Anti-Müllerian Hormone Concentrations for Determining Resumption of Sertoli Cell Function following Removal of a 4.7 mg Deslorelin Implant in Tomcats
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Serum Testosterone and AMH Determination
2.2. Statistical Analysis
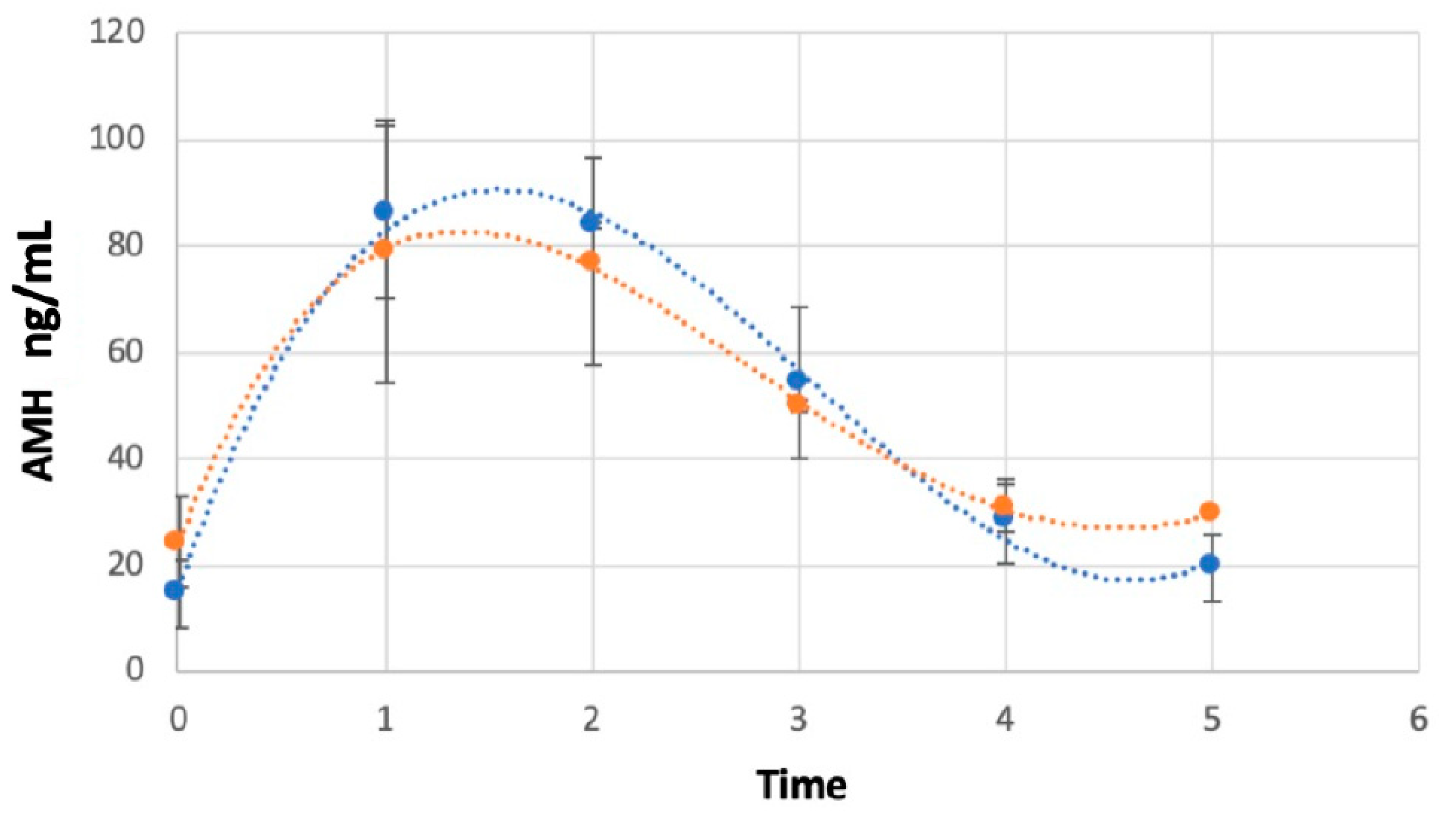
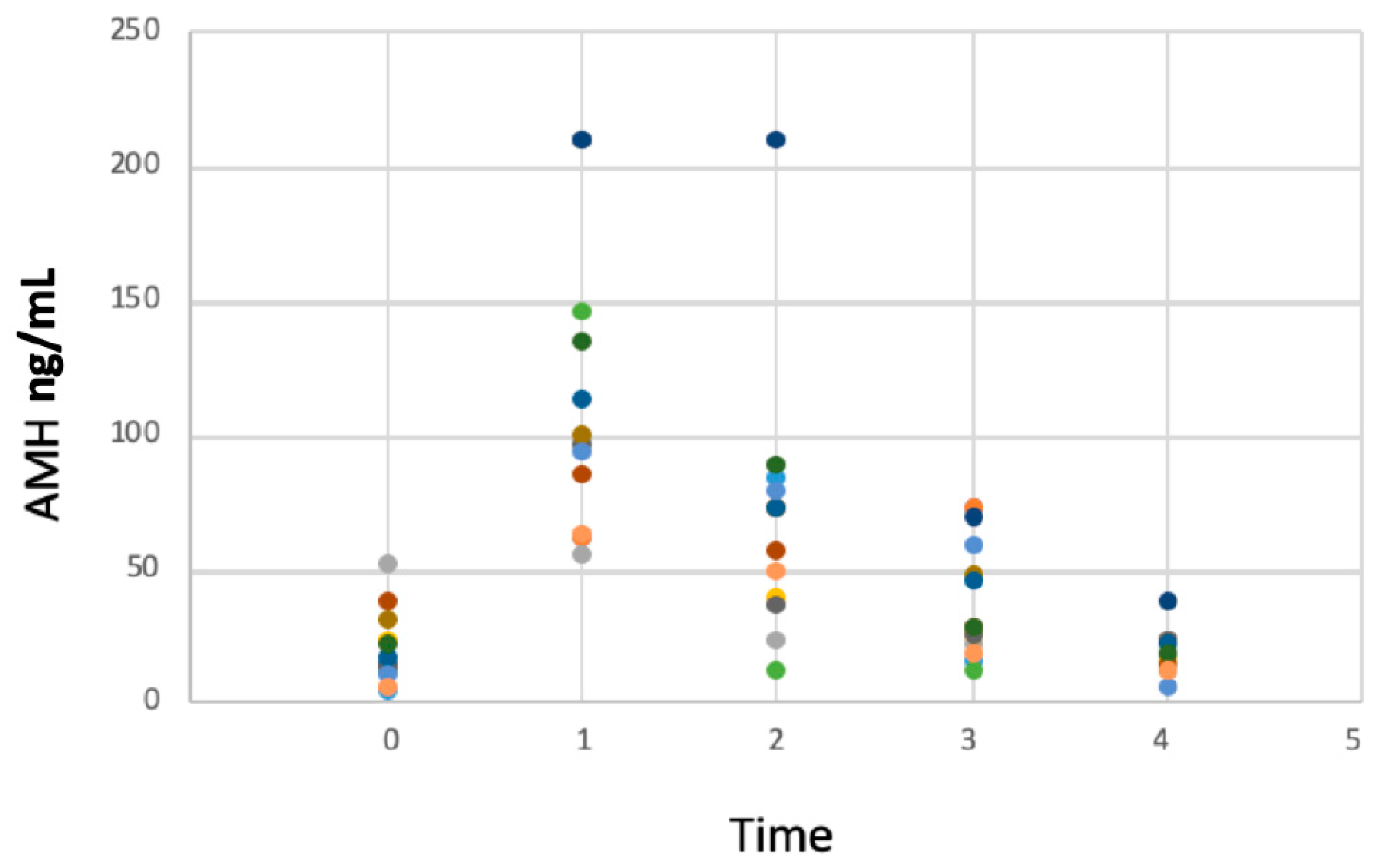
3. Results
AMH Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cate, R.L.; Mattaliano, R.J.; Hession, C.; Tizard, R.; Farber, N.M.; Cheung, A.; Ninfa, E.G.; Frey, A.Z.; Gash, D.J.; Chow, E.P.; et al. Isolation of the Bovine and Human Genes for Müllerian Inhibiting Substance and Expression of the Human Gene in Animal Cells. Cell 1986, 45, 685–698. [Google Scholar] [CrossRef]
- Place, N.J.; Hansen, B.S.; Cheraskin, J.L.; Cudney, S.E.; Flanders, J.A.; Newmark, A.D.; Barry, B.; Scarlett, J.M. Measurement of Serum Anti-Müllerian Hormone Concentration in Female Dogs and Cats before and after Ovariohysterectomy. J. Vet. Diagn. Investig. 2011, 23, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Axnér, E.; Ström Holst, B. Concentrations of Anti-Müllerian Hormone in the Domestic Cat. Relation with Spay or Neuter Status and Serum Estradiol. Theriogenology 2015, 83, 817–821. [Google Scholar] [CrossRef]
- Alm, H.; Holst, B.S. Identifying Ovarian Tissue in the Bitch Using Anti-Müllerian Hormone (AMH) or Luteinizing Hormone (LH). Theriogenology 2018, 106, 15–20. [Google Scholar] [CrossRef]
- Pir Yagci, I.; Pekcan, M.; Polat, I.M.; Kalender, H.; Macun, H.C. Does Serum Anti-Müllerian Hormone Levels Always Discriminate Presence of the Ovaries in Adult Bitches? Comparison of Two ELISA Kits. Reprod. Domest. Anim. 2016, 51, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Turna Yilmaz, Ö.; Toydemir, T.S.F.; Kirsan, I.; Gunay Ucmak, Z.; Caliskan Karacam, E. Anti-Müllerian Hormone as a Diagnostic Tool for Ovarian Remnant Syndrome in Bitches. Vet. Res. Commun. 2015, 39, 159–162. [Google Scholar] [CrossRef]
- Rey, R. Anti-Müllerian Hormone in Disorders of Sex Determination and Differentiation. Arq. Bras. Endocrinol. Metabol. 2005, 49, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Josso, N. Antimüllerian Hormone: New Perspectives for a Sexist Molecule. Endocr. Rev. 1986, 7, 421–433. [Google Scholar] [CrossRef]
- Young, J.; Chanson, P.; Salenave, S.; Noël, M.; Brailly, S.; O’Flaherty, M.; Schaison, G.; Rey, R. Testicular Anti-Mullerian Hormone Secretion Is Stimulated by Recombinant Human FSH in Patients with Congenital Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2005, 90, 724–728. [Google Scholar] [CrossRef]
- Edelsztein, N.Y.; Rey, R.A. Importance of the Androgen Receptor Signaling in Gene Transactivation and Transrepression for Pubertal Maturation of the Testis. Cells 2019, 8, 861. [Google Scholar] [CrossRef]
- Josso, N.; Di Clemente, N.; Gouédard, L. Anti-Müllerian Hormone and Its Receptors. Mol. Cell. Endocrinol. 2001, 179, 25–32. [Google Scholar] [CrossRef]
- Rey, R. Endocrine, Paracrine and Cellular Regulation of Postnatal Anti-Müllerian Hormone Secretion by Sertoli Cells. Trends Endocrinol. Metab. 1998, 9, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Zhang, H.X.; Xiao, Z.; Qiao, J.; Li, R. Regulation of Anti-Müllerian Hormone (AMH) in Males and the Associations of Serum AMH with the Disorders of Male Fertility. Asian J. Androl. 2019, 21, 109–114. [Google Scholar] [CrossRef]
- Balogh, O.; Somoskői, B.; Kollár, E.; Kowalewski, M.P.; Gram, A.; Reichler, I.M.; Klein, R.; Kawate, N.; Mester, L.; Walter, B.; et al. Anti-Müllerian Hormone, Testosterone, and Insulin-like Peptide 3 as Biomarkers of Sertoli and Leydig Cell Function during Deslorelin-Induced Testicular Downregulation in the Dog. Theriogenology 2021, 175, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Hermanowicz, A.; Komarowska, M.; Debek, W. Serum AMH in Physiology and Pathology of Male Gonads. Int. J. Endocrinol. 2013, 2013, 128907. [Google Scholar] [CrossRef] [PubMed]
- Giudice, C.; Banco, B.; Veronesi, M.C.; Ferrari, A.; Di Nardo, A.; Grieco, V. Immunohistochemical Expression of Markers of Immaturity in Sertoli and Seminal Cells in Canine Testicular Atrophy. J. Comp. Pathol. 2014, 150, 208–215. [Google Scholar] [CrossRef]
- Holst, B.S.; Dreimanis, U. Anti-Müllerian Hormone: A Potentially Useful Biomarker for the Diagnosis of Canine Sertoli Cell Tumours. BMC Vet. Res. 2015, 11, 166. [Google Scholar] [CrossRef]
- Trigg, T.E.; Doyle, A.G.; Walsh, J.D.; Swangchan-uthai, T. A Review of Advances in the Use of the GnRH Agonist Deslorelin in Control of Reproduction. Theriogenology 2006, 66, 1507–1512. [Google Scholar] [CrossRef]
- Romagnoli, S.; Siminica, A.; Sontas, B.; Milani, C.; Mollo, A.; Stelletta, C. Semen Quality and Onset of Sterility Following Administration of a 4.7-Mg Deslorelin Implant in Adult Male Dogs. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 389–392. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Romagnoli, S. Reversible Control of Reproduction in Tomcats: Medical Options for Manipulating Libido and Fertility. J. Feline Med. Surg. 2023, 25, 1098612X231171406. [Google Scholar] [CrossRef]
- Sirivaidyapong, S.; Mehl, N.S.; Trigg, T.E. Delay of Puberty and Reproductive Performance in Male Dogs Following the Implantation of 4.7 and 9.4 Mg GnRH-Agonist Deslorelin at an Early Pre-Pubertal Age. Reprod. Domest. Anim. 2012, 47, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C. Long-Term Contraception in a Small Implant: A Review of Suprelorin (Deslorelin) Studies in Cats. J. Feline Med. Surg. 2015, 17, 766–771. [Google Scholar] [CrossRef] [PubMed]
- van Zeeland, Y.R.A.; Pabon, M.; Roest, J.; Schoemaker, N.J. Use of a GnRH Agonist Implant as Alternative for Surgical Neutering in Pet Ferrets. Vet. Rec. 2014, 175, 66. [Google Scholar] [CrossRef]
- Vinke, C.M.; van Deijk, R.; Houx, B.B.; Schoemaker, N.J. The Effects of Surgical and Chemical Castration on Intermale Aggression, Sexual Behaviour and Play Behaviour in the Male Ferret (Mustela Putorius Furo). Appl. Anim. Behav. Sci. 2008, 115, 104–121. [Google Scholar] [CrossRef][Green Version]
- Novotny, R.; Cizek, P.; Vitasek, R.; Bartoskova, A.; Prinosilova, P.; Janosovska, M. Reversible Suppression of Sexual Activity in Tomcats with Deslorelin Implant. Theriogenology 2012, 78, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Lucas, X. Clinical Use of Deslorelin (GnRH Agonist) in Companion Animals: A Review. Reprod. Domest. Anim. 2014, 49, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Nuñez Favre, R.; García, M.F.; García Mitacek, M.C.; Rearte, R.; Fontaine, C.; de la Sota, R.L.; Stornelli, M.A. Reestablishment of Sperm Quality after Long-Term Deslorelin Suppression in Tomcats. Anim. Reprod. Sci. 2018, 195, 302–308. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Georgiev, P.; Antonov, A.; Vodenicharov, A.; Navarro, C.; Wehrend, A. Reversibility of Germinative and Endocrine Testicular Function after Long-Term Contraception with a GnRH-Agonist Implant in the Tom-a Follow-up Study. Theriogenology 2014, 81, 941–946. [Google Scholar] [CrossRef]
- Padula, A.M. GnRH Analogues—Agonists and Antagonists. Anim. Reprod. Sci. 2005, 88, 115–126. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Georgiev, P.; Fasulkov, I.; Vodenicharov, A.; Wehrend, A. Basal Testosterone Concentrations after the Application of a Slow-Release GnRH Agonist Implant Are Associated with a Loss of Response to Buserelin, a Short-Term GnRH Agonist, in the Tom Cat. Theriogenology 2013, 80, 65–69. [Google Scholar] [CrossRef]
- Romagnoli, S.; Baldan, A.; Righetti, C.; Milani, C.; Mollo, A.; Stelletta, C. Semen Quality and Interval to Sterility in Tomcats Treated with a 9.4 Mg Deslorelin Implant. J. Feline Med. Surg. 2017, 19, 194–199. [Google Scholar] [CrossRef]
- Rhodes, L. New Approaches to Non-Surgical Sterilization for Dogs and Cats: Opportunities and Challenges. Reprod. Domest. Anim. 2017, 52, 327–331. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Wehrend, A.; Georgiev, P. Suppression of Fertility in Adult Cats. Reprod. Domest. Anim. 2014, 49, 33–40. [Google Scholar] [CrossRef]
- Goericke-Pesch, S.; Georgiev, P.; Antonov, A.; Albouy, M.; Wehrend, A. Clinical Efficacy of a GnRH-Agonist Implant Containing 4.7 Mg Deslorelin, Suprelorin®, Regarding Suppression of Reproductive Function in Tomcats. Theriogenology 2011, 75, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ferré-Dolcet, L.; Carniello, L.; Ferro, S.; Cattai, A.; Romagnoli, S.; Mollo, A. Interval between Removal of a 4.7 Mg Deslorelin Implant after a 3-, 6-, and 9-Month Treatment and Restoration of Testicular Function in Tomcats. Animals 2020, 10, 1559. [Google Scholar] [CrossRef]
- Ferré-Dolcet, L.; Ferro, S.; Contiero, B.; Fontaine, C.; Badon, T.; Gelli, D.; Romagnoli, S. Clinical Use of Anti-Müllerian Hormone to Monitor Resumption of Ovarian Activity Following Removal of a 4.7 Mg Deslorelin Implant in Queens. Vet. Res. Commun. 2022, 46, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Paltiel, H.J.; Diamond, D.A.; Di Canzio, J.; Zurakowski, D.; Borer, J.G.; Atala, A. Testicular Volume: Comparison of Orchidometer and US Measurements in Dogs. Radiology 2002, 222, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Saito, K.; Oohta, M.; Inoue, K.; Ogawa, Y.; Yoshida, H. Testicular Volume Measurement: Comparison of Ultrasonography, Orchidometry, and Water Displacement. Urology 2007, 69, 152–157. [Google Scholar] [CrossRef]
- Inomata, T.; Ninomiya, H.; Sakita, K.; Kashiwazaki, N.; Ito, J.; Ariga, M.; Inoue, S. Developmental Changes of Müllerian and Wolffian Ducts in Domestic Cat Fetuses. Exp. Anim. 2009, 58, 41–45. [Google Scholar] [CrossRef] [PubMed]
- García Mitacek, M.C.; Stornelli, M.C.; Praderio, R.G.; de la Sota, R.L.; Stornelli, M.A. Ultrasonographic and Progesterone Changes during Days 21 to 63 of Pregnancy in Queens. Theriogenology 2015, 84, 1131–1141. [Google Scholar] [CrossRef]
- França, L.R.; Godinho, C.L. Testis Morphometry, Seminiferous Epithelium Cycle Length, and Daily Sperm Production in Domestic Cats (Felis Catus). Biol. Reprod. 2003, 68, 1554–1561. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferré-Dolcet, L.; Bordogna, M.; Contiero, B.; Fontaine, C.; Bedin, S.; Romagnoli, S. Anti-Müllerian Hormone Concentrations for Determining Resumption of Sertoli Cell Function following Removal of a 4.7 mg Deslorelin Implant in Tomcats. Animals 2023, 13, 2552. https://doi.org/10.3390/ani13162552
Ferré-Dolcet L, Bordogna M, Contiero B, Fontaine C, Bedin S, Romagnoli S. Anti-Müllerian Hormone Concentrations for Determining Resumption of Sertoli Cell Function following Removal of a 4.7 mg Deslorelin Implant in Tomcats. Animals. 2023; 13(16):2552. https://doi.org/10.3390/ani13162552
Chicago/Turabian StyleFerré-Dolcet, Lluis, Matteo Bordogna, Barbara Contiero, Christelle Fontaine, Silvia Bedin, and Stefano Romagnoli. 2023. "Anti-Müllerian Hormone Concentrations for Determining Resumption of Sertoli Cell Function following Removal of a 4.7 mg Deslorelin Implant in Tomcats" Animals 13, no. 16: 2552. https://doi.org/10.3390/ani13162552
APA StyleFerré-Dolcet, L., Bordogna, M., Contiero, B., Fontaine, C., Bedin, S., & Romagnoli, S. (2023). Anti-Müllerian Hormone Concentrations for Determining Resumption of Sertoli Cell Function following Removal of a 4.7 mg Deslorelin Implant in Tomcats. Animals, 13(16), 2552. https://doi.org/10.3390/ani13162552






