Nest-Site Features and Breeding Ecology of Chestnut-Vented Nuthatch Sitta nagaensis in Southwestern China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
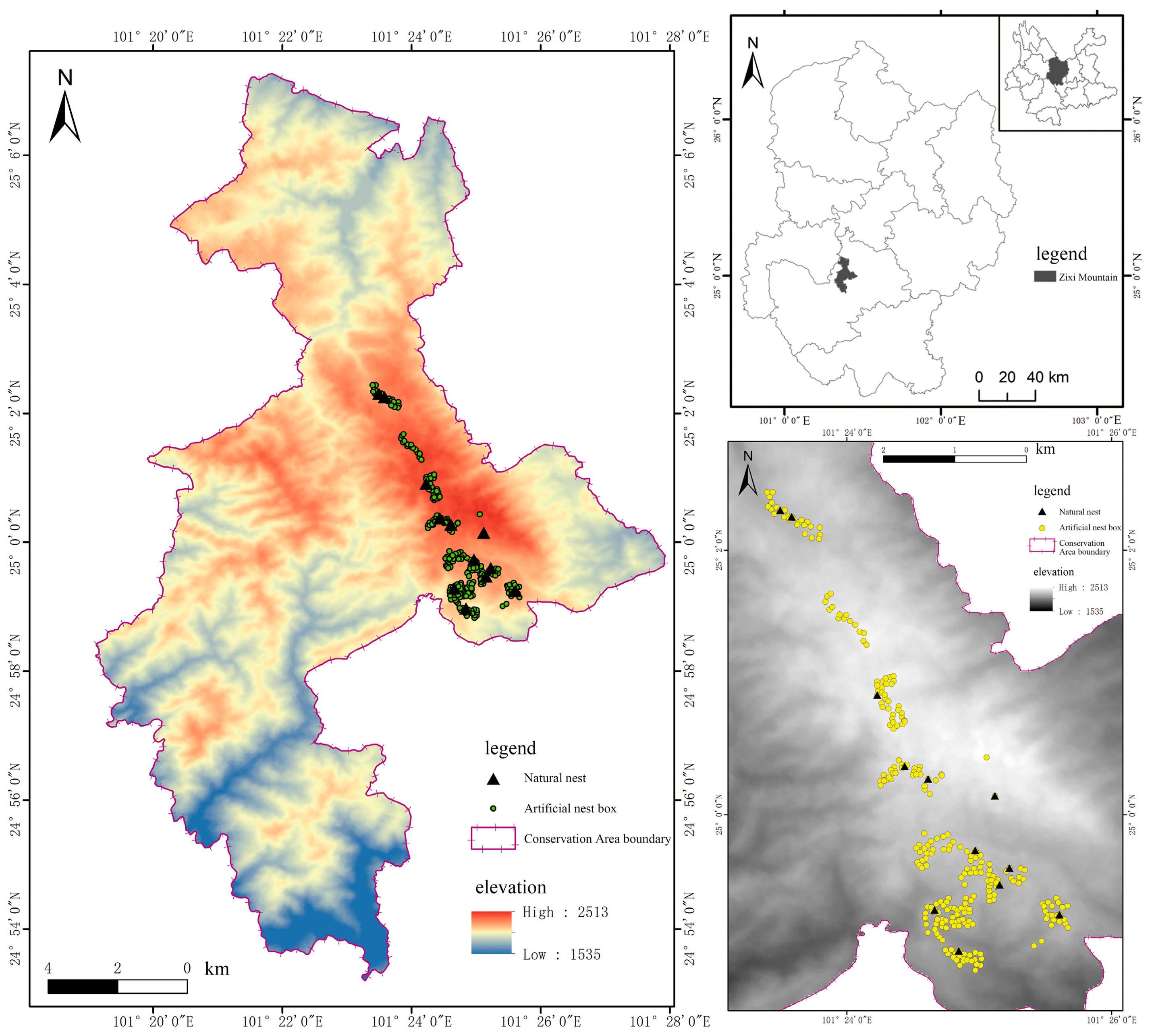
2.1. Study Area
2.2. Field Work
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Nests and Nest-Site Features
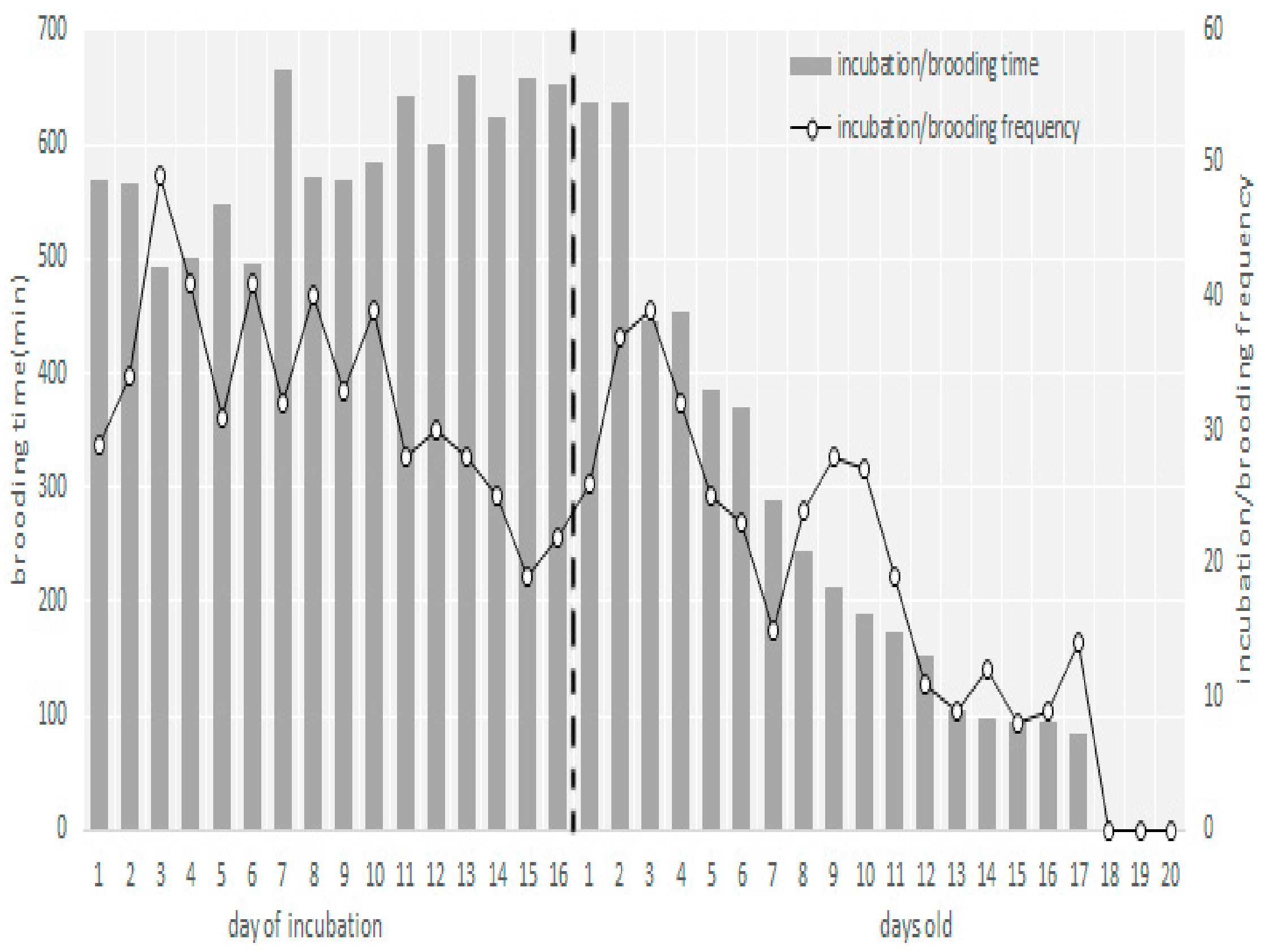
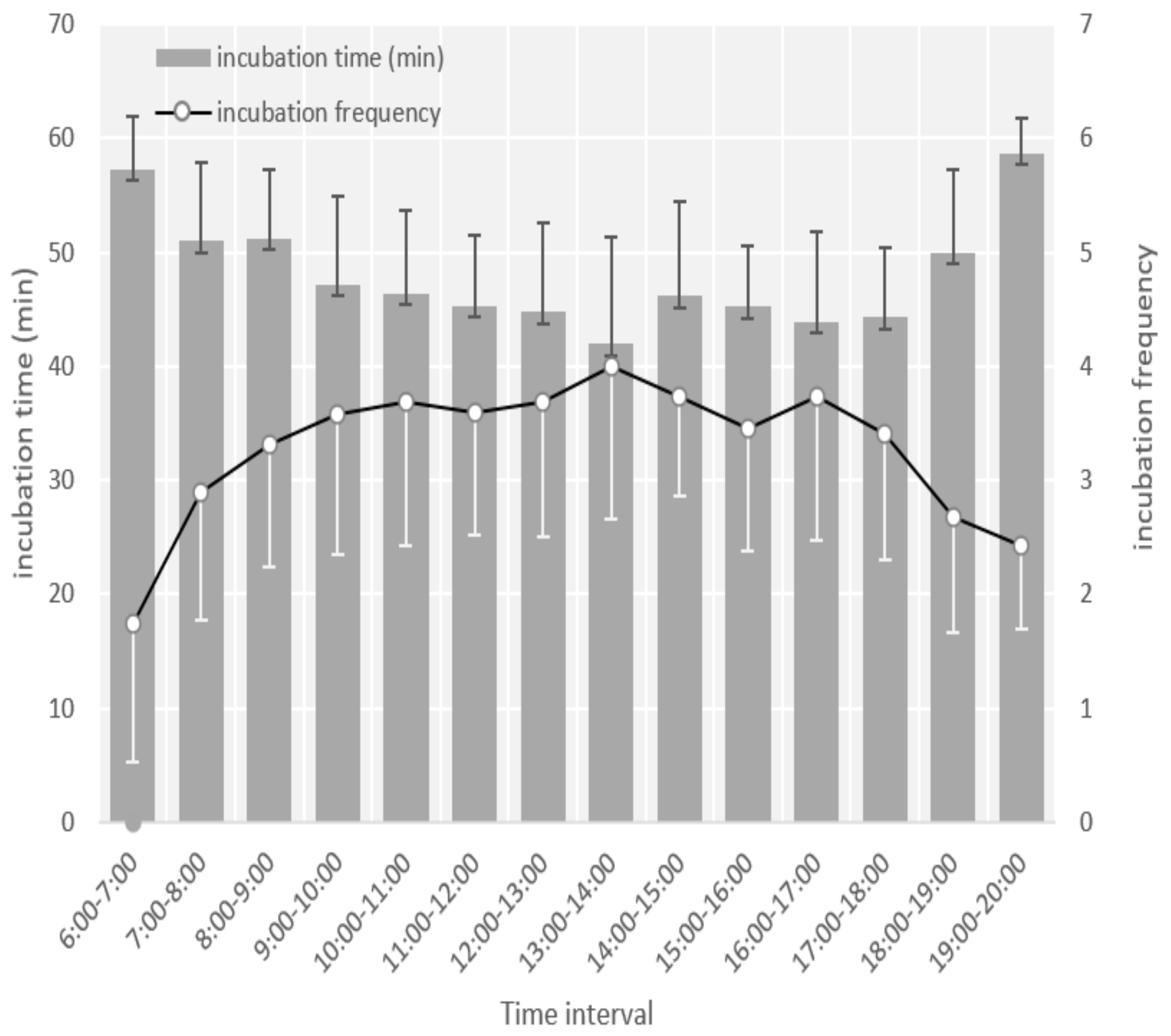
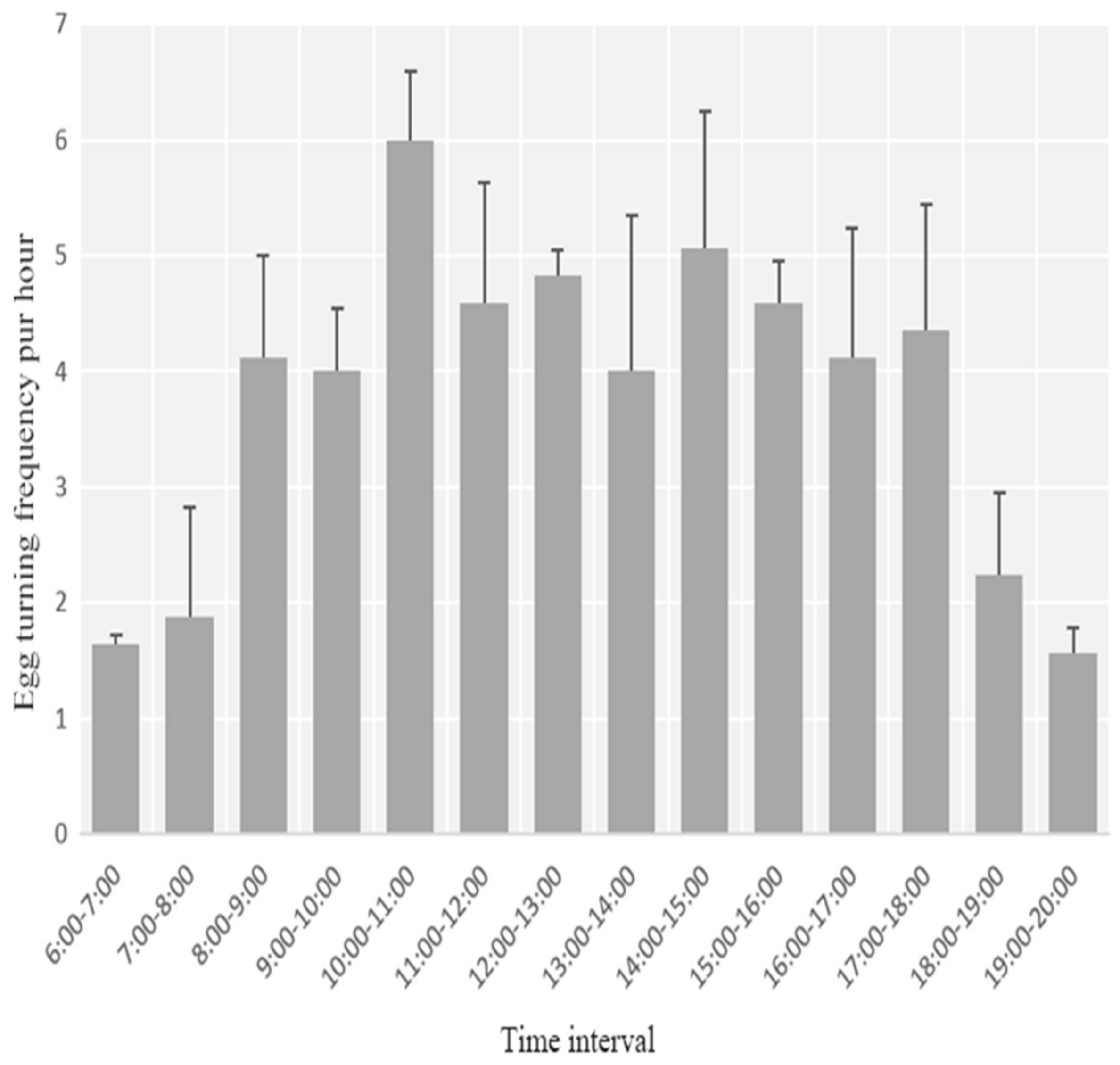
3.2. Egg Laying, Clutch Size, and Incubation Behavior
3.3. Nestling Brooding and Feeding Behavior
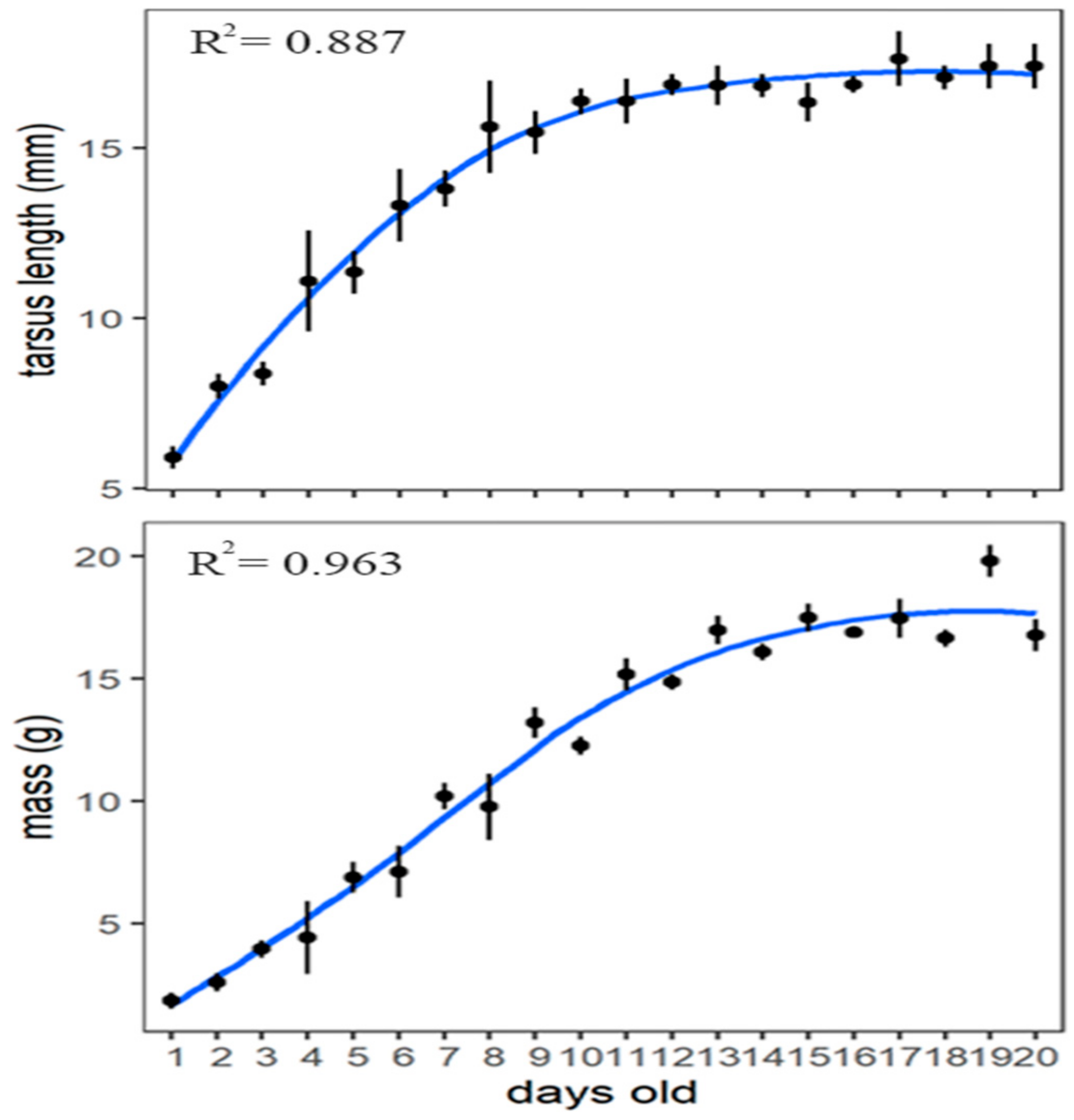
3.4. Nestling Development
3.5. Breeding Success and Predation
4. Discussion
4.1. Nests and Nest-Site Features
4.2. Breeding Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, T.E. Avian Life History Evolution in Relation to Nest Sites, Nest Predation, and Food. Ecol. Monogr. 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Dobson, F.S. A lifestyle view of life-history evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 17565–17566. [Google Scholar] [CrossRef]
- Gadgil, M.; Bossert, W.H. Life Historical Consequences of Natural Selection. Am. Nat. 1970, 104, 1–24. [Google Scholar] [CrossRef]
- Bennett, P.M.; Owens, I.P.F. Evolutionary Ecology of Birds: Life Histories, Mating Systems, and Extinction; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Zheng, G.M. Ornithology; Beijing Normal University Press: Beijing, China, 2012. [Google Scholar]
- May, R.M.; Godfrey, J. Biological Diversity: Differences between Land and Sea [and Discussion]. Philos. Trans. R. Soc. B 1994, 343, 105–111. [Google Scholar]
- Liang, D.; Gao, G.; Han, L.X.; Luo, X. Breeding Biology of Fire-Tailed Myzornis (Myzornis pyrrhoura) in an Alpine Environment in Southwestern China. Wilson J. Ornithol. 2017, 129, 568–575. [Google Scholar] [CrossRef]
- Zarybnicka, M.; Riegert, J.; Stastny, K. Seasonal habitat-dependent change in nest box occupation by Tengmalm’s owl associated with a corresponding change in nest predation. Popul. Ecol. 2017, 59, 65–70. [Google Scholar] [CrossRef]
- Libois, E.; Gimenez, O.; Oro, D.; Mínguez, E.; Pradel, R.; Aguilar, A.S. Nest boxes: A successful management tool for the conservation of an endangered seabird. Biol. Conserv. 2012, 155, 39–43. [Google Scholar]
- Butler, M.W.; Whitman, B.A.; Dufty, A.M. Nest Box Temperature and Hatching Success of American Kestrels Varies with Nest Box Orientation. Wilson J. Ornithol. 2009, 121, 778–782. [Google Scholar] [CrossRef]
- Mänd, R.; Tilgar, V.; Lõhmus, A.; Leivits, A. Providing nest boxes for hole-nesting birds—Does habitat matter? Biodivers. Conserv. 2005, 14, 1823–1840. [Google Scholar] [CrossRef]
- Gao, W. Studies on reproduction and feeding of Nuthatches. Acta Zool. Sin. 1978, 3, 260–268. [Google Scholar]
- Li, S.H.; Zhang, L.S.; Sun, Y.F.; Jiang, Y.L.; Yao, J.Y. Hatching input of female nuthatch. J. Northeast Norm. Univ. Nat. Sci. 2018, 50, 105–108. [Google Scholar]
- Yi, G.D.; Yang, Z.J.; Gao, W.; Li, Y. Growth and Development of Nestling in Nuthatch Brood Sizes; Northeast Normal University: Changchun, China, 2004; pp. 105–110. [Google Scholar]
- John, M.J.N.; Karen, P.; He, F.Q. Field Handbook of Chinese Birds; Hunan Education Publishing House: Changsha, China, 2000. [Google Scholar]
- An, K. Present Status of Zixishan Nature Reserve and Corresponding Countermeasures of Protection and Management Nature Reserve. For. Inventory Plan. 2018, 43, 126–133, (In Chinese with English Abstract). [Google Scholar]
- Feng, Y.Y.; Li, Q.S.; Liang, D.; Jiang, D.M.; Wu, X.R.; Gao, G.; Wang, X.W.; Fan, L.Z.; Luo, X. Annual variation of bird richness in winter in Zixi Mountain, Yunnan Province. J. Southwest For. Univ. Nat. Sci. 2019, 39, 110–115. [Google Scholar]
- Zhang, L.; Bai, L.M.; Wang, J.; Wan, D.M.; Liang, W. Occupation rates of artificial nest boxes by secondary cavity-nesting birds: The influence of nest site characteristics. J. Nat. Conserv. 2021, 63, 12605. [Google Scholar]
- eFloras. 2008. Available online: http://www.efloras.org (accessed on 22 March 2022).
- Hoyt, D.F. Practical Methods of Estimating Volume and Fresh Weight of Bird Eggs. Auk 1979, 96, 73–77. [Google Scholar]
- Liang, D.; Liu, Y.; Gao, G.; Luo, X. Breeding Biology of a High Altitudinal Aethopyga Sunbird in Southwestern China. J. Nat. Hist. 2020, 54, 2381–2390. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, L.; Yu, H.Y.; Zhao, X.Y.; Zhang, N.; An, J.; Ding, C.Q. Selection of artificial nest boxes by secondary cavity nesting birds in different habitats. Sci. Silvae Sin. 2021, 57, 99–107. [Google Scholar]
- Fretwell, S.D.; Lucas, H.L. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1969, 19, 16–36. [Google Scholar] [CrossRef]
- Potti, J.; Camacho, C.; Canal, D.; Martínez-Padilla, J. Three decades of crimes and misdemeanours in the nest box life of European Pied Flycatchers Ficedula hypoleuca. Ardeola 2021, 68, 315–333. [Google Scholar]
- Doligez, B.; Pärt, T.; Danchin, E.; Clobert, J.; Gustafsson, L. Availability and use of public information and conspecific density for settlement decisions in the collared flycatcher. J. Anim. Ecol. 2004, 73, 75–87. [Google Scholar] [CrossRef]
- Parejo, D.; White, J.; Clobert, J.; Dreiss, A.; Danchin, E. Blue Tits Use Fledgling Quantity and Quality as Public Information in Breeding Site Choice. Ecology 2007, 88, 2373–2382. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Breeding behaviour of Nuthatch Sitta europaeain relation to natural hole attributes in a primeval forest. Bird Study 2004, 51, 143–155. [Google Scholar] [CrossRef]
- Cantarero, A.; López-Arrabé, J.; Moreno, J. Selection of Nest Site and Nesting Material in the Eurasian Nuthatch Sitta europaea. Ardea 2015, 103, 91–94. [Google Scholar] [CrossRef]
- Löhrl, H. Das Verhalten des Kleibers (Sitta europaea caesia Wolf). Z. Tierpsychol. 1958, 15, 191–252. [Google Scholar]
- Conway, C.J.; Martin, T.E. Evolution of passerine incubation behavior: Influence of food, temperature, and nest predation. Evolution 2000, 54, 670–685. [Google Scholar] [PubMed]
- Deeming, D.C. Avian Incubation: Behaviour, Environment, and Evolution; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Da Oliveira, G.; Dos Santos, V.M.; Rodrigues, J.C.; Nascimento, S.T. Effects of different egg turning frequencies on incubation efficiency parameters. Poult. Sci. 2020, 99, 4417–4420. [Google Scholar]
- Martin, T.E.; Llyod, P.; Bosque, C.; Barton, D.C.; Biancucci, A.L.; Cheng, Y.-R.; Ton, R. Growth rate variation among passerine species in tropical and temperate sites: An antagonistic interaction between parental food provisioning and nest predation risk. Evolution 2011, 65, 1607–1622. [Google Scholar] [CrossRef]
- Gauthier, G. The Effect of Experience and Timing on Reproductive Performance in Buffleheads. Auk 1989, 106, 568–576. [Google Scholar]




| Characteristic | 2020 | 2021 | 2022 | |||
|---|---|---|---|---|---|---|
| Natural nest | Nest-box nest | Natural nest | Nest-box nest | Natural nest | Nest-box nest | |
| Number of nests | 3 | 2 | 7 | 7 | 8 | 5 |
| Clutch size (range) | 4 | 3.5 ± 0.5 | 3.5 ± 0.76 | 3.43 ± 0.73 | 3.75 ± 0.43 | 3.6 ± 0.49 |
| 4 | 3–4 | 2–4 | 3–4 | 3–4 | 3–4 | |
| Number of fledglings (all nests) | 5 | 7 | 17 | 14 | 20 | 10 |
| First egg laying date (earliest and latest) | 27 March | 5 April | 26 March | 15 April | 25 March | 11 April |
| 29 March | 15 April | 28 March | 5 May | 1 April | 7 May | |
| Hatching success | 71.42% | 87.5% | 70.08% | 58.33% | 75% | 52.63% |
| 5/7 | 7/8 | 17/24 | 14/24 | 20/26 | 10/19 | |
| Fledging success | 100% | 100% | 76.47% | 85.71% | 100% | 100% |
| 5/5 | 7/7 | 13/17 | 12/14 | 20/20 | 10/10 | |
| Reproductive success | 100% | 100% | 85.71% | 57.14% | 87.5% | 60% |
| 3/3 | 2/2 | 6/7 | 4/7 | 6/8 | 3/5 | |
| Year | Average Daily Temperature | Proportion of Bad Weather * |
|---|---|---|
| 2020 | 26.32 ± 2.74 °C range 12.8–23.6 °C, n = 55 | 32.72% |
| 2021 | 25.99 ± 1.97 °C range 18.8–28.1 °C, n = 63 | 39.68% |
| 2022 | 20.73 ± 4.25 °C range 12.8–23.6 °C, n = 71 | 45.07% |
| Body Length (mm) | Bill Length (mm) | Tarsus Length (mm) | Mass (g) | Number | |
|---|---|---|---|---|---|
| Adult | 121.40 ± 4.94 | 16.16 ± 1.72 | 17.77 ± 1.40 | 14.79 ± 0.54 | 19 |
| Fledgling | 102.39 ± 4.44 | 14.44 ± 1.79 | 17.29 ± 0.52 | 17.51 ± 1.79 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, R.; Li, Y.; Yuan, Q.; He, M.; Xu, X.; Chen, G.; Zhang, W.; Duan, Y. Nest-Site Features and Breeding Ecology of Chestnut-Vented Nuthatch Sitta nagaensis in Southwestern China. Animals 2023, 13, 2034. https://doi.org/10.3390/ani13122034
Mo R, Li Y, Yuan Q, He M, Xu X, Chen G, Zhang W, Duan Y. Nest-Site Features and Breeding Ecology of Chestnut-Vented Nuthatch Sitta nagaensis in Southwestern China. Animals. 2023; 13(12):2034. https://doi.org/10.3390/ani13122034
Chicago/Turabian StyleMo, Ruixin, Yu Li, Qingmiao Yuan, Mingyun He, Xianyin Xu, Guangjian Chen, Wenwen Zhang, and Yubao Duan. 2023. "Nest-Site Features and Breeding Ecology of Chestnut-Vented Nuthatch Sitta nagaensis in Southwestern China" Animals 13, no. 12: 2034. https://doi.org/10.3390/ani13122034
APA StyleMo, R., Li, Y., Yuan, Q., He, M., Xu, X., Chen, G., Zhang, W., & Duan, Y. (2023). Nest-Site Features and Breeding Ecology of Chestnut-Vented Nuthatch Sitta nagaensis in Southwestern China. Animals, 13(12), 2034. https://doi.org/10.3390/ani13122034





