Simple Summary
The aim of this study was to evaluate the antimicrobial efficacy of cinnamon essential oil (CEO) against Escherichia coli (E. coli) strains isolated from poultry with colibacillosis. One hundred and seventeen strains isolated from laying hens, broilers, and turkeys and belonging to serogroups O78, O2, O128, O139, which are often responsible for avian colibacillosis, were analyzed. The minimum inhibitory concentration (MIC)50 and MIC90 of CEO were evaluated by testing each bacterial strain at cell densities of 108 CFU/mL and 106 CFU/mL, respectively. At 108 CFU/mL, MIC50 and MIC90 were, respectively, 0.4 and 0.5 µL/mL for the strains from laying hens, and 0.5 and 0.6 µL/mL for strains from turkeys. MIC50 and MIC90 corresponded to 0.5 µL/mL for the strains isolated from broilers. Grouping the strains according to the serogroup, MIC50 and MIC90 were 0.4 and 0.5 µL/mL for strains belonging to serogroups O78, O2, and O128. A concentration of 0.5 µL/mL of CEO corresponded to both MIC50 and MIC90 for strains belonging to serogroup O139. MIC50 and MIC90 of CEO were 0.3 and 0.4 µL/mL respectively for strains tested at the cell density of 106 CFU/mL, regardless of the bird species of origin. According to the serogroups, MIC50 and MIC90 were 0.3 and 0.4 µL/mL for strains belonging to serogroups O78 and O2. A concentration of 0.4 µL/mL of CEO corresponded both to MIC50 and MIC90 for strains belonging to serogroups O139 and O128. This study showed that CEO has effective antibacterial activity against pathogenic E. coli in poultry.
Abstract
Colibacillosis, caused by E. coli, is responsible for economic losses in the poultry industry due to mortality, decreased production, and the cost of antibiotic treatments. Prevention of colibacillosis is based on improved biosecurity measures and the use of the vaccine performed with O78 E. coli strains, which is responsible for most cases of colibacillosis. Recently, there has been increased interest in other infection control methods, such as the use of natural compounds. The aim of this study was to evaluate the antimicrobial efficacy of cinnamon essential oil (CEO) against E. coli strains isolated from poultry. The MIC50 and MIC90 of CEO were determined by testing 117 strains belonging to serogroups O78, O2, O128, O139, isolated from laying hens (91 strains), broilers (10 strains), and turkeys (16 strains). The bacterial strains were tested at cell densities of 108 and 106 CFU/mL. At the cell density of 108 CFU/mL, MIC50 and MIC90 were 0.4 and 0.5 µL/mL for most of the tested strains, while they corresponded to 0.5 µL/mL for all strains isolated from broilers and for strains belonging to serogroup O139. At the cell density of 106 CFU/mL, MIC50 and MIC90 were 0.3 and 0.4 µL/mL, regardless of bird species of origin and for strains belonging to serogroups O78 and O2. In addition, a concentration of 0.04 µL/mL of CEO corresponded both to MIC50 and MIC90 for strains belonging to serogroups O139 and O128. Based on these results, cinnamon essential oil showed an effective antibacterial activity against E. coli strains from poultry and could find field application for the prevention of colibacillosis.
1. Introduction
Escherichia coli (E. coli) is a commensal microorganism that colonizes the lower gastrointestinal tract of mammals and birds shortly after birth and acts as a symbiont involved in the synthesis of vitamins needed by the hosts [1]. E. coli can also enrich its accessory genome with virulence genes (VG) that allow it to adapt to unfavorable environmental conditions by colonizing even extra-intestinal organ niches [2]. Certain serotypes, generally having different virulence factors [3,4] are more frequently associated to colibacillosis. Therefore, E. coli strains are classified into two main groups: intestinal pathogenic E. coli (InPEC) and pathogenic E. coli extra-intestinal (ExPEC) [5]. A subgroup of the latter, which is called avian pathogenic E. coli (APEC), is particularly relevant to the poultry industry because it causes colibacillosis, a disease with significant economic losses due to mortality and decreased productivity of the affected birds. The most frequent clinical forms of colibacillosis are as follows: yolk sac infection (omphalitis) in broiler chicks; septicemia in broiler chicks and adults; reproductive tract infections (salpingitis) in laying hens; respiratory infections, predominantly air sacculitis in both broilers and layers [5,6], and lesions in other visceral organs [6]. APEC strains use several virulence and pathogenesis factors, mainly adhesins, iron acquisition systems, and toxins [7]. These factors facilitate the evasion of host immune responses and systemic spread of APEC, enabling infection in chickens [7]. In addition to these factors, secretion systems (type III and VI), quorum sensing (QS) systems, transcriptional regulators, two-component systems, and metabolism-associated genes contribute to the APEC pathogenesis in chickens [8,9,10,11]. APEC virulence factors are also involved in mechanisms of resistance to antibiotics, such as β-lactams and colistin, which may pose a high risk to humans due to the transmission of antibiotic-resistant bacteria and genes through the food chain [12]. O1, O2, and O78 are the most common antigenic serotypes of APEC in poultry [13,14]. However, other serotypes are associated with colibacillosis in poultry, such as E. coli O111, which causes severe septicemia and polyserositis in hens [15], E. coli O128 and O139 isolated from broilers with a history of respiratory symptoms and pericarditis, peri-hepatitis, and air sacculitis [16,17]. A major predisposing factor for systemic APEC infections is stress, which can be induced by a variety of agents or by inappropriate husbandry practices [18]. Poor hygiene and lack of biosecurity measures in herd management and among farms may promote the spread of the infections and virulence determinants.
Candidate vaccines produced using pathogenic strains isolated from birds of affected flocks have been tested for prevention of colibacillosis [19,20,21,22]. Recently, a commercial live vaccine performed with O78 E. coli strain and registered in the EU in 2013 [23] was made available in most countries namely Italy, Germany, Spain, and France where laying hens are intensively reared. The vaccine provides effective protection in case of challenge with O78 wild strains [24]. However, it is less effective against infections due to strains belonging to other serogroups [25]. A study using the live commercial O78 vaccine and an inactivated candidate vaccine containing E. coli strains O18, O78, and O111 showed a better level of protection against colibacillosis conferred by combining the two types of vaccines rather than administering them separately [26]. Control of colibacillosis has historically been achieved using different classes of antimicrobials. However, considering the increase in antimicrobial resistance related to the excessive use of antibiotics, the potential risk of transmission of resistant bacteria to humans through the consumption of foods of animal origin, and the possible exposure of veterinarians and farmers to animals contaminated with antibiotic-resistant bacteria [27], alternative control strategies are recommended as part of a “One Health” approach that relies on integrated and unifying prevention measures to protect animal and public health [28,29]. In addition, economic losses occur in poultry farms due to compliance with the withdrawal period during and after the treatment.
Therefore, the poultry industry is currently focused on eliminating the use of antibiotics, thus seeking innovative management systems [30]. The production of antibiotic-free broiler has increased due to consumer perception that poultry meat is qualitatively superior to conventionally produced meat [31,32]. Alternative methods to antibiotics for the control of bacterial diseases in poultry farms could be natural substances such as some essential oils that, due to their potential antimicrobial properties, could find application in poultry phytotherapy [33]. Cinnamon has potential antibacterial activity that seems to be related to cinnamaldehyde, also known as cinnamic aldehyde, which is the main chemical constituent of cinnamon plants. The concentration of cinnamic aldehyde depends on the species of cinnamon and the part of the plant used to extract the essential oil [34,35,36]. Other bioactive compounds such as coumarins, alkaloids, tannins, and phenols that are constituents, although in smaller amounts than cinnamaldehyde, may contribute to the beneficial effects of cinnamon plants [37,38]. The aim of this study was to evaluate the antimicrobial efficacy of cinnamon essential oil (CEO) against APEC strains isolated from laying hens with colibacillosis.
2. Material and Methods
2.1. Bacterial Strains Used for the Experiment
One hundred and seventeen E. coli strains were used for the analysis. They were from the bacterial strain collection of the Avian Diseases section, Department of Veterinary Medicine, Valenzano, BA, Italy. All strains were isolated between 2001 and 2022 from laying hens, broilers, and turkey that died because of colibacillosis. Strains were grown on MacConkey agar (Oxoid, Basingstoke, UK) and incubated at 37 °C for 24 h. Each colony morphologically compatible with E. coli was selected and transferred onto nutrient agar (Oxoid) and incubated at 37 °C for 24 h. The colonies were tested for indole and gas production, and oxidase activity. Gas/indole-positive and oxidase-negative colonies were identified as E. coli using the API-20E biochemical gallery (bio Mérieux, Marcy l’Étoile, Lyon, France). Each strain was stored in Brucella broth (Oxoid) at −20 °C and glycerol (10%). The strains selected for the experiment belonged to some serogroups frequently responsible for colibacillosis in poultry. In detail, 54 strains belonged to serogroup O78, 37 to serogroup O2, 19 to serogroup O139, and 7 to serogroup O128.
2.2. Preparation of Bacterial Suspensions
Before analysis, all strains were grown on tryptic soy agar (TSA) (Oxoid, Basingstoke, UK) at 37 °C overnight. A bacterial suspension of 0.5 McFarland standard corresponding to 1–2 × 108 CFU/mL [39] was prepared from each strain, using sterile saline solution (0.9%). Starting with a cell density of 108 CFU/mL, bacterial suspensions of 106 CFU/mL were obtained using stepwise dilutions.
2.3. Cinnamon Essential Oil and Preparation of Medium
Commercial (ERBA VITA GROUP S.p.A., Chiesanuova, San Marino) hydro-distilled pure cinnamon (Cinnamomum zeylanicum Blume) essential oil 100% pure (CEO) was used for the experiment.
The efficacy tests were performed on Muller–Hinton agar (Oxoid) reconstituted according to the manufacturer’s instructions and autoclaved at 121 °C for 15 min. The broth was heated to 50 °C in a thermostatic bath before adding CEO in different volumes according to the concentrations to be tested.
2.4. Preliminary Test
A preliminary efficacy test was performed using 4 strains of E. coli belonging to serogroups O2 (2 strains) and O78 (2 strains). Bacterial suspensions with cell density of 108 CFU/mL and 106 CFU/mL, respectively, were prepared from each strain.
According to a previous study [40], 10 µL of suspension with cell density of 108 CFU/mL were spot inoculated on Muller–Hinton agar containing essential oil in concentrations from 0.01 to 1% (0.01, 0.05, 0.1, 0.5, 1%) corresponding to 0.1 µL/mL to 10 µL/mL (0.1, 0.5, 1, 5, 10 µL/mL), respectively.
Ten µL of 106 CFU/mL bacterial suspension was spot inoculated on the oil-containing medium in concentrations ranging from 0.005 to 0.5% (0.005, 0.01, 0.05, 0.1, 0.5%) corresponding to 0.05 to 5 µL/mL (0.05, 0.1, 0.5, 1, 5 µL/mL).
Each suspension was inoculated simultaneously on oil-free Muller–Hinton agar, as positive control for bacterial growth. All plates were incubated at 37 °C for 24 h under aerobic conditions. The inhibitory activity of CEO was assessed by bacterial growth or nongrowth in the spot.
2.5. Preparation of the Efficacy Tests
Based on the results obtained in the preliminary test, concentrations of CEO from 0.01 to 0.08% (0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08%) corresponding to 0.1 to 0.8 µL/mL (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8 µL/mL) and 0.01 to 0.05% (0.01, 0.02, 0.03, 0.04, 0.05%) corresponding to 0.1 to 0.5 µL/mL (0.1, 0.2, 0.3, 0.4, 0.5 µL/mL) were chosen to assess the sensitivity of each strain at cell densities of 108 CFU/mL and 106 CFU/mL, respectively. The bacterial suspensions were spot inoculated on the medium. Each suspension was inoculated simultaneously on oil-free Muller–Hinton agar, as positive control for bacterial growth. After 24 h at 37 °C in aerobic conditions, the results were read by evaluating the efficacy of CEO based on strain growth/no growth of the strains in the spot. A numbered grid placed under the plate was used for both inoculation and strain identification (Figure 1). Each experiment was carried out twice on two different days.

Figure 1.
(A). Growth of strains in the corresponding spots; (B). Numbered grid for strain identification.
2.6. Statistical Analysis
Inhibition data were analyzed by univariate statistical analysis (Pearson’s chi-square test and Fisher’s exact test for independence). Values of p < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 13 software for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Preliminary Tests
All strains tested at a cell density of 108 CFU/mL were inhibited by essential oil at concentrations ranging from 10 to 1 µL/mL (10, 5, 1 µL/mL). Only one strain belonging to serotype O78 grew in the presence of 0.5 µL/mL essential oil. All strains grew with 0.1 µL/mL of essential oil.
At the cell density of 106 CFU/mL, all strains were inhibited by CEO from 5 to 0.5 µL/mL, while they grew with concentrations of 0.1 and 0.05 µL/mL of CEO.
3.2. Efficacy Tests
MIC50 and MIC90 were determined by testing different cell densities of E. coli strains. At 108 CFU/mL, MIC50 and MIC90 of CEO were 0.4 and 0.5 µL/mL, respectively (Figure 2) (Table 1).
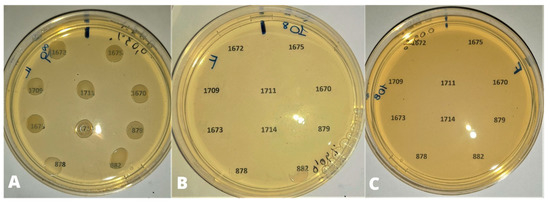
Figure 2.
Bacterial density of 108 UFC/mL. (A) 0.03% (0.3 µL/mL) of CEO: no inhibition of strain growth; (B) 0.04% (0.4 µL/mL) of CEO: inhibition of growth except for strains 878 and 882 (partial inhibition); (C) 0.05% (0.5 µL/mL) of CEO: inhibition of bacterial growth.

Table 1.
Inhibitory effect of cinnamon essential oil against E. coli strains (108 CFU/mL) grouped according to the bird species of origin.
Considering the whole strain pool, only four strains out of 117 strains (3.41% of the population) were inhibited at concentration of 0.3 µL/mL. All tested strains were inhibited at concentrations higher than 0.5 µL/mL. Grouping the bacterial strains according to the bird species of origin, MIC50 and MIC90 of CEO were, respectively, 0.4 and 0.5 µL/mL for strains from laying hens, and 0.5 and 0.6 µL/mL for strains from turkeys. MIC50 and MIC90 corresponded to 0.5 µL/mL for all strains isolated from broilers.
Grouping the strains according to the serogroup, (Table 2), MIC50 and MIC90 were 0.4 and 0.5 µL/mL for strains belonging to serogroups O78, O2, and O128. One (2.7%) and three (5.56%) strains belonging respectively to serogroups O2 and O78 were inhibited by 0.3 µL/mL of CEO. Concentration of 0.5 µL/mL of CEO corresponded to both MIC50 and MIC90 for strains belonging to serogroup O139.

Table 2.
Inhibitory effect of cinnamon essential oil against E. coli strains (108 CFU/mL) grouped according to the serogroup.
MIC50 and MIC90 of CEO were 0.3 and 0.4 µL/mL respectively for bacterial strains tested at the cell density of 106 CFU/mL (Figure 3) (Table 3), regardless of bird species of origin.
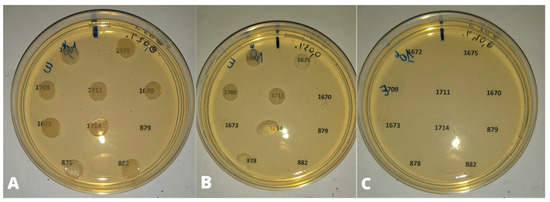
Figure 3.
Bacterial density of 106 UFC/mL. (A) 0.02% (0.2 µL/mL) of CEO: no inhibition of bacterial growth except for strain 879; (B) 0.03% (0.3 µL/mL) of CEO: growth inhibition of strains 1670, 1673, 879, 882; (C) 0.04% (0.4 µL/mL) of CEO: inhibition of bacterial growth.

Table 3.
Inhibitory effect of cinnamon essential oil against E. coli strains (106 CFU/mL) grouped according to the bird species of origin.
Eleven strains out of 117 (9.4%), including nine strains from laying hens, one from turkeys and one from broilers were inhibited by 0.2 µL/mL of CEO. All strains were inhibited by 0.4 µL/mL of CEO.
According to the serogroups (Table 4), the MIC50 and MIC90 were 0.3 and 0.4 µL/mL for strains belonging to serogroups O78 and O2. Seven strains out of 54 and four out of 37, belonging respectively to serogroups O78 and O2, were inhibited by 0.2 µL/mL of CEO (Table 4). Concentration of 0.4 µL/mL of CEO corresponded both to MIC50 and MIC90 for strains belonging to serogroups O139 and O128.

Table 4.
Inhibitory effect of cinnamon essential oil against E. coli strains (106 CFU/mL) grouped according to the serogroup.
4. Discussion
Based on the data obtained, cinnamon essential oil (CEO) has effective antibacterial effects against pathogenic E. coli in poultry, regardless of the bacterial cell density used in the experiments.
Concerning Cinnamomum zeylanicum, used in the experiments, the major components of the essential oil are cinnamaldehyde (88.2%), benzyl alcohol (8.0%), and eugenol (1.0%), which have synergistic or additive effects [41,42]. The combination of these components allows cinnamaldehyde to penetrate the phospholipid bilayer of bacterial cell walls more easily and bind more readily to proteins, preventing them from performing normal functions and causing cytoplasmic coagulation, denaturation of enzymes and proteins, and loss of metabolites and ions [43,44]. The efficacy of CEO against the strains tested is of particular interest considering that E. coli, which is Gram-negative, has a thick outer membrane layer of lipopolysaccharides covering the cell wall, potentially making it more resistant to hydrophobic substances than Gram-positive bacteria [45,46,47]. Cinnamaldehyde has important anti-adhesive properties against pathogenic E. coli [48]. By testing E. coli ATCC 25,922 [49], it has been shown that cinnamaldehyde may suppress bacterial growth prolonging the lag phase, may increase cell membrane permeability causing its collapse and leakage of cell contents, and may cause oxidative damage to the bacterial cell membrane. On the other hand, regarding E. coli and other Gram-negative bacteria such as Salmonella spp., Pseudomonas spp., and Vibrio spp., cinnamon is able not only to alter ATP-ase activity and thus the permeability of cell membranes, but also to interfere with mitochondrial functions and cell division mechanisms of bacterial cells [50]. In addition, cinnamaldehyde can downregulate genes associated with the flagellar system and biofilm formation [36,51]. In the case of E. coli ATCC 8735, exposure to low concentrations of cinnamaldehyde changes its structure and morphology, altering its fatty acid composition and binding directly to genomic DNA [52].
The MIC values found in our study ranged from 0.2 to 0.5 µL/mL and 0.3 to 0.8 µL/mL for the strains tested with a cell density of 106 UFC/mL and 108 UFC/mL, respectively, in agreement with a previous study [53]. Variable MIC values were reported in other studies. MICs ranged from 0.8 to 3.2 mg/mL of cinnamaldehyde for E. coli strains tested with a cell density of 105 UFC/mL [54], and 1 mg/mL [47] or 2.5 mg/mL [55] for a bacterial cell density of 107 UFC/mL. Finally, 6.25 mg/mL was the minimum concentration of cinnamaldehyde effective in inhibiting the growth of bacteria with a cell density of 108 UFC/mL [56]. The different MIC values found in the experiments could be related to the method used to extract the essential oil, as the amount of cinnamaldehyde in the oil may vary depending on the solvent and the pressure and temperature parameters used in the extraction method [57,58,59]. In addition, the laboratory conditions, such as exposition to high temperatures [60,61], prolonged air contact, and exposure to light, while performing experiments, can cause degradation of cinnamaldehyde and affect its efficacy [61,62,63]. The 100% pure essential oil used in this study was obtained by hydro distillation of cinnamon bark, as it provides a high cinnamaldehyde content (between 52% and 81%), which is mainly responsible for cinnamon antibacterial activity [64,65].
In our study, CEO showed efficacy, with no relevant differences in MIC values depending on the serogroup of strains, against APEC strains isolated from layers dead from colibacillosis. Moreover, high antimicrobial efficacy was found by testing the strains at a cell density of 108 UFC/mL, usually found in poultry with colibacillosis [66]. Based on these results, CEO may be useful also for therapeutic purposes other than colibacillosis prevention.
Although there are possible differences depending on the serogroup they belong to [67], APEC strains are more frequently endowed with several genes, associated with pathogenic potential, that encode the bacterium’s resistance to attack by the host’s immune system than non-pathogenic fecal strains [13,68,69]. A close relationship was also found between virulence factors and antibiotic resistance. In fact, genes responsible for antimicrobial resistance are often included in conjugative plasmids that may also carry virulence factor determinants [51]. Considering that antibiotic therapy is one of the main control strategies to reduce morbidity and mortality caused by APEC infections and that frequent use of antibiotics may lead to the selection of resistant strains, cinnamon could be useful in limiting antibiotic use. In addition, natural compounds and cinnamon particularly have different targets in the bacterial cell that require the development of more complex resistance mechanisms [43,70]. Another interesting point seems to be the combination of cinnamon essential oil with common antibiotics to reduce their dosage, preserve their effectiveness, and boost their antibacterial efficacy [71]. Previous studies have shown the synergistic activity of CEO in combination with ampicillin and chloramphenicol against Staphylococcus aureus (S. aureus), and with chloramphenicol against E. coli, resulting in a decrease of MICs of the antibiotics [72]. The synergistic interaction of CEO with piperacillin against E. coli [73], and with colistin against multidrug resistant strains of Pseudomonas aeruginosa (P. aeruginosa) [74] was also found. Similarly, thyme essential oil increases the antibacterial efficacy of piperacillin, cefepime, and meropenem against P. aeruginosa compared with the efficacy of the individual drugs [75]. In addition, Italian strawflower (Helichrysum italicum) essential oil increases the efficacy of B-lactams, quinolones, and chloramphenicol against Enterobacter aerogenes, E. coli, and P. aeruginosa, reducing their respective MIC values [76]. The combination of tobramycin and tea tree oil and a mixture of fennel essential oil, cefoxitin, mupirocin, co-trimoxazole, and ciprofloxacin showed relevant antibacterial efficacy against ATCC strains of E. coli and S. aureus [77] and multidrug resistant S. aureus strains [78].
In addition, natural plant-derived compounds such as thymol [79] and cinnamaldehyde [80,81], are rapidly excreted through the kidneys and have a short half-life, making animal products safe for human consumption.
Finally, the antimicrobial efficacy of cinnamon could be enhanced by the association with other natural substances, as previously reported for other essential oils. The combination of essential oil of eucalyptus (Eucalyptus deves) and coriander (Coriandrum Sativum) showed synergy against Yersinia Enterocolitica [82]. The combination of three plant-derived phenolic principles, thymol (contained in large quantities in thyme), carvacrol (in thyme and oregano), and eugenol (in cinnamon and cloves) showed greater antimicrobial efficacy against E. coli, Salmonella enteritidis, S. aureus, and P. aeruginosa than the individual compounds [83]. Various combinations of oregano, thyme, basil, and marjoram also exhibited an additional efficacy against Bacillus cereus, E. coli, and P. aeruginosa [84].
Interestingly, cinnamon is one of the phytogenic feed additives (PFAs) approved by the Food and Drug Administration as an additive in poultry feed [85]. PFAs, in addition to their antimicrobial effect, can stimulate the growth of commensal bacteria in the poultry gut, with beneficial effects on the microbiota [36,86]. Also, the use of CEO as a supplement in the diet of broilers leads to the improvement of intestinal immunocompetence and the increase of villi in the gut mucosal surface [87,88,89]. These effects can indirectly contribute to defending against intestinal infection.
5. Conclusions
In conclusion, this study highlighted the antimicrobial efficacy of CEO against E. coli belonging to some serogroups which are among the most frequently responsible for avian colibacillosis. Considering that colibacillosis is one of the most recurrent and relevant disease in poultry leading to frequent antibiotic use on farms, cinnamon could be a valid option for preventing the infection, especially when combined with other methods such as the increase of biosecurity measures and the use of vaccines. Based on the results of this study, cinnamon could be also useful for the treatment of avian colibacillosis by minimizing the use of antibiotics. However, further studies are needed to better assess this aspect. In addition, another relevant step, should be the evaluation of the most suitable route of CEO administration to birds under field conditions.
Author Contributions
Conceptualization, E.C.; Methodology, G.C. and E.C.; Formal analysis, F.R.D.; Investigation, G.C., A.B., R.L., M.M.D. and E.C.; Resources, G.B.; Data curation, G.C., F.R.D., A.B. and E.C.; Writing—original draft, G.C., F.R.D., F.D. and E.C.; Writing—review & editing, E.C.; Visualization, G.B. and A.C.; Supervision, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- La Ragione, R.; Woodward, M. Virulence factors of Escherichia coli serotypes associated with avian colisepticaemia. Res. Vet. Sci. 2002, 73, 27–35. [Google Scholar] [CrossRef]
- Schouler, C.; Schaeffer, B.; Bree, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic Strategy for Identifying Avian Pathogenic Escherichia coli Based on Four Patterns of Virulence Genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Nolan, L.K.; Vaillancourt, J.P.; Barbieri, N.L.; Logue, C.M. Diseases of Poultry, 14th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 770–830. [Google Scholar]
- Dziva, F.; Stevens, M.P. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef]
- Palaniyandi, S.; Mitra, A.; Herren, C.D.; Zhu, X.; Mukhopadhyay, S. LuxS contributes to virulence in avian pathogenic Escherichia coli O78:K80:H9. Vet. Microbiol. 2013, 166, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bao, Y.; Sun, M.; Dong, W.; Pan, Z.; Zhang, W.; Lu, C.; Yao, H. Two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways. Infect. Immun. 2014, 82, 3867–3879. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Liu, X.; Wang, D.; Liang, H.; Wu, X.; Tian, M.; Ding, C.; Wang, G.; Yu, S. Escherichia coli type III secretion system 2 regulator EtrA promotes virulence of avian pathogenic Escherichia coli. Microbiology 2017, 163, 1515–1524. [Google Scholar] [CrossRef]
- Li, Q.; Yin, L.; Xue, M.; Wang, Z.; Song, X.; Shao, Y.; Liu, H.; Tu, J.; Qi, K. The transcriptional regulator PhoP mediates the tolC molecular mechanism on APEC biofilm formation and pathogenicity. Avian Pathol. 2020, 49, 211–220. [Google Scholar] [CrossRef]
- Osman, K.M.; Kappell, A.D.; Elhadidy, M.; El-Mougy, F.; El-Ghany, W.A.; Orabi, A.; Mubarak, A.S.; Dawoud, T.M.; Hemeg, H.A.; Moussa, I.M.; et al. Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: A risk to public health and food safety. Sci. Rep. 2018, 8, 5859. [Google Scholar] [CrossRef]
- Circella, E.; Pennelli, D.; Tagliabue, S.; Camarda, A. Virulence-associated genes in avian pathogenic Escherichia coli from laying hens in Apulia, southern Italy. Br. Poult. Sci. 2012, 53, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M. Human and avian extraintestinal pathogenic Escherichia coli infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Alborali, G.L.; Bardotti, M.; Candotti, P.; Guadagnini, P.F.; Anna Martino, P.; Stonfer, M. Severe Escherichia coli O111 septicaemia and polyserositis in hens at the start of lay. Avian Pathol. 2000, 29, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, A.; Eid, S.; Mohammed, R. Phenotypic Characterization of Escherichia coli Isolated from Broiler Chicken. SCVMJ 2019, 24, 143–150. [Google Scholar] [CrossRef]
- Giovanardi, D.; Campagnari, E.; Ruffoni Sperati, L.; Pesente, P.; Ortali, G.; Furlattini, V. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian Pathol. 2005, 34, 313–318. [Google Scholar] [CrossRef]
- Barnes, H.J.; Gross, W.B. Diseases of Poultry, 10th ed.; Wiley & Sons: Ames, IA, USA, 1997; pp. 131–141. [Google Scholar]
- Gomis, S.; Babiuk, L.; Allan, B.; Willson, P.; Waters, E.; Hecker, R.; Potter, A. Protection of chickens against a lethal challenge of Escherichia coli by a vaccine containing cpg oligodeoxynucleotides (cpg-odn) as an adjuvant. Avian Dis. 2007, 51, 78–83. [Google Scholar] [CrossRef]
- Gregersen, R.H.; Christensen, H.; Ewers, C.; Bisgaard, M. Impact of Escherichia coli vaccine on parent stock mortality, first week mortality of broilers and population diversity of E. coli in vaccinated flocks. Avian Path. 2010, 39, 287–295. [Google Scholar] [CrossRef]
- Hera, A.; Bures, J. Veterinary autogenous vaccines. Dev. Biol. 2004, 117, 19–25. [Google Scholar]
- Landman, W.J.; Van Eck, J.H. The efficacy of inactivated Escherichia coli autogenous vaccines against the E. coli peritonitis syndrome in layers. Avian Path. 2017, 46, 658–665. [Google Scholar] [CrossRef]
- European Medicine Agency. Summary of the European Public Assessment Report (EPAR) for Poulvac E. coli. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/poulvac-e-coli (accessed on 4 May 2020).
- Galal, H.M.; Tawfek, A.M.; Abdrabou, M.I.; Hessain, A.M.; Alhaaji, J.H.; Kabli, S.A.; Elbehiry, A.; Alwarhi, W.K.; Moussa, I.M. Recent approaches for control of E. coli and respiratory complex in Middle East. Saudi J. Biol. Sci. 2018, 25, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef] [PubMed]
- Koutsianos, D.; Gantelet, H.; Franzo, G.; Lecoupeur, M.; Thibault, E.; Cecchinato, M.; Koutoulis, K.C. An Assessment of the Level of Protection Against Colibacillosis Conferred by Several Autogenous and/or Commercial Vaccination Programs in Conventional Pullets upon Experimental Challenge. Vet. Sci. 2020, 7, 80. [Google Scholar] [CrossRef]
- Casalino, G.; Bozzo, G.; Dinardo, F.R.; D’Amico, F.; Dimuccio, M.M.; Camarda, A.; Ceci, E.; Romito, D.; Circella, E. Prevalence and antimicrobial resistence of Campylobacter jejuni and Campylobacter coli from laying hens housed in different rearing systems. Animals 2022, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 18 November 2016).
- European Union Regulation (EU) 2018/848: Of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0848 (accessed on 14 June 2018).
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.H.; Sarker, S.; Islam, M.S.; Islam, M.A.; Karim, M.R.; Kayesh, M.E.; Shiddiky, M.J.; Anwer, M.S. Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology 2020, 9, 411. [Google Scholar] [CrossRef]
- Farooq, M.; Smoglica, C.; Ruffini, F.; Soldati, L.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance Genes Occurrence in Conventional and Antibiotic-Free Poultry Farming, Italy. Animals 2022, 12, 2310. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Szczerbińska, D. Use of Essential Oils in Broiler Chicken Production—A Review. Ann. Anim. Sci. 2017, 17, 317–335. [Google Scholar] [CrossRef]
- Bae, K.H.; Ji, J.M.; Park, K.L. The antibacterial component from Cinnamomic Cortex against a cariogenic bacterium Streptococcus mutans OMZ 176. Arch. Pharm. Res. 1992, 15, 239–241. [Google Scholar] [CrossRef]
- Dayananda, K.R.; Senanayake, U.M.; Wijesekera. Cinnamon and Cassia. The Genus Cinnamomum, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 130–156. [Google Scholar]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Thomas, J.; Kuruvilla, K.M. Cinnamon. Handb. Herbs Spices 2012, 1, 182–196. [Google Scholar]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 1 January 2020).
- Circella, E.; Casalino, G.; D’Amico, F.; Pugliese, N.; Dimuccio, M.M.; Camarda, A.; Bozzo, G. In vitro antimicrobial effectiveness tests using Garlic (Allium sativum) against Salmonella enterica subspecies enterica serovar Enteritidis. Antibiotics 2022, 11, 1481. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.M.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanism of membrane toxicity of hydrocarbons. Micobiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Hsouna, A.B.; Trigui, M.; Mansour, R.B.; Jarraya, R.M.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Pereira, W.A.; Pereira, C.D.; Assunção, R.G.; da Silva, I.S.; Rego, F.S.; Alves, L.S.; Santos, J.S.; Nogueira, F.J.; Zagmignan, A.; Thomsen, T.T.; et al. New insights into the antimicrobial action of cinnamaldehyde towards Escherichia coli and its effects on intestinal colonization of mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- He, T.F.; Wang, L.H.; Niu, D.B.; Wen, Q.H.; Zeng, X.A. Cinnamaldehyde inhibit Escherichia coli associated with membrane disruption and oxidative damage. Arch. Microbiol. 2019, 201, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Mendonça, N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 2012, 3, 18–28. [Google Scholar] [CrossRef]
- He, T.F.; Zhang, Z.H.; Zeng, X.A.; Wang, L.H.; Brennan, C.S. Determination of membrane disruption and genomic DNA binding of cinnamaldehyde to Escherichia coli by use of microbiological and spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2018, 178, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Usha, M.; Ragini, S.; Naqvi, S.M. Antibacterial activity of acetone and ethanol extracts of cinnamon (Cinnamomum zeylanicum) and Ajowan (Trachyspermum ammi) on four food spoilage bacteria. I. Res. J. Biol. Sci. 2012, 1, 7–11. [Google Scholar]
- Prabuseenivasan, S.; Javakumar, M.; Ignacimuthu, S. In vitro antibacterial activuty of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Tajik, H.; Yarahmadi, A.; Sanginabadi, S. Antimicrobial Effect of Cinnamon Essential Oil Against Escherichia Coli and Staphylococcus aureus. Health Scope 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Shangguan, W.; Chen, S.; Zhong, Q. Antibiofilm activities of the cinnamon extract against Vibrio parahaemolyticus and Escherichia coli. Arch. Microbiol. 2021, 203, 125–135. [Google Scholar] [CrossRef]
- Wardatun, S.; Rustiani, E.; Alfiani, N.; Rissani, D. Study effect type of extraction method and type of solvent to cinnamaldehyde and trans-cinnamic acid dry extract cinnamon (Cinnamomum burmanii [Nees & T, Nees] Blume). J. Young Pharm. 2017, 9, 49–51. [Google Scholar]
- Masghati, S.; Ghoreishi, S.M. Supercritical CO2 extraction of cinnamaldehyde and eugenol from cinnamon bark: Optimization of operating conditions via response surface methodology. J. Supercrit. Fluids 2018, 140, 62–71. [Google Scholar] [CrossRef]
- Baseri, H.; Haghighi-Asl, A.; Lotfollahi, M.N. Effects of Operating Parameters on the Cinnamaldehyde Content of Extracted Essential Oil Using Various Methods. Chem. Eng. Technol. 2010, 33, 267–274. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Qi, B.; Harindintwali, J.D.; Koko, M.Y.; Zhang, S.; Li, Y. Encapsulation of cinnamaldehyde: An insight on delivery systems and food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 2521–2543. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, Y.L.; Liang, M.; Dai, S.Y.; Ma, L.; Li, W.G.; Lai, F.; Liu, X.M. Characteristics and hazards of the cinnamaldehyde oxidation process. RSC Adv. 2020, 10, 19124–19133. [Google Scholar] [CrossRef]
- Sedaghat, D.; Dewettinck, K.; Devlieghere, F.; Van der Meeren, P. Influence of non-ionic emulsifier type on the stability of cinnamaldehyde nanoemulsions: A comparison of polysorbate 80 and hydrophobically modified inulin. Food Chem. 2018, 258, 237–244. [Google Scholar] [CrossRef]
- Kazemi, M.; Mokhtariniya, S. Essential oil composition of bark of Cinnamomum zeylanicum. J. Essent. Oil-Bear. Plants 2016, 19, 786–789. [Google Scholar] [CrossRef]
- Boughendjioua, H.; Djeddi, S. Study of The Organoleptic and Physicochemical Properties of Cinnamon Essential Oil (Cinnamomum zeylanicum). Am. J. Life Sci. Res. 2018, 6, 123–130. [Google Scholar]
- Asdrubali, G.; Bolognesi, P.G.; Cabassi, C.S.; Camarda, A.; Catelli, E.; Circella, E.; Cringoli, G.; Delogu, M.; Di Modugno, G.; Dipineto, L.; et al. Manuale di Patologia Aviare, 1st ed.; Le Point Vétérinaire Italie srl: Milan, Italy, 2009; pp. 77–84. [Google Scholar]
- Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Rodríguez-Moctezuma, J.R.; Domínguez-Trejo, P.; Vaca-Paniagua, F.; Vaca, S. Virulence factors, antibiotic resistance phenotypes and O-serogroups of Escherichia coli strains isolated from community acquired urinary tract infection patients in Mexico. J. Microbiol. Immunol. Infect. 2017, 50, 478–485. [Google Scholar] [CrossRef]
- Nakazato, G.; Campos, T.A.; Stehling, E.G.; Brocchi, M.; Silveira, W.D. Virulence factors of avian pathogenic Escherichia coli (APEC). Pesqui. Vet. Bras. 2009, 29, 479–486. [Google Scholar] [CrossRef]
- Mellata, M.; Dho-Moulin, M.; Dozois, C.M.; Curtiss, R.; Brown, P.K.; Arnè, P.; Bréè, A.; Desautels, C.; Fairbrother, J.M. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect. Immun. 2003, 71, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Becerril, R.; Nerín, C.; Gómez-Lus, R. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathog. Dis. 2012, 9, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Shehata, M.A.; Rafeek, E. Virulence genes content and antimicrobial resistance in Escherichia colifrom broiler chickens. Vet. Med. Int. 2014, 2014, 195189. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Yap, P.S.; Lim, S.H.; Hu, C.P.; Yiap, B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef]
- El-Hosseiny, L.; El-Shenawy, M.; Haroun, M.; Abdullah, F. Comparative evaluation of the inhibitory effect of some essential oils with antibiotics against Pseudomonas aeruginosa. Int. J. Antibiot. 2014, 2014, 586252. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzięcioł, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The effect of fennel essential oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef]
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.M.; Caldwell, J. Studies on trans-cinnamaldehyde. I. The influence of dose size and sex on its disposition in the rat and mouse. Food Chem. Toxicol. 1994, 32, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Santiesteban-López, A.; Palou, E.; López-Malo, A. Susceptibility of food-borne bacteria to binary combinations of antimicrobials at selected awand pH. J. Appl. Microbiol. 2007, 102, 485–497. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. Cinnamon: A Natural Feed Additive for Poultry Health and Production—A Review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef]
- Jang, I.S.; Ko, Y.H.; Kang, S.Y.; Lee, C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47. [Google Scholar] [CrossRef]
- Kettunen, H.; Ouwehand, A.; Schulze, H.; Rautonen, N. Dietary essential oil supplementation enhanced intestinal immunocompetence in young broiler chick. Reprod. Nutr. Dev. 2006, 46, S101. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).