Metagenetic Analysis of the Pregnant Microbiome in Horses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. DNA Sequencing
2.3. Bioinformatics and Statistics
3. Results
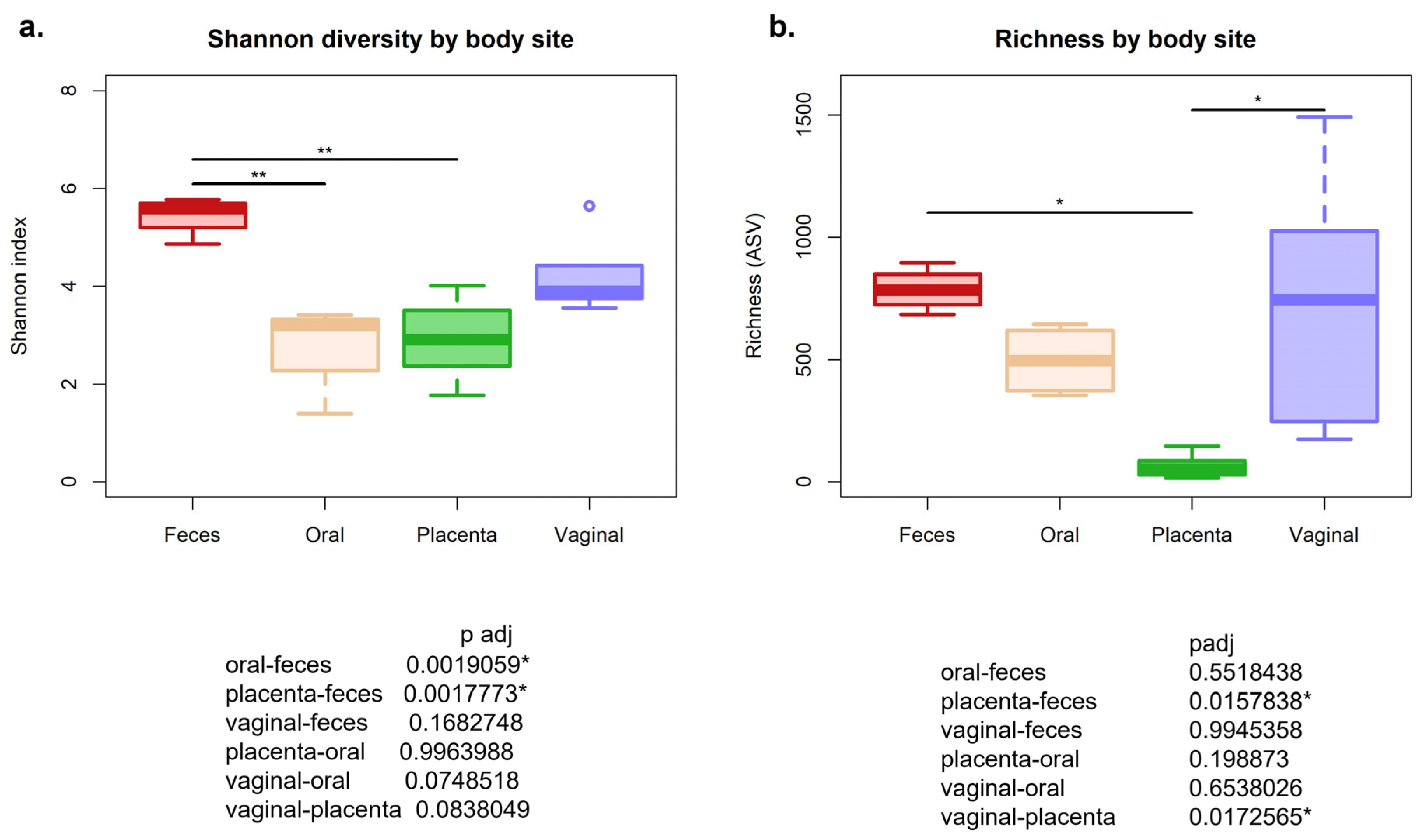
3.1. Alpha Diversity Using Shannon Diversity Matrix Was Significantly Different between Body Sites
3.2. Bacterial Community Compositions Were Different among Body Sites When Assessing Beta Diversity
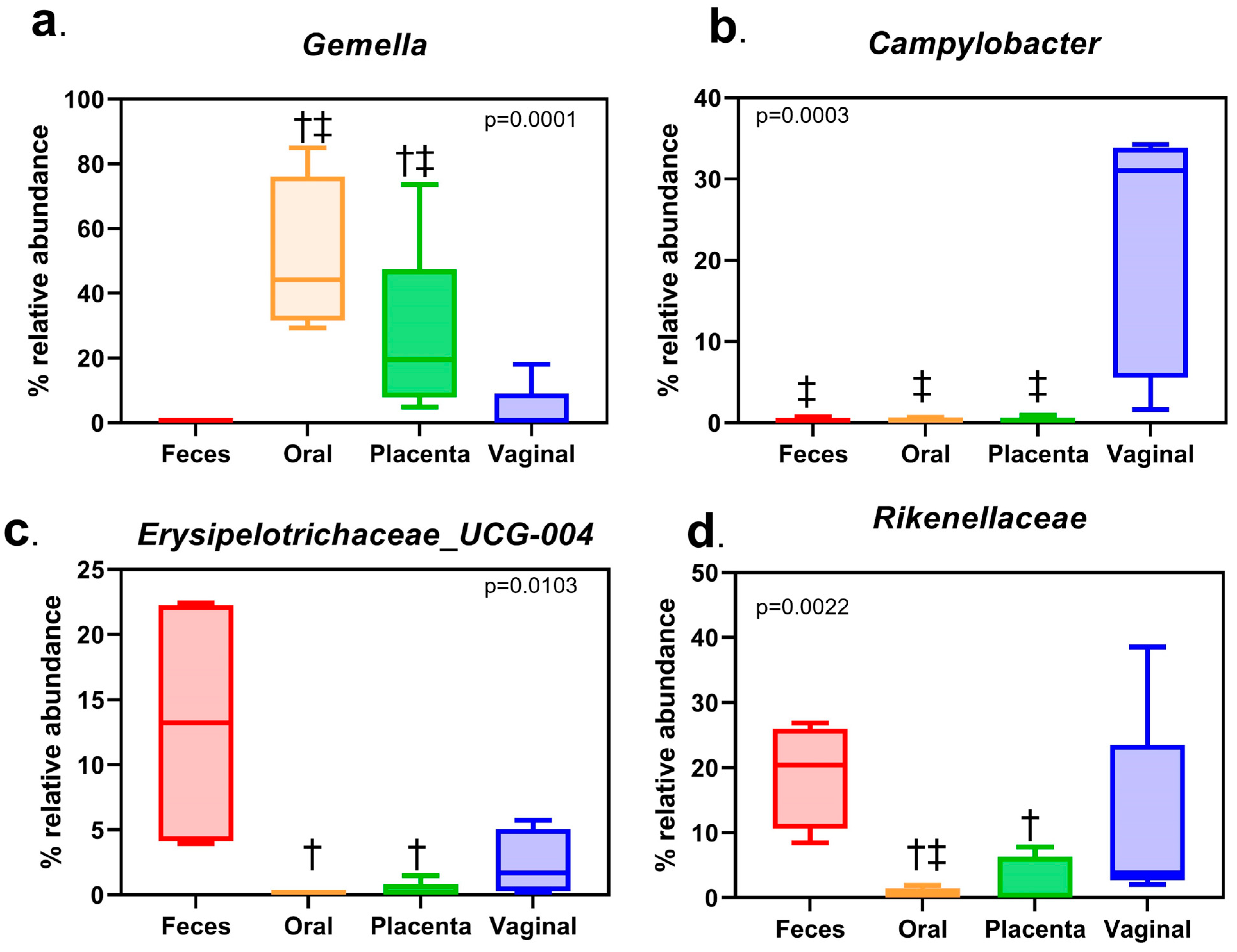
3.3. Difference in Relative Abundance Found at Phyla and Genus Level
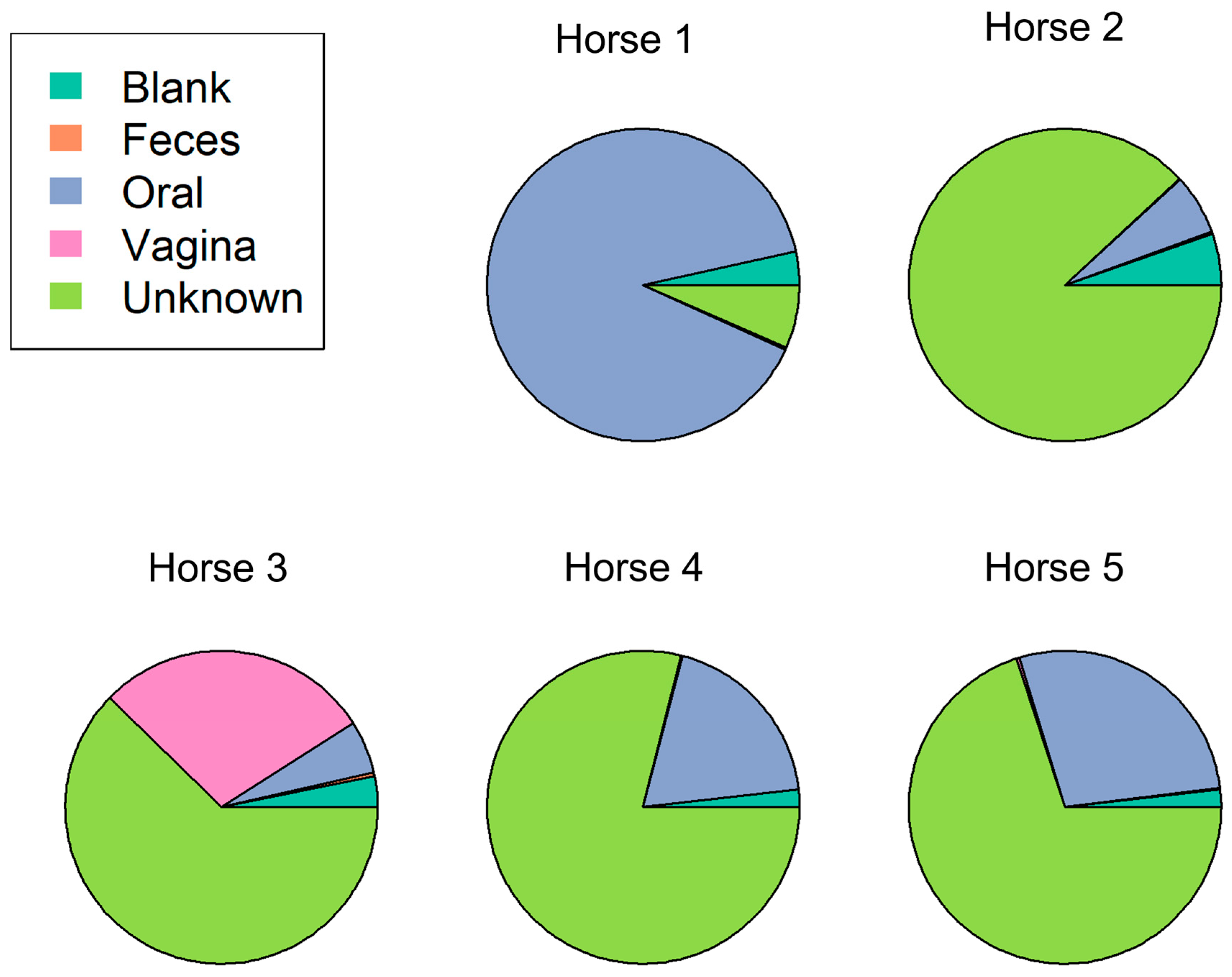
3.4. The Estimated Bacterial Source of the Placental Microbiome Is Mainly of Unknown Origin
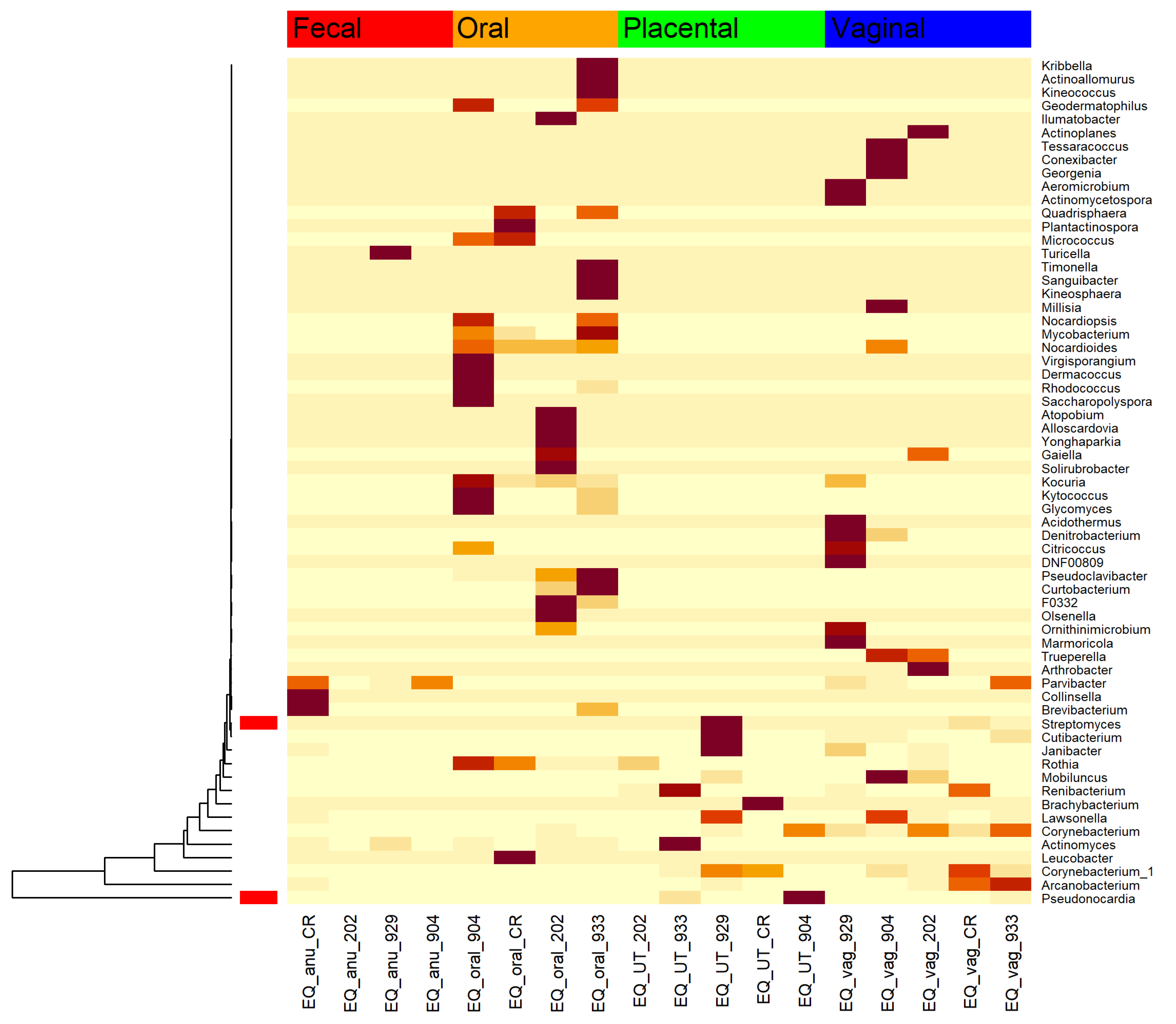
3.5. Microbial Relatives of the Known Nocardioform Pathogens Were Found within These Healthy Horse Placentas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.; Campling, G. Functions of the placenta. Anaesth. Intensive Care Med. 2014, 15, 136–139. [Google Scholar] [CrossRef]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016, 4, 29. [Google Scholar] [CrossRef]
- Leiby, J.S.; McCormick, K.; Sherrill-Mix, S.; Clarke, E.L.; Kessler, L.R.; Taylor, L.J.; Hofstaedter, C.E.; Roche, A.M.; Mattei, L.M.; Bittinger, K.; et al. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 2018, 6, 196. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804. [Google Scholar] [CrossRef]
- Shapira, M. Gut microbiotas and host evolution: Scaling up symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human gut microbiome: Function matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Day, A.S.; Huinao, K.D.; Leach, S.T.; Lemberg, D.A.; Dowd, S.E.; Mitchell, H.M. Microbial dysbiosis in pediatric patients with Crohn’s disease. J. Clin. Microbiol. 2012, 50, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. In Metabolic Interaction in Infection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 459–476. [Google Scholar]
- McElrath, T.F.; Hecht, J.L.; Dammann, O.; Boggess, K.; Onderdonk, A.; Markenson, G.; Harper, M.; Delpapa, E.; Allred, E.N.; Leviton, A. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. Am. J. Epidemiol. 2008, 168, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.A.; Allen, W.R.; Steven, D.H. Studies on the equine placenta. J. Reprod. Fertil. 1974, 41, 441–445. [Google Scholar] [CrossRef]
- Pozor, M. Equine placenta—A clinician’s perspective. Part 1: Normal placenta—Physiology and evaluation. Equine Vet. Educ. 2016, 28, 327–334. [Google Scholar] [CrossRef]
- Sathe, S.; Leiken, A.; Plummer, P. Metagenomic sequencing of the uterine microbial environment during estrus and early pregnancy in mares. Clin. Theriogenol. 2017, 9, 453. [Google Scholar]
- Barba, M.; Martínez-Boví, R.; Quereda, J.J.; Mocé, M.L.; Plaza-Dávila, M.; Jiménez-Trigos, E.; Gómez-Martín, Á.; González-Torres, P.; Carbonetto, B.; García-Roselló, E. Vaginal microbiota is stable throughout the estrous cycle in Arabian mares. Animals 2020, 10, 2020. [Google Scholar] [CrossRef]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genomics 2019, 51, 390–399. [Google Scholar] [CrossRef]
- Murcia, P.R. Clinical insights: The equine microbiome. Equine Vet. J. 2019, 51, 714–715. [Google Scholar] [CrossRef]
- Husso, A.; Jalanka, J.; Alipour, M.J.; Huhti, P.; Kareskoski, M.; Pessa-Morikawa, T.; Iivanainen, A.; Niku, M. The composition of the perinatal intestinal microbiota in horse. Sci. Rep. 2020, 10, 441. [Google Scholar] [CrossRef]
- Jacquay, E. Colonization and Maturation of the Foal Fecal Microbiota from Birth through Weaning and the Effect of Weaning Method. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2017. [Google Scholar]
- Xia, Y.W.; Cornelius, A.J.; Donnelly, C.G.; Bicalho, R.C.; Cheong, S.H.; Sones, J.L. Metagenomic analysis of the equine placental microbiome. Clin. Theriogenol. 2017, 9, 452. [Google Scholar]
- Van Heule, M.; Monteiro, H.F.; Bazzazan, A.; Scoggin, K.; Rolston, M.; Ali, H.E.S.; Weimer, B.C.; Ball, B.; Daels, P.; Dini, P. Characterization of the equine placental microbial population in healthy pregnancies. Theriogenology 2023, 206, 60–70. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘vegan’. Community Ecology Package Version. 2013. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 7 June 2023).
- Anderson, M.J. Permutational Multivariate Analysis of Variance; Department of Statistics, University of Auckland: Auckland, New Zealand, 2005; Available online: http://img2.timg.co.il/forums/1_124959686.pdf (accessed on 25 August 2016).
- Knights, D.; Kuczynski, J.; Charlson, E.S.; Zaneveld, J.; Mozer, M.C.; Collman, R.G.; Bushman, F.D.; Knight, R.; Kelley, S.T. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 2011, 8, 761–763. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Chen, T.; Wu, T.; Lin, T.; Jiang, L.; Lang, S.; Liu, L.; Natarajan, L.; Tu, J.X.; et al. A semiparametric model for between-subject attributes: Applications to beta-diversity of microbiome data. Biometrics 2022, 78, 950–962. [Google Scholar] [CrossRef]
- Canisso, I.; Ball, B.A.; Erol, E.; Squires, E.L.; Troedsson, M.H.; Dact, D. Comprehensive review on equine placentitis. Proc. Am. Assoc. Equine Pract. 2015, 61, 490–509. [Google Scholar]
- Kennedy, R.; Lappin, D.F.; Dixon, P.M.; Buijs, M.J.; Zaura, E.; Crielaard, W.; O’donnell, L.; Bennett, D.; Brandt, B.W.; Riggio, M.P. The microbiome associated with equine periodontitis and oral health. Vet. Res. 2016, 47, 49. [Google Scholar] [CrossRef]
- Theelen, M.J.; Luiken, R.E.; Wagenaar, J.A.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Rossen, J.W.; Zomer, A.L. The equine faecal microbiota of healthy horses and ponies in the Netherlands: Impact of host and environmental factors. Animals 2021, 11, 1762. [Google Scholar] [CrossRef]
- Weinert-Nelson, J.R.; Biddle, A.S.; Williams, C.A. Fecal microbiome of horses transitioning between warm-season and cool-season grass pasture within integrated rotational grazing systems. Anim. Microbiome 2022, 4, 41. [Google Scholar] [CrossRef]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Guo, J.; Sun, H.; Maimai, T.; Zhao, C.; Cao, Y.; Zhang, N.; Hu, X.; Fu, Y. Characterization of bacterial community of rumen from bovine during laminitis challenge by high-throughput sequencing. Research Square 2020. preprint. [Google Scholar] [CrossRef]
- Edwards, J.E.; Shetty, S.A.; Van Den Berg, P.; Burden, F.; Van Doorn, D.A.; Pellikaan, W.F.; Dijkstra, J.; Smidt, H. Multi-kingdom characterization of the core equine fecal microbiota based on multiple equine (sub) species. Anim. Microbiome 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Heil, B.A.; Thompson, S.K.; Kearns, T.A.; Davolli, G.M.; King, G.; Sones, J.L. Metagenetic characterization of the resident equine uterine microbiome using multiple techniques. J. Equine Vet. Sci. 2018, 66, 111. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.B.; de Freitas, S.L.; da Costa, A.N.L.; Bicalho, R.C.; Lima, S.; et al. Uterine microbiota progression from calving until establishment of metritis in dairy cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef]
- Liu, C.J.; Liang, X.; Niu, Z.Y.; Jin, Q.; Zeng, X.Q.; Wang, W.X.; Li, M.Y.; Chen, X.R.; Meng, H.Y.; Shen, R.; et al. Is the delivery mode a critical factor for the microbial communities in the meconium? EBioMedicine 2019, 49, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Scoggin, K.E.; Ruby, R.E.; Erol, E.; Ball, B.A. Clinical, pathologic, and epidemiologic features of nocardioform placentitis in the mare. Theriogenology 2021, 171, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.B.; Donahue, J.M.; Giles, R.C., Jr.; Petrites-Murphy, M.B.; Poonacha, K.B.; Roberts, A.W.; Smith, B.J.; Tramontin, R.R.; Tuttle, P.A.; Swerczek, T.W. Etiology and pathology of equine placentitis. J. Vet. Diagn. Investig. 1993, 5, 56–63. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Troedsson, M.H. The immune response to equine ascending placentitis: A narrative review. Theriogenology 2023, 203, 11–20. [Google Scholar] [CrossRef]
- Holyoak, G.R.; Premathilake, H.U.; Lyman, C.C.; Sones, J.L.; Gunn, A.; Wieneke, X.; DeSilva, U. The healthy equine uterus harbors a distinct core microbiome plus a rich and diverse microbiome that varies with geographical location. Sci. Rep. 2022, 12, 14790. [Google Scholar] [CrossRef]
- Macpherson, M.L. Treatment strategies for mares with placentitis. Theriogenology 2005, 64, 528–534. [Google Scholar] [CrossRef]



| ID | Age (Years) | Gestation (Days) |
|---|---|---|
| Horse 1 | 8 | 96–98 |
| Horse 2 | 8 | 98–100 |
| Horse 3 | 6 | 100–102 |
| Horse 4 | 4 | 98–99 |
| Horse 5 | 5 | 98–126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckers, K.F.; Gomes, V.C.L.; Crissman, K.R.; Liu, C.-C.; Schulz, C.J.; Childers, G.W.; Sones, J.L. Metagenetic Analysis of the Pregnant Microbiome in Horses. Animals 2023, 13, 1999. https://doi.org/10.3390/ani13121999
Beckers KF, Gomes VCL, Crissman KR, Liu C-C, Schulz CJ, Childers GW, Sones JL. Metagenetic Analysis of the Pregnant Microbiome in Horses. Animals. 2023; 13(12):1999. https://doi.org/10.3390/ani13121999
Chicago/Turabian StyleBeckers, Kalie F., Viviane C. L. Gomes, Kassandra R. Crissman, Chin-Chi Liu, Christopher J. Schulz, Gary W. Childers, and Jenny L. Sones. 2023. "Metagenetic Analysis of the Pregnant Microbiome in Horses" Animals 13, no. 12: 1999. https://doi.org/10.3390/ani13121999
APA StyleBeckers, K. F., Gomes, V. C. L., Crissman, K. R., Liu, C.-C., Schulz, C. J., Childers, G. W., & Sones, J. L. (2023). Metagenetic Analysis of the Pregnant Microbiome in Horses. Animals, 13(12), 1999. https://doi.org/10.3390/ani13121999







