on milk production, nitrogen utilization, and amino acid metabolism of lactating dairy cows. J. Dairy Sci. 2019, 102, 7102–7117. [Google Scholar] [CrossRef]
- Grossi, S.; Compiani, R.; Rossi, L.; Dell’Anno, M.; Castillo, I.; Sgoifo Rossi, C.A. Effect of Slow-Release Urea Administration on Production Performance, Health Status, Diet Digestibility, and Environmental Sustainability in Lactating Dairy Cows. Animals 2021, 11, 2405. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Leung, K.W.; Yang, S.; Wang, X.; Tang, K.; Hu, J. Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai-Tibetan Plateau. Biology 2021, 10, 1081. [Google Scholar] [CrossRef]
- Pang, K.; Chai, S.; Yang, Y.; Wang, X.; Liu, S.; Wang, S. Dietary forage to concentrate ratios impact on yak ruminal microbiota and metabolites. Front. Microbiol. 2022, 13, 964564. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Xu, N.; Yang, F.; Yoon, I.; Chung, Y.; Liu, J.; Wang, J. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotechnol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Kowalski, Z.M.; Andrieu, S.; Micek, P. On farm impact: Optigen®in diets fed high yielding dairy cows. In Proceedings of the Alltech’s 23rd Annual Symposium, Lexington, KY, USA, 20–23 May 2007; Lyons, T.P., Jacques, K.A., Eds.; Alltech: Nicholasville, KY, USA, 2010. [Google Scholar]
- Giallongo, F.; Hristov, A.N.; Oh, J.; Frederick, T.; Weeks, H.; Werner, J.; Lapierre, H.; Patton, R.A.; Gehman, A.; Parys, C. Effects of slow-release urea and rumen-protected methionine and histidine on performance of dairy cows. J. Dairy Sci. 2015, 98, 3292–3308. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, L.; Lu, Y.; Wu, B.; Gong, X.; Liu, M.; Wang, J.; Sun, Q.; Vierling, E.; Xu, S. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lei, Q.; Ma, H.; Jiang, M.; Yang, T.; Ma, Q.; Datsomor, O.; Zhan, K.; Zhao, G. Phloretin Protects Bovine Rumen Epithelial Cells from LPS-Induced Injury. Toxins 2022, 14, 337. [Google Scholar] [CrossRef]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Z.; Yu, Z.; Zhu, W. Effects of dietary replacement of soybean meal with dried distillers grains with solubles on the microbiota occupying different ecological niches in the rumen of growing Hu lambs. J. Anim. Sci. Biotechnol. 2020, 11, 93. [Google Scholar] [CrossRef]
- Varijakshapanicker, P.; McKune, S.; Miller, L.; Hendrickx, S.; Balehegn, M.; Dahl, G.E.; Adesogan, A.T. Sustainable livestock systems to improve human health, nutrition, and economic status. Anim. Front. 2019, 9, 39–50. [Google Scholar] [CrossRef]
- Azizi, A.; Sharifi, A.; Azarfar, A.; Kiani, A.; Jolazadeh, A. Performance and ruminal parameters of fattening Moghani lambs fed recycled poultry bedding. Anim. Nutr. 2017, 3, 145–150. [Google Scholar] [CrossRef]
| Items | Soybean Meal Group (SM) | Slow-Release Urea Group (SRU) |
|---|---|---|
| Ingredient, % of DM | ||
| Alfalfa hay | 8.5 | 8.5 |
| Oaten hay | 6.5 | 6.5 |
| Corn silage | 59.2 | 59.6 |
| Corn grain | 5.3 | 5.3 |
| Soybean meal | 4.0 | 3.5 |
| Slow-release urea | 0 | 0.1 |
| DDGS | 3.7 | 3.7 |
| Oatmeal | 4 | 4 |
| Rootlet | 1.6 | 1.6 |
| Spray corn husk | 2 | 2 |
| Corn germ meal | 3 | 3 |
| Premix 1 | 2.2 | 2.2 |
| Total | 100 | 100 |
| Nutrient composition | ||
| DM, % | 51.38 | 51.03 |
| Ash, % of DM | 6.98 | 5.67 |
| Crude protein, % of DM | 14.93 | 15.14 |
| Crude fat, % of DM | 2.21 | 2.37 |
| NDF, % of DM | 37.56 | 37.08 |
| ADF, % of DM | 21.15 | 20.95 |
| Ca, % of DM | 0.65 | 0.68 |
| P, % of DM | 0.32 | 0.35 |
| NEL 2, Mcal/kg of DM | 1.57 | 1.53 |
| Item | Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| SM | SRU | |||
| Rectal temperature (°C) | ||||
| 08:00 | 38.50 | 38.42 | 0.05 | 0.433 |
| 14:00 | 39.21 | 39.08 | 0.06 | 0.392 |
| 20:00 | 38.92 | 38.73 | 0.12 | 0.083 |
| Average | 38.88 | 38.74 | 0.08 | 0.224 |
| Respiration rate (breaths/min) | ||||
| 08:00 | 50.06 | 48.83 | 1.70 | 0.581 |
| 14:00 | 70.10 | 64.77 | 1.53 | 0.144 |
| 20:00 | 59.55 | 54.63 | 1.03 | 0.071 |
| Average | 59.91 | 56.08 | 1.55 | 0.153 |
| Item | Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| SM | SRU | |||
| DMI, kg/d | 22.45 | 21.47 | 0.49 | 0.342 |
| Milk yield, kg/d | 23.52 | 25.25 | 0.35 | 0.081 |
| ECM 2, kg/d | 25.80 | 27.70 | 0.52 | 0.423 |
| Milk fat, % | 4.03 | 4.04 | 0.08 | 0.310 |
| Milk protein, % | 3.52 | 3.54 | 0.11 | 0.611 |
| Milk lactose, % | 5.23 | 5.21 | 0.19 | 0.481 |
| Total solids, % | 17.09 | 16.85 | 0.63 | 0.302 |
| SCC, ×103/mL | 181.21 | 217.88 | 12.58 | 0.582 |
| MUN 3, mg/dL | 13.99 | 15.09 | 0.25 | <0.001 |
| Item | Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| SM | SRU | |||
| AST, U/L | 70.08 | 96.32 | 5.08 | 0.007 |
| ALT, U/L | 23.04 | 28.69 | 1.11 | 0.008 |
| ALP, U/L | 35.68 | 32.37 | 1.96 | 0.411 |
| γ-GT, U/L | 20.60 | 29.15 | 1.64 | 0.006 |
| T-AA, μmol/L | 3.50 | 3.64 | 0.06 | 0.234 |
| SOD, U/mL | 84.87 | 83.09 | 2.27 | 0.704 |
| GSH-PX, U/mL | 350.42 | 365.10 | 6.82 | 0.292 |
| CAT, U/mL | 24.04 | 25.54 | 1.29 | 0.573 |
| TAOC, U/mL | 5.65 | 5.91 | 0.34 | 0.705 |
| MDA, nmol/mL | 4.84 | 4.59 | 0.14 | 0.382 |
| HSP-70, pg/mL | 343.11 | 299.79 | 9.13 | 0.014 |
| HSP-90α, pg/mL | 49.09 | 40.86 | 1.41 | 0.002 |
| Item | Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| SM | SRU | |||
| Rumen pH | 6.58 | 6.65 | 0.06 | 0.595 |
| TVFA (mM) | 93.41 | 104.86 | 3.38 | 0.090 |
| Acetate (mM) | 59.38 | 68.53 | 2.25 | 0.034 |
| Propionate (mM) | 19.67 | 21.44 | 1.00 | 0.403 |
| Butyrate (mM) | 10.94 | 12.10 | 0.41 | 0.161 |
| Isobutyrate (mM) | 0.88 | 0.85 | 0.05 | 0.897 |
| Valerate (mM) | 1.28 | 1.14 | 0.06 | 0.294 |
| Isovalerate (mM) | 1.29 | 0.79 | 0.11 | 0.010 |
| Acetate/Propionate | 3.07 | 3.23 | 0.09 | 0.402 |
| NH3 (mg/dL) | 12.99 | 17.50 | 1.55 | 0.153 |
| Item | Treatment 1 | SEM | p-Value | |
|---|---|---|---|---|
| SM | SRU | |||
| CH4, g/d | 432.30 | 413.56 | 9.44 | 0.336 |
| CH4, g/L milk | 18.38 | 16.37 | 0.45 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Zhang, X.; Wang, K.; Datsomor, O.; Li, X.; Lin, M.; Feng, C.; Zhao, G.; Zhan, K. Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows. Animals 2023, 13, 2771. https://doi.org/10.3390/ani13172771
Jiang M, Zhang X, Wang K, Datsomor O, Li X, Lin M, Feng C, Zhao G, Zhan K. Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows. Animals. 2023; 13(17):2771. https://doi.org/10.3390/ani13172771
Chicago/Turabian StyleJiang, Maocheng, Xuelei Zhang, Kexin Wang, Osmond Datsomor, Xue Li, Miao Lin, Chunyan Feng, Guoqi Zhao, and Kang Zhan. 2023. "Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows" Animals 13, no. 17: 2771. https://doi.org/10.3390/ani13172771
APA StyleJiang, M., Zhang, X., Wang, K., Datsomor, O., Li, X., Lin, M., Feng, C., Zhao, G., & Zhan, K. (2023). Effect of Slow-Release Urea Partial Replacement of Soybean Meal on Lactation Performance, Heat Shock Signal Molecules, and Rumen Fermentation in Heat-Stressed Mid-Lactation Dairy Cows. Animals, 13(17), 2771. https://doi.org/10.3390/ani13172771







