Assessment of Harbour Porpoise Bycatch along the Portuguese and Galician Coast: Insights from Strandings over Two Decades
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strandings in Western Iberia
2.1.1. Data Collection
2.1.2. Data Analysis
2.2. Bycatch Assessment and Potential Biological Removal: Insights from Portuguese Data
3. Results
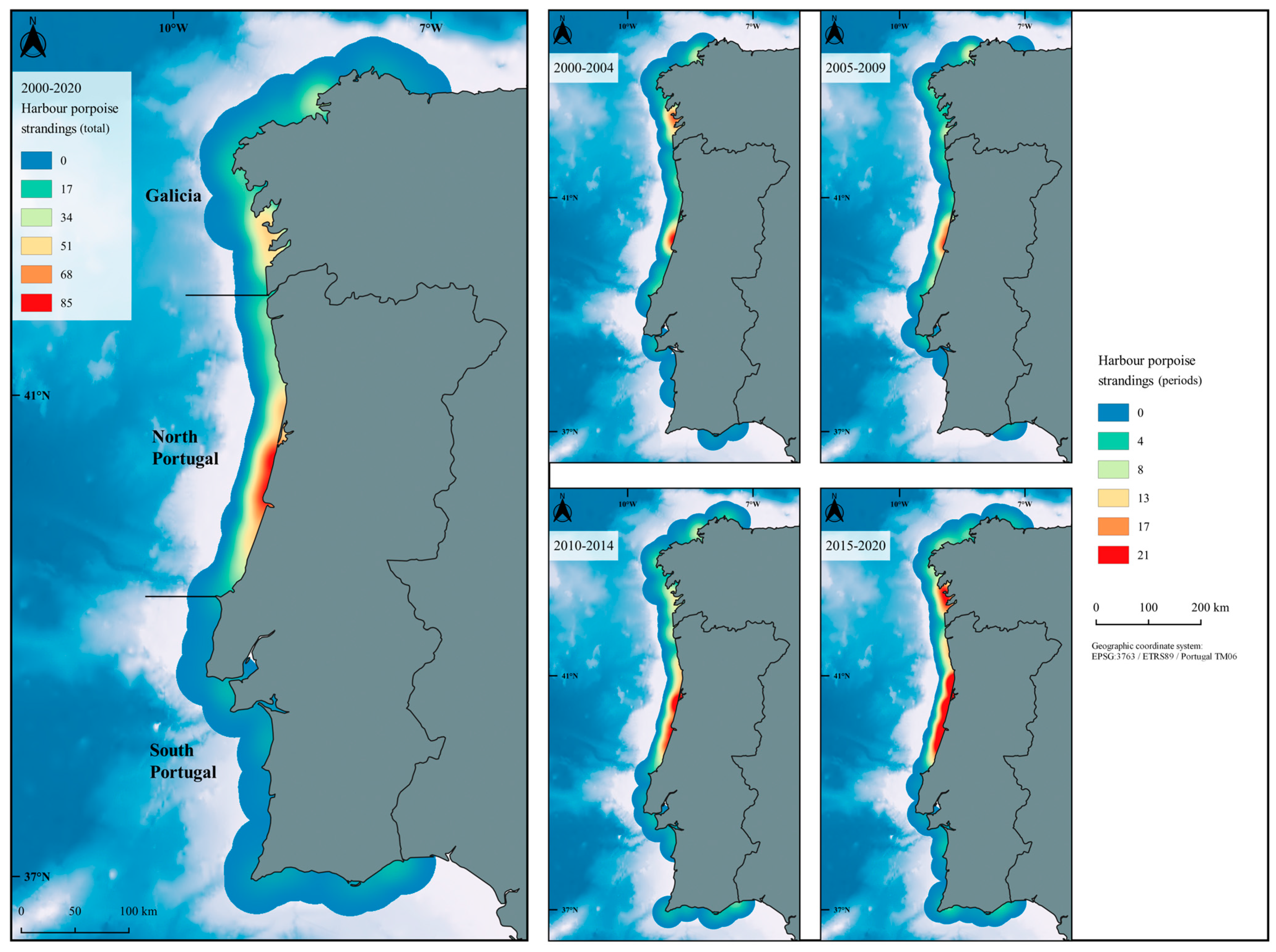
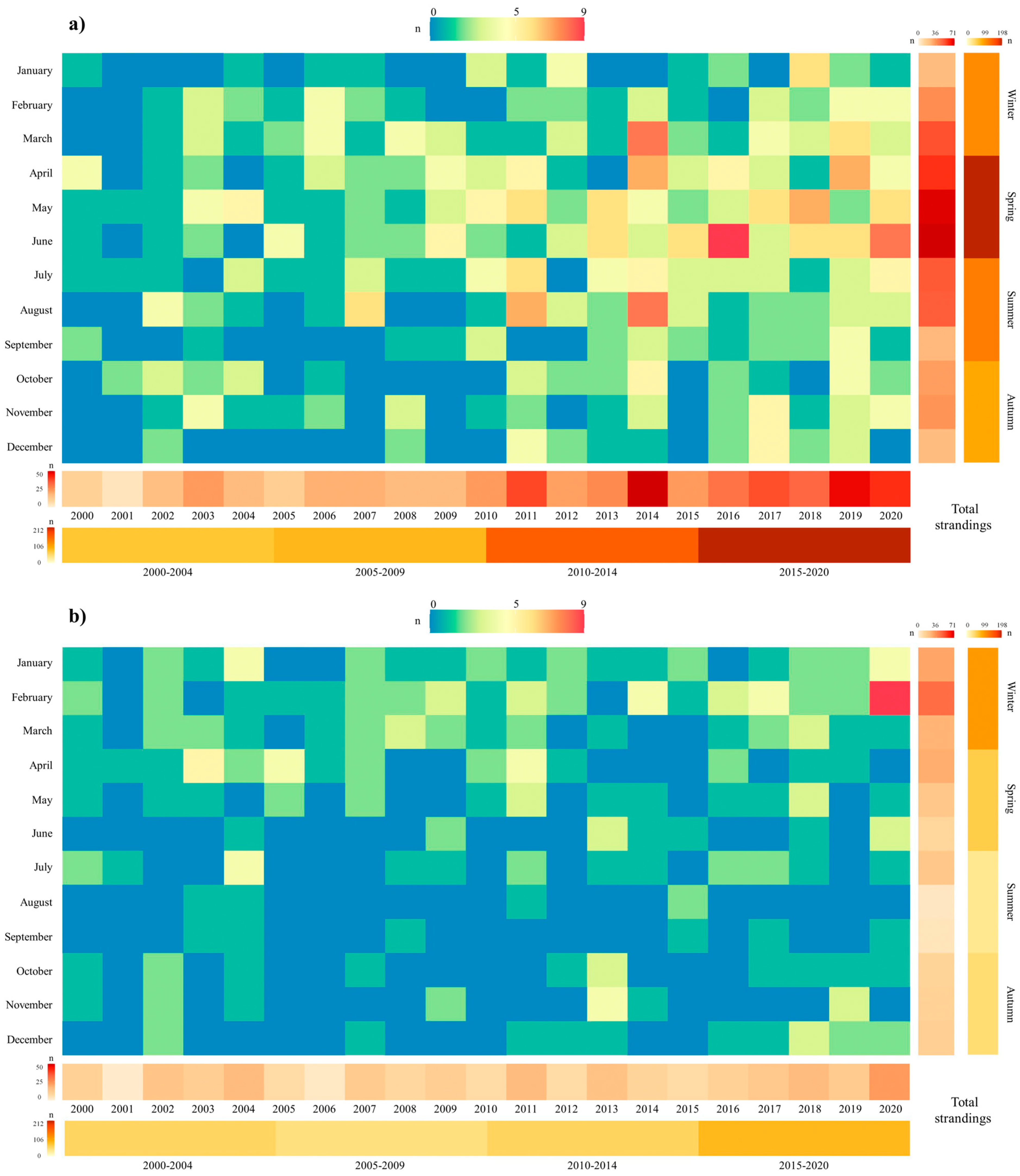
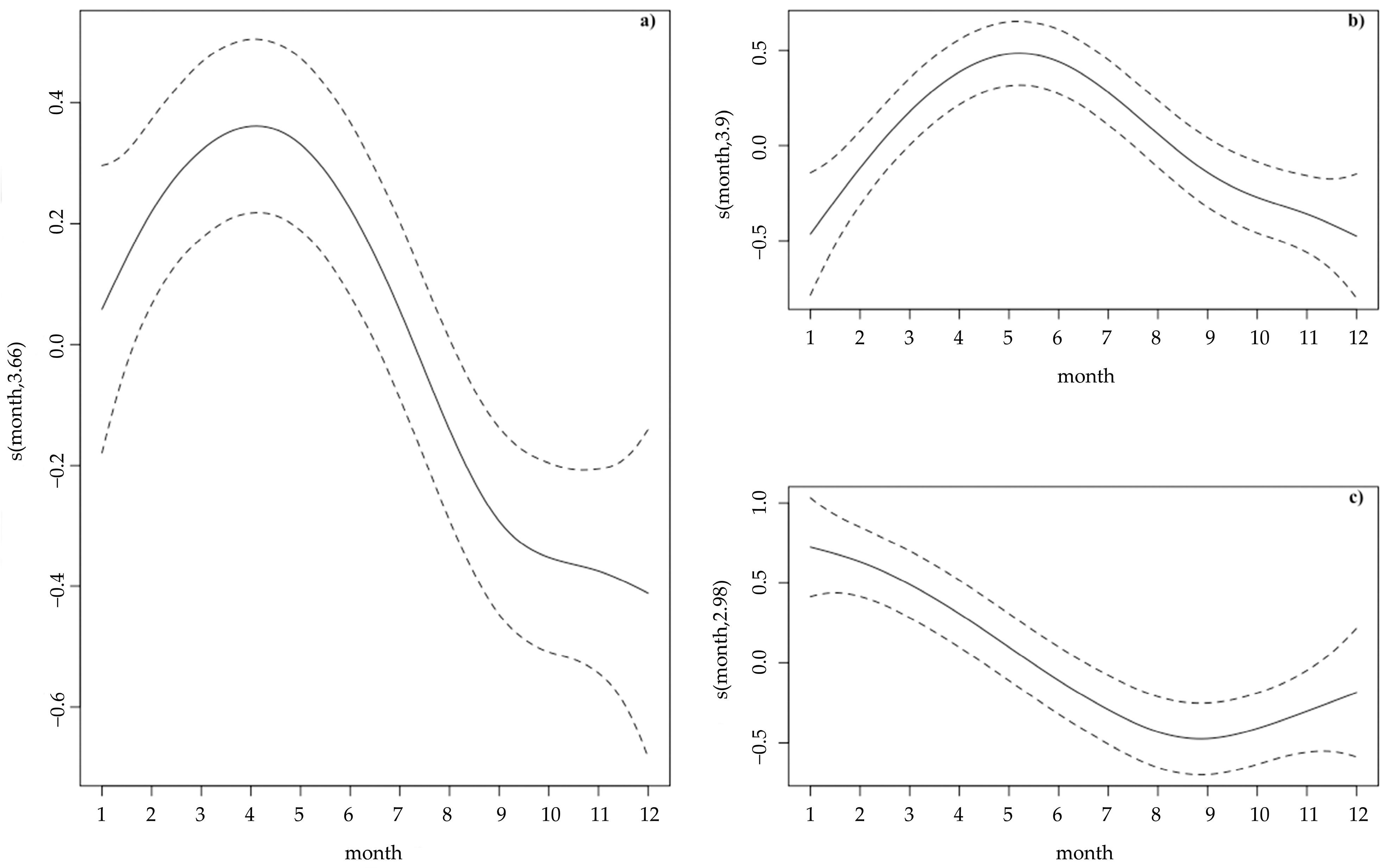
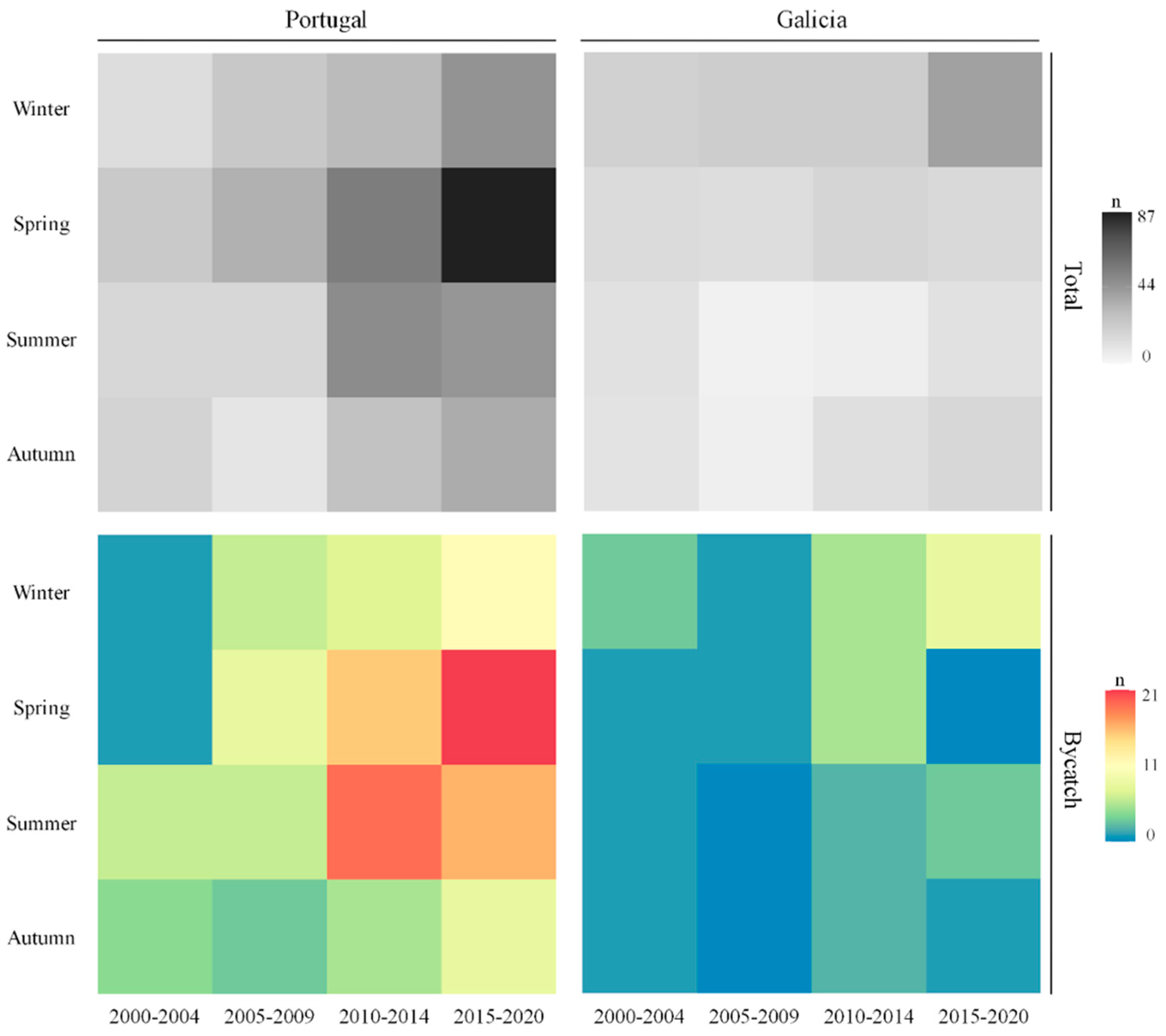
3.1. Strandings in Western Iberia
3.2. Bycatch Assessment and Potential Biological Removal: Insights from Portuguese Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontaine, M.; Roland, K.; Calves, I.; Austerlitz, F.; Palstra, F.P.; Tolley, K.A.; Ryan, S.; Ferreira, M.; Jauniaux, T.; Llavona, A.; et al. Postglacial climate changes and rise of three ecotypes of harbour porpoises, Phocoena phocoena, in western Palearctic waters. Mol. Ecol. 2014, 23, 3306–3321. [Google Scholar] [CrossRef]
- Chehida, Y.B.; Stelwagen, T.; Hoekendijk, J.P.A.; Ferreira, M.; Eira, C.; Pereira, A.T.; Nicolau, L.; Marçalo, A.; Thumloup, J.; Fontaine, M.C. Harbor porpoise losing its edges: Genetic time series suggests a rapid population decline in Iberian waters over the last 30 years. BioRxiv 2021. [Google Scholar] [CrossRef]
- Hammond, P.S.; Lacey, C.; Gille, A.; Viquerat, S.; Börjesson, P.; Macleod, K.; Ridoux, V.; Santos, M.B.; Scheidat, M.; Teilmann, J.; et al. Estimates of Cetacean Abundance in European Atlantic Waters in Summer 2016 from the SCANS-III Aerial and Shipboard Surveys. Final Report 2021. p. 42. Available online: https://scans3.wp.st-andrews.ac.uk/files/2021/06/SCANS-III_design-based_estimates_final_report_revised_June_2021.pdf (accessed on 1 March 2022).
- Torres-Pereira, A.; Ferreira, M.; Eira, C.; López, A.; Sequeira, M. Phocoena phocoena boto. In Livro Vermelho dos Mamíferos de Portugal Continental; Mathias, M.L., Fonseca, C., Rodrigues, L., Grilo, C., Lopes-Fernandes, M., Palmeirim, J.M., Santos-Reis, M., Alves, P.C., Cabral, J.A., Ferreira, M., et al., Eds.; Associação para a Investigação e Desenvolvimento de Ciências and Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2023; pp. 190–191. [Google Scholar]
- BOE-A-2020-15296. Boletín Oficial del Estado, Ministerio para la Transición Ecológica y el Reto Demográfico, Spain. 2020. No. 314. pp. 108167–108171. Available online: https://www.boe.es (accessed on 15 March 2022).
- Read, A.J.; Drinker, P.; Northridge, S. Bycatch of marine mammals in US and global fisheries. Conserv. Biol. 2006, 20, 163–169. [Google Scholar] [CrossRef]
- Reeves, R.R.; McClellan, K.; Werner, T.B. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger. Species Res. 2013, 20, 71–97. [Google Scholar] [CrossRef]
- Brownell, R.L., Jr.; Reeves, R.R.; Read, A.J.; Smith, B.D.; Thomas, P.O.; Ralls, K.; Amano, M.; Berggren, P.; Chit, A.M.; Collins, T.; et al. Bycatch in gillnet fisheries threatens Critically Endangered small cetaceans and other aquatic megafauna. Endanger. Species Res. 2019, 40, 285–296. [Google Scholar] [CrossRef]
- Alonso-Fernández, A.; Otero, J.; Bañón, R.; Campelos, J.M.; Quintero, F.; Ribó, J.; Filguera, F.; Juncal, L.; Lamas, F.; Gancedo, A.; et al. Inferring abundance trends of key species from a highly developed small-scale fishery off NE Atlantic. Fish. Res. 2019, 209, 101–116. [Google Scholar] [CrossRef]
- Directorate-General for Natural Resources, Safety and Maritime Services—DGRM. Annual Report Portuguese Fishing Fleet—2019. In Fleet Report 2019; DGRM: Lisboa, Portugal, 2019; p. 21. Available online: https://www.dgrm.mm.gov.pt/en/web/guest/relatorios (accessed on 17 June 2023).
- International Council for the Exploration of the Sea—ICES. Bay of Biscay and Iberian Coast Ecoregion—Fisheries Overview, Including Mixed-Fisheries Considerations. In Report of the ICES Advisory Committee. 2020. Available online: https://ices-library.figshare.com/articles/report/Bay_of_Biscay_and_Iberian_Coast_ecoregion_Fisheries_overview_including_mixed-fisheries_considerations/18637877 (accessed on 17 June 2023). [CrossRef]
- Pascual-Fernández, J.J.; Florido-de-Corral, D.; Cruz-Modino, R.; Villasante, S. Small-Scale Fisheries in Spain: Diversity and Challenges. In Small-Scale Fisheries in Europe: Status, Resilience and Governance; Pascual-Fernández, J.J., Pita, C., Bavinck, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Volume 23, pp. 253–288. [Google Scholar]
- Pita, C.; Gaspar, M. Small-scale fisheries in Portugal: Current situation, challenges and opportunities for the future. In Small-Scale Fisheries in Europe: Status, Resilience and Governance; Pascual-Fernández, J.J., Pita, C., Bavinck, M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Volume 23, pp. 253–288. [Google Scholar]
- Suuronen, P.; Gilman, E. Monitoring and managing fisheries discards: New technologies and approaches. Marine Policy 2020, 116, 103554. [Google Scholar] [CrossRef]
- Tuck, G.N. Are bycatch rates sufficient as the principal fishery performance measure and method of assessment for seabirds? Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 412–422. [Google Scholar] [CrossRef]
- Moore, J.E.; Curtis, K.A.; Lewison, R.L.; Dillingham, P.W.; Cope, J.M.; Fordham, S.V.; Heppell, S.S.; Pardo, S.A.; Simpfendorfer, C.A.; Tuck, G.N.; et al. Evaluating sustainability of fisheries bycatch mortality for marine megafauna: A review of conservation reference points for data-limited populations. Environ. Conserv. 2013, 40, 329–344. [Google Scholar] [CrossRef]
- Leeney, R.H.; Amies, R.; Broderick, A.C.; Witt, M.J.; Loveridge, J.; Doyle, J.; Godley, B.J. Spatio-temporal analysis of cetacean strandings and bycatch in a UK fisheries hotspot. Biodivers. Conserv. 2008, 17, 2323–2338. [Google Scholar] [CrossRef]
- Peltier, H.; Jepson, P.D.; Dabin, W.; Deaville, R.; Daniel, P.; Van Canneyt, O.; Ridoux, V. The contribution of stranding data to monitoring and conservation strategies for cetaceans: Developing spatially explicit mortality indicators for common dolphins (Delphinus delphis) in the eastern North-Atlantic. Ecol. Indic. 2014, 39, 203–214. [Google Scholar] [CrossRef]
- Peltier, H.; Authier, M.; Deaville, R.; Dabin, W.; Jepson, P.D.; van Canneyt, O.; Daniel, P.; Ridoux, V. Small cetacean bycatch as estimated from stranding schemes: The common dolphin case in the northeast Atlantic. Environ. Sci. Policy 2016, 63, 7–18. [Google Scholar] [CrossRef]
- Saavedra, C.; Pierce, G.J.; Gago, J.; Jusufovski, D.; Cabrero, A.; Cerviño, S.; López, A.; Martínez-Cedeira, J.A.; Santos, M.B. Factors driving patterns and trends in strandings of small cetaceans. Mar. Biol. 2017, 164, 1–17. [Google Scholar] [CrossRef]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, O.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef]
- Coombs, E.J.; Deaville, R.; Sabin, R.C.; Allan, L.; O’Connell, M.; Berrow, S.; Smith, B.; Brownlow, A.; Doeschate, M.T.; Penrose, R.; et al. What can cetacean stranding records tell us? A study of UK and Irish cetacean diversity over the past 100 years. Mar. Mammal Sci. 2019, 35, 1527–1555. [Google Scholar] [CrossRef]
- Williams, R.; Gero, S.; Bejder, L.; Calambokidis, J.; Kraus, S.D.; Lusseau, D.; Read, A.J.; Robbins, J. Underestimating the damage: Interpreting cetacean carcass recoveries in the context of the Deepwater Horizon/BP incident. Conserv. Lett. 2011, 4, 228–233. [Google Scholar] [CrossRef]
- Peltier, H.; Dabin, W.; Daniel, P.; Van Canneyt, O.; Dorémus, G.; Huon, M.; Ridoux, V. The significance of stranding data as indicators of cetacean populations at sea: Modelling the drift of cetacean carcasses. Ecol. Indic. 2012, 18, 278–290. [Google Scholar] [CrossRef]
- Pietroluongo, G.; Corazzola, G.; Centelleghe, C.; Mazzariol, S. Diagnostic framework for the assessment of fishery interaction in stranded marine mammals. In LIFE DELFI—Dolphin Experience: Lowering Fishing Interactions; LIFE18 NAT/IT/000942; LIFE DELFI: Rome, Italy, 2021. [Google Scholar]
- Bernaldo de Quirós, Y.; Hartwick, M.; Rotstein, D.S.; Garner, M.M.; Bogomolni, A.; Greer, W.; Niemeyer, M.E.; Early, G.; Wenzel, F.; Moore, M. Discrimination between bycatch and other causes of cetacean and pinniped stranding. Dis. Aquat. Org. 2018, 127, 83–95. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Scheidat, M.; Siemensma, M.L.; Couperus, B.; Leopold, M.F.; Morell, M.; Grone, A.; Kik, M.J. Challenges in the assessment of bycatch: Postmortem findings in harbor porpoises (Phocoena phocoena) retrieved from gillnets. Vet. Pathol. 2021, 58, 405–415. [Google Scholar] [CrossRef]
- Kuiken, T.; Garcia Hartmann, M. Cetacean Pathology: Dissection Techniques and Tissue sampling. In Proceedings of the first European Cetacean Society Workshop, ECS, Leiden, The Netherlands, 13–14 September 1991. [Google Scholar]
- Kuiken, T. (Ed.) A review of the criteria for the diagnosis of by-catch in cetaceans. In Diagnosis of Bycatch in Cetaceans, Proceedings of the Second European Cetacean Society Workshop on Cetacean Pathology, Montpelier, France, 2 March 1994; European Cetacean Society: Saskatoon, SK, Canada, 1994. [Google Scholar]
- Geraci, R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; p. 371. [Google Scholar]
- Moore, M.J.; van der Hoop, J.; Barco, S.G.; Costidis, A.M.; Gulland, F.M.; Jepson, P.D.; Moore, K.T.; Raverty, S.; McLellan, W.A. Criteria and case definitions for serious innjury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Org. 2013, 103, 229–264. [Google Scholar] [CrossRef]
- Ijsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling–Joint ACCOBAMS and ASCOBANS Document. ACCOBAMS-MOP7/2019/Doc 33. 2019. Available online: https://accobams.org/wp-content/uploads/2019/04/MOP7.Doc33_Best-practices-on-cetacean-post-mortem-investigation.pdf (accessed on 10 August 2023).
- Read, F. Understanding Cetacean and Fisheries Interactions in the North-West Iberian Peninsula. Ph.D. Thesis, University of Vigo, Vigo, Spain, 16 September 2015. [Google Scholar]
- Camarão, B.C. Estudo da Reprodução de Pequenos Cetáceos Através da Morfologia do Ovário. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2017. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project 2022. Available online: http://qgis.osgeo.org (accessed on 1 November 2022).
- Sbrocco, E.J.; Barber, P.H. MARSPEC: Ocean climate layers for marine spatial ecology. Ecology 2013, 94, 979. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. Available online: http://www.respond2articles.com/MEE/ (accessed on 1 January 2023).
- Wood, S.N.; Pya, N.; Saefken, B. Smoothing parameter and model selection for general smooth models (with discussion). J. Am. Stat. Assoc. 2016, 111, 1548–1575. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007; pp. 97–124. [Google Scholar]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 November 2022).
- Torres-Pereira, A.; Araújo, H.; Matos, F.L.; Bastos-Santos, J.; Sá, S.; Ferreira, M.; Martínez-Cedeira, J.; López, A.; Sequeira, M.; Vingada, J.; et al. Harbour Porpoise Abundance in Portugal over a 5-Year Period and Estimates of Potential Distribution. Animals 2022, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Wade, P.R. Calculating limits to the allowable human-caused mortality of cetaceans and pinnipeds. Mar. Mammal Sci. 1998, 14, 1–37. [Google Scholar] [CrossRef]
- National Marine Fisheries Service—NMFS. Revisions to Guidelines for Assessing Marine Mammal Stocks (GAMMS II). NMFS and NOAA General Counsel. 2005; p. 24. Available online: https://media.fisheries.noaa.gov/dam-migration/guidelines_for_preparing_stock_assessment_reports_2005_revision_gamms_ii.pdf (accessed on 1 November 2022).
- Authier, M.; Peltier, H.; Dorémus, G.; Dabin, W.; Van Canneyt, O.; Ridoux, V. How much are stranding records affected by variation in reporting rates? A case study of small delphinids in the Bay of Biscay. Biodivers. Conserv. 2014, 23, 2591–2612. [Google Scholar] [CrossRef]
- Foord, C.S.; Rowe, K.M.C.; Robb, K. Cetacean biodiversity, spatial and temporal trends based on stranding records (1920-2016), Victoria, Australia. PLoS ONE 2019, 14, e0223712. [Google Scholar] [CrossRef]
- Vingada, J.; Eira, C. Conservation of Cetaceans and Seabirds in Continental Portugal. In The LIFE + MarPro Project; Rainho & Neves, Lda.: Aveiro, Portugal, 2018; p. 257. [Google Scholar]
- Consello Económico e Social de Galicia—CES. Rexistro de Buques Pesqueiros da Comunidade Autónoma. Available online: https://www.pescadegalicia.gal/rexbuque/ (accessed on 1 May 2022).
- Martínez-Cedeira, J.; López, A. Actualización del Estado de Conservación de la Marsopa en las Demarcaciones Marinas Noratlántica y Sudatlántica y Elaboración del Borrador del Plan de Conservación; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018; p. 217. [Google Scholar]
- Martínez-Cedeira, J.; Izquierdo Ferreiro, I. Informe embarques a bordo de barcos de pesca, Proyecto VIRADA. CEMMA 2021, 50. [Google Scholar]
- INE—Instituto Nacional de Estatística–Estatísticas da Pesca. Technical Note 2020; INE: Lisboa, Portugal, 2021; p. 149. Available online: https://www.ine.pt/xurl/pub/280980980 (accessed on 1 April 2022)ISBN 978-989-25-0566-4.
- Martins, R.; Carneiro, M.; Rebordão, F.R.; Sobral, M. A Pesca Com a Arte de Xávega; Scientific and Technical Report; Instituto de Investigação das Pescas e do Mar—IPIMAR: Lisboa, Portugal, 1999; p. 32. [Google Scholar]
- Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas—ASCOBANS. Resolution No. 3 Incidental Take of Small Cetaceans. In Proceedings of the ASCOBANS Meeting of Parties 3, Bristol, UK, 26–28 July 2000.
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the EU Action Plan: Protecting and Restoring Marine Ecosystems for Sustainable and Resilient Fisheries, Document 52023DC0102. 21 February 2023, p. 24. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52023DC0102 (accessed on 8 August 2023).
| GAL | PT | Total | PT North | PT South | |
|---|---|---|---|---|---|
| Sex | |||||
| nsex | 232 | 524 | 756 | 453 | 71 |
| Male | 113, 48.71% | 209, 39.89% | 322, 42.59% | 191, 42.16% | 18, 25.35% |
| Female | 90, 38.79% | 202, 38.55% | 292, 38.62% | 186, 41.06% | 16, 22.54% |
| ni | 29, 12.50% | 113, 21.56% | 142, 18.78% | 76, 16.78% | 37, 52.11% |
| Age Class | |||||
| nage | 105 | 406 | 511 | 383 | 23 |
| Foetus | 2, 1.90% | 17, 4.19% | 19, 3.72% | 17, 4.44% | 0, 0.00% |
| Neonate | 7, 6.67% | 31, 7.64% | 38, 7.44% | 30, 7.83% | 1, 4.35% |
| Calf | 15, 14.29% | 27, 6.65% | 42, 8.22% | 23, 6.01% | 4, 17.39% |
| Juvenile | 50, 47.62% | 169, 41.63% | 219, 42.85% | 166, 43.34% | 3, 13.04% |
| Adult | 12, 11.43% | 118, 29.06% | 130, 25.44% | 107, 27.94% | 11, 47.83% |
| ni | 19, 18.10% | 44, 10.84% | 63, 12.33% | 40, 10.44% | 4, 17.39% |
| nexcl 1 | 127 | 118 | 245 | 70 | 48 |
| Cause of stranding | |||||
| ncause | 83 | 281 | 364 | 264 | 17 |
| Bycatch | 34, 40.96% | 137, 48.75% | 171, 46.98% | 136, 51.51% | 1, 5.88% |
| Probable bycatch | 6, 7.23% | 34, 12.10% | 40, 10.99% | 32, 12.12% | 2, 11.76% |
| Diseases | 3, 3.61% | 19, 6.76% | 22, 6.04% | 18, 6.82% | 1, 5.88% |
| Other | 8, 9.64% | 30, 10.68% | 38, 10.44% | 28, 10.61% | 2, 11.76% |
| ni | 32, 38.55% | 61, 21.71% | 93, 25.55% | 50, 18.94% | 11, 64.71% |
| nexcl 2 | 149 | 243 | 392 | 189 | 54 |
| Period | Strandings | Nbycatch | Estimated Annual Mortality (EAM) | Carcass Detection Rate (CDR) | Estimated Annual Mortality from Bycatch (EAMbycatch) | Annual Population Removal (APR) |
|---|---|---|---|---|---|---|
| Mr × N | Strandings/EAM (%) | Nbycatch/CDR | EAMbycatch/N (%) | |||
| 2011 | 24 | 10 | 215 (82–564) | 11.15 (4.25–29.24) | 90 (34–235) | 7.50 (2.86–19.66) |
| 2012 | 15 | 6 | 539 (273–1065) | 2.78 (1.41–5.50) | 216 (109–426) | 7.20 (3.64–14.22) |
| 2013 | 18 | 10 | 577 (276–1209) | 3.12 (1.49–6.53) | 321 (153–672) | 10.00 (4.77–20.95) |
| 2014 | 26 | 12 | 298 (129–686) | 8.74 (3.79–20.15) | 137 (60–316) | 8.31 (3.60–19.14) |
| 2015 | 13 | 11 | 386 (166–899) | 3.36 (1.45–7.82) | 327 (141–761) | 15.23 (6.55–35.45) |
| 2011–2015 | Mean: 19 | Mean: 10 | 406 (232–711) | 4.73 (2.70–8.29) | 207 (118–363) | 9.19 (5.25–16.10) |
| Period | N (CI) | CV | Nmin (CI) | PBR (CI) |
|---|---|---|---|---|
| 2011 | 1196 (456–3135) | 0.5070 | 968 (376–2586) | 10 (4–26) |
| 2012 | 2995 (1516–5917) | 0.3495 | 2718 (1376–5370) | 27 (14–54) |
| 2013 | 3207 (1531–6718) | 0.3814 | 2860 (1366–5992) | 29 (14–60) |
| 2014 | 1653 (717–3809) | 0.4327 | 1431 (621–3296) | 14 (6–33) |
| 2015 | 2147 (923–4997) | 0.4386 | 1851 (796–4309) | 19 (8–43) |
| 2011–2015 | 2254 (1287–3949) | 0.2199 | 2166 (1237–3795) | 22 (12–43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Pereira, A.; Araújo, H.; Monteiro, S.S.; Ferreira, M.; Bastos-Santos, J.; Sá, S.; Nicolau, L.; Marçalo, A.; Marques, C.; Tavares, A.S.; et al. Assessment of Harbour Porpoise Bycatch along the Portuguese and Galician Coast: Insights from Strandings over Two Decades. Animals 2023, 13, 2632. https://doi.org/10.3390/ani13162632
Torres-Pereira A, Araújo H, Monteiro SS, Ferreira M, Bastos-Santos J, Sá S, Nicolau L, Marçalo A, Marques C, Tavares AS, et al. Assessment of Harbour Porpoise Bycatch along the Portuguese and Galician Coast: Insights from Strandings over Two Decades. Animals. 2023; 13(16):2632. https://doi.org/10.3390/ani13162632
Chicago/Turabian StyleTorres-Pereira, Andreia, Hélder Araújo, Silvia Silva Monteiro, Marisa Ferreira, Jorge Bastos-Santos, Sara Sá, Lídia Nicolau, Ana Marçalo, Carina Marques, Ana Sofia Tavares, and et al. 2023. "Assessment of Harbour Porpoise Bycatch along the Portuguese and Galician Coast: Insights from Strandings over Two Decades" Animals 13, no. 16: 2632. https://doi.org/10.3390/ani13162632
APA StyleTorres-Pereira, A., Araújo, H., Monteiro, S. S., Ferreira, M., Bastos-Santos, J., Sá, S., Nicolau, L., Marçalo, A., Marques, C., Tavares, A. S., De Bonis, M., Covelo, P., Martínez-Cedeira, J., López, A., Sequeira, M., Vingada, J., & Eira, C. (2023). Assessment of Harbour Porpoise Bycatch along the Portuguese and Galician Coast: Insights from Strandings over Two Decades. Animals, 13(16), 2632. https://doi.org/10.3390/ani13162632





