Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
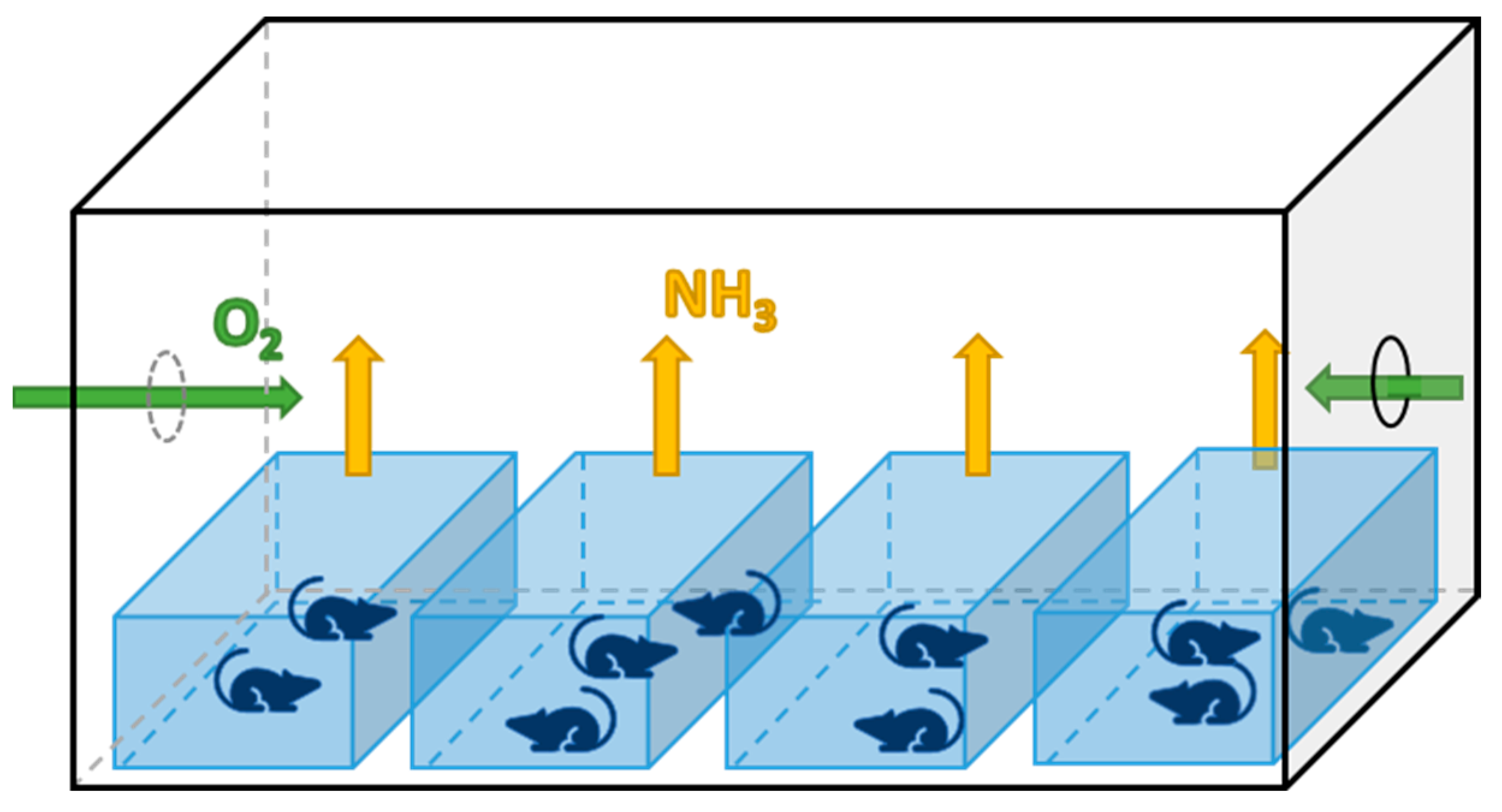
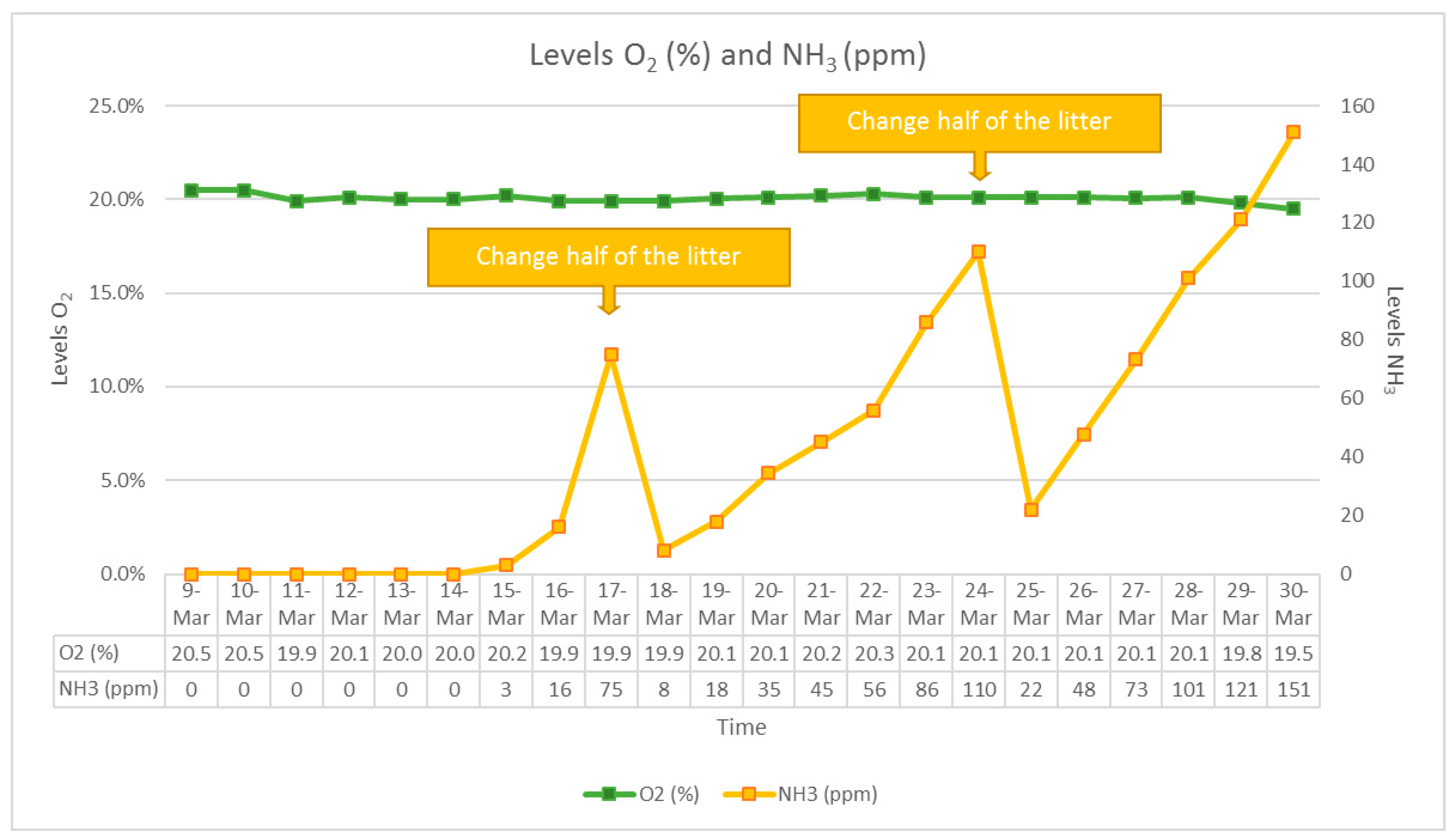
2.1. Animals and Sampling Procedures
2.2. Histopathological Analyses
2.3. Immunohistochemical Analyses
2.4. Statistical Analysis
3. Results
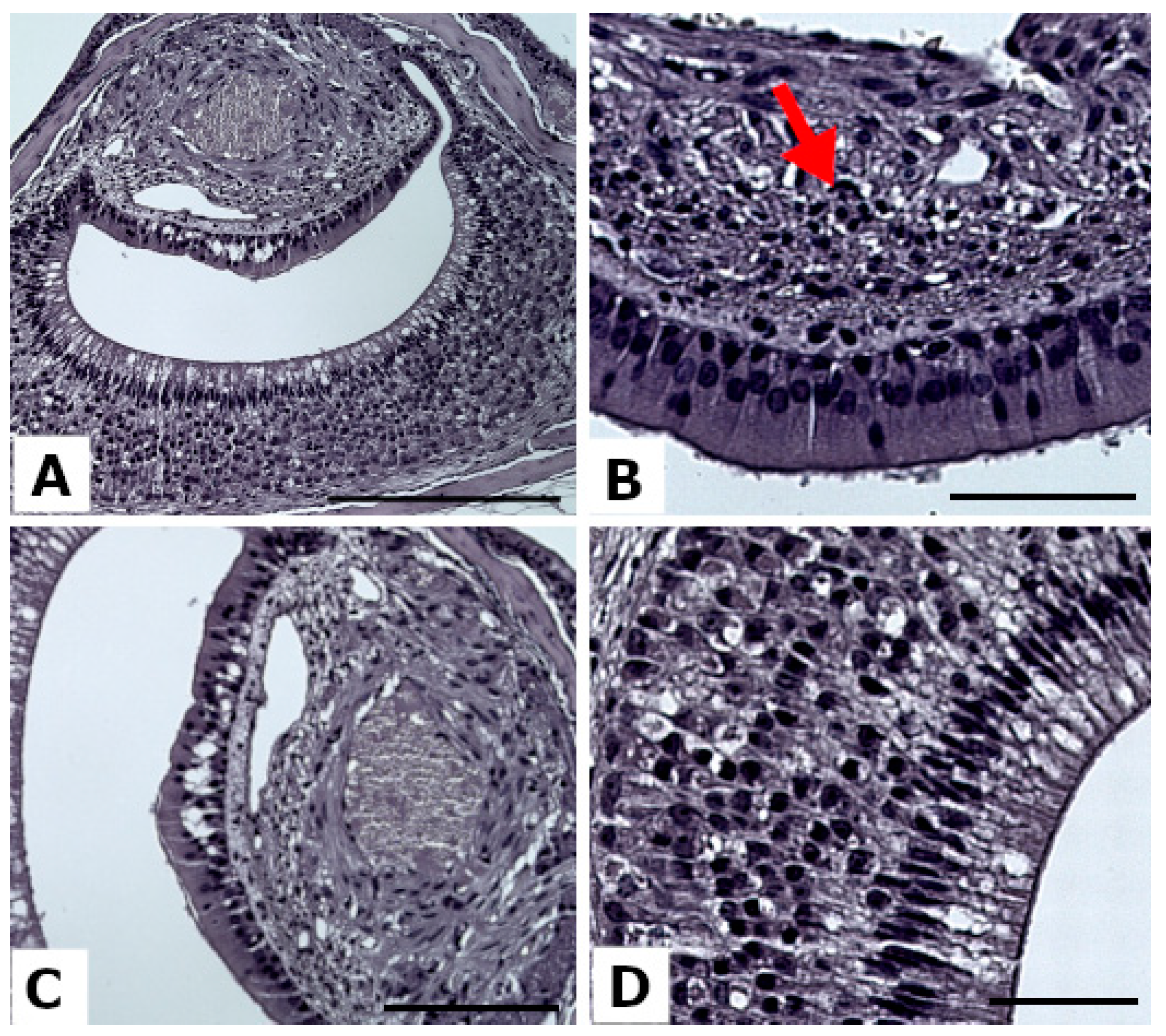
3.1. VNO Alteration
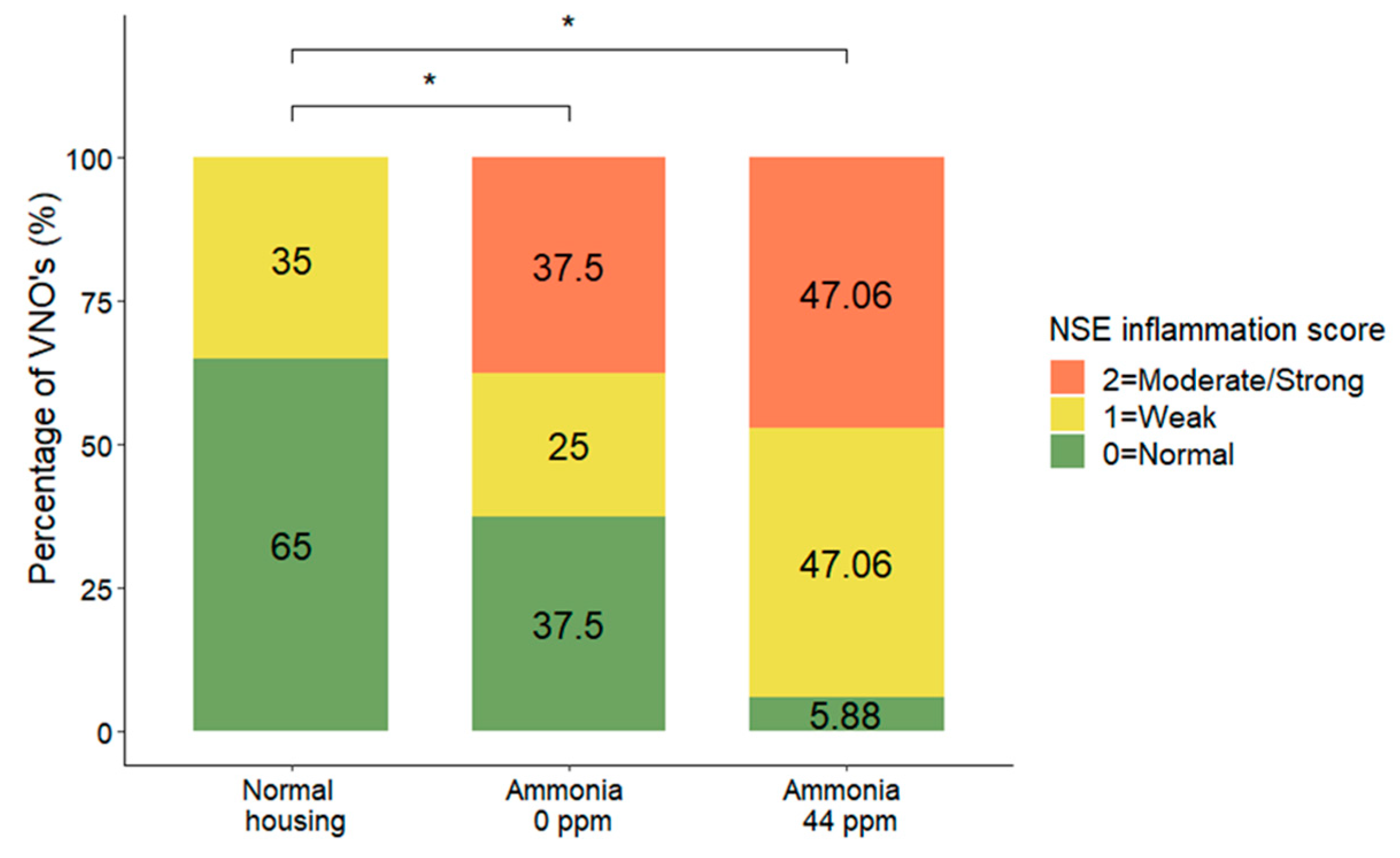
3.2. NSE Inflammation
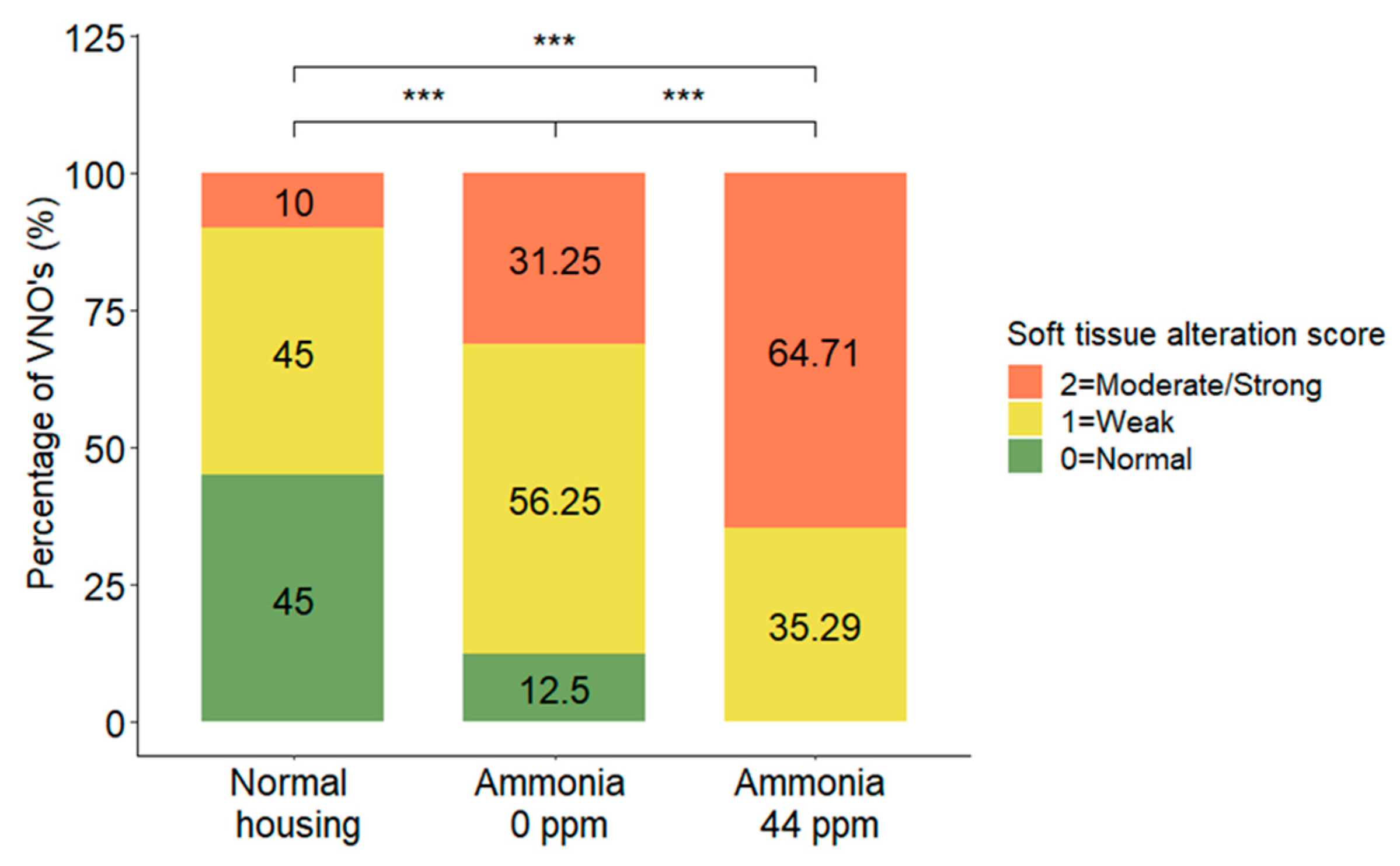
3.3. Soft Tissue Alteration
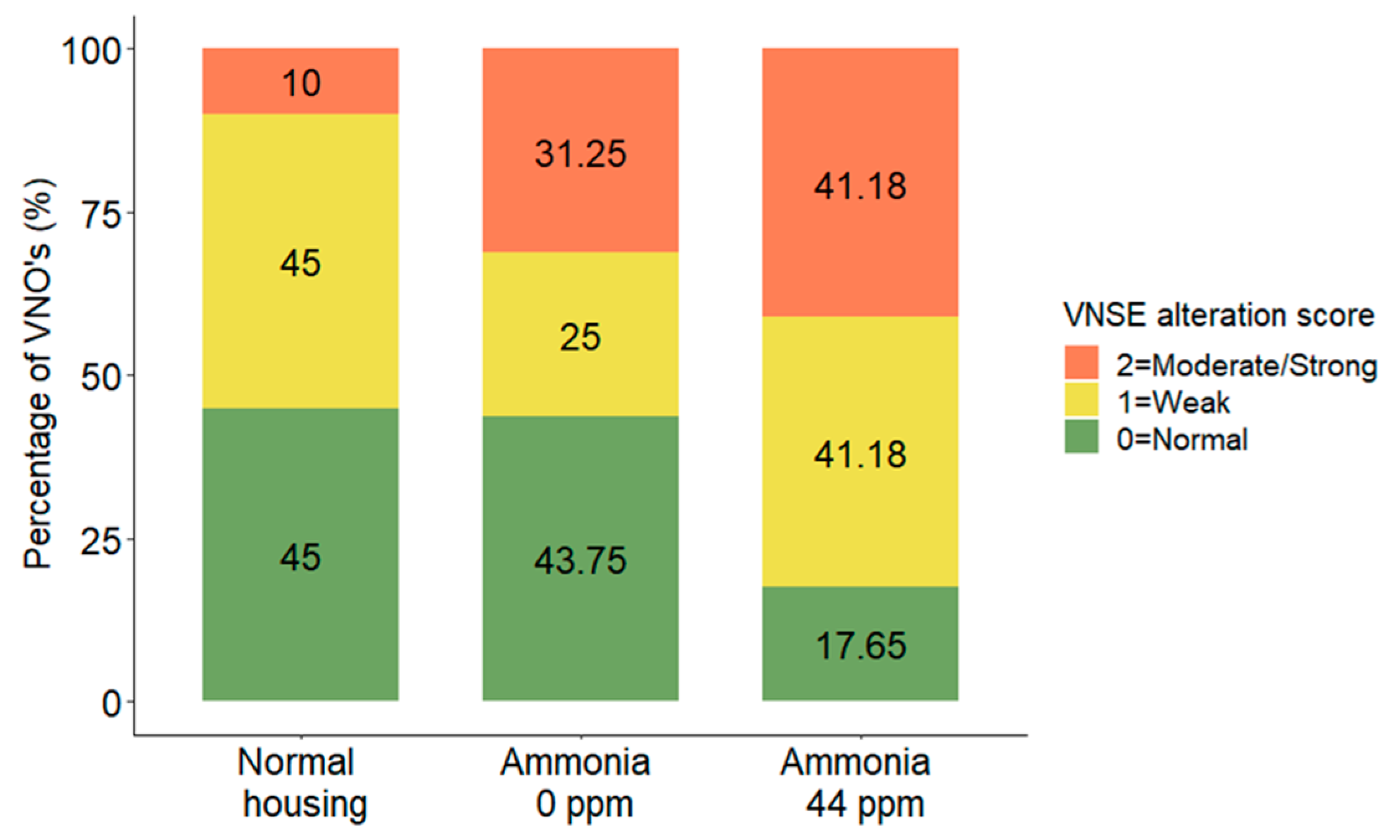
3.4. VNSE Alteration
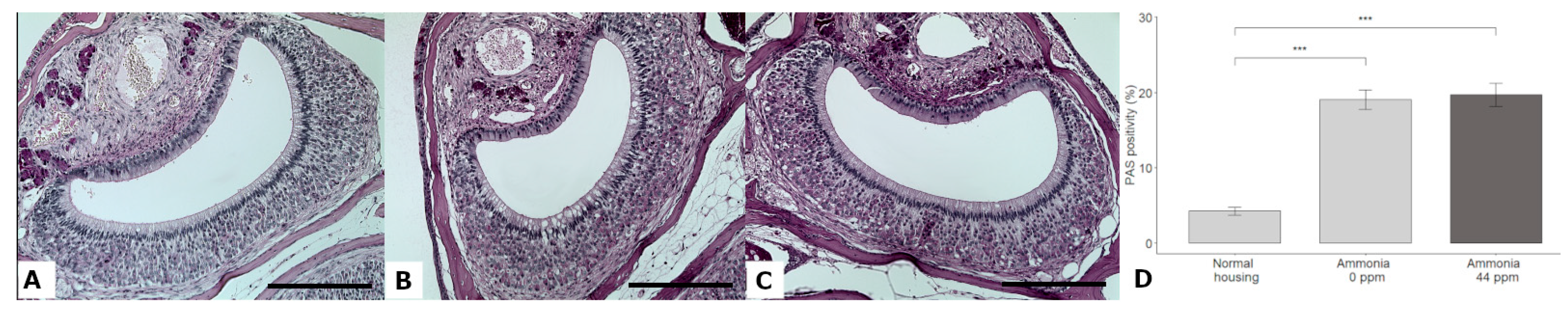
3.5. Glycogen Accumulation
3.6. OMP Expression in the VNSE
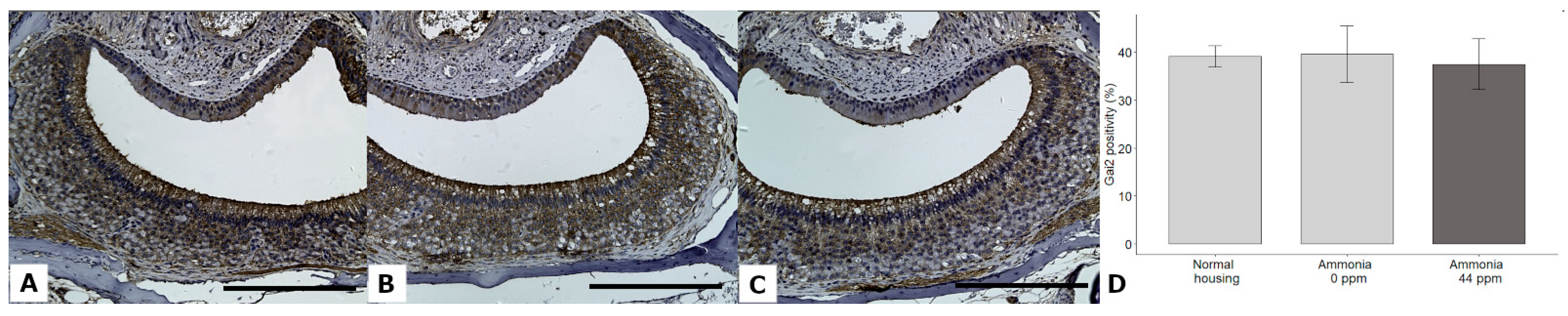
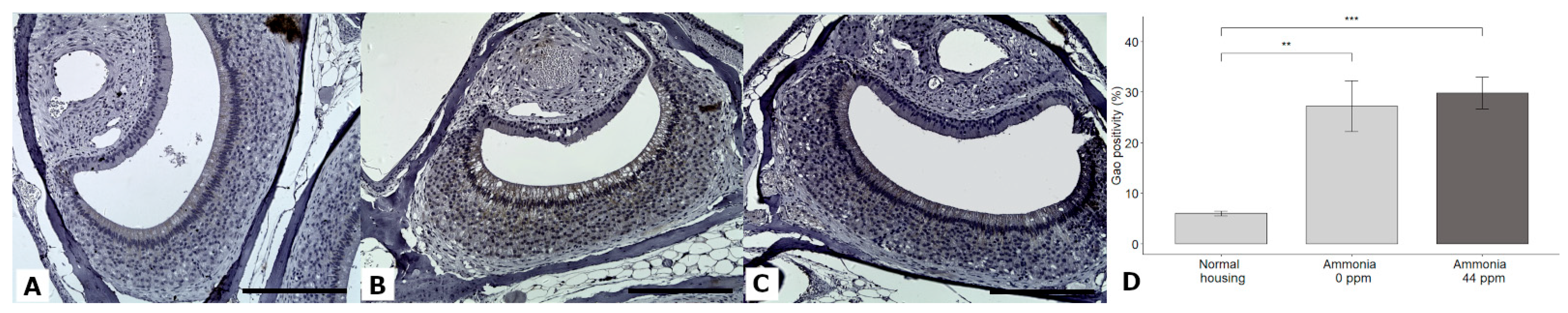
3.7. Gαi2 and Gαo Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zufall, F.; Kelliher, K.R.; Leinders-Zufall, T. Pheromone detection by mammalian vomeronasal neurons. Microsc. Res. Tech. 2002, 58, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Martínez-Marcos, A. Structure and function of the vomeronasal system: An update. Prog. Neurobiol. 2003, 70, 245–318. [Google Scholar] [CrossRef]
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 2009, 459, 574–577. [Google Scholar] [CrossRef]
- Isogai, Y.; Si, S.; Pont-Lezica, L.; Tan, T.; Kapoor, V.; Murthy, V.N.; Dulac, C. Molecular Organization of Vomeronasal Chemoreception. Nature 2012, 478, 241–245. [Google Scholar] [CrossRef]
- Rodriguez, I. Vomeronasal receptors: V1Rs, V2Rs, and FPRs. In The Detection of Odors, Tastes and Other Chemostimuli; Academic Press: Cambridge, MA, USA, 2016; pp. 175–190. [Google Scholar]
- Dulac, C.; Axel, R. A novel family of genes encoding putative pheromone receptors in mammals. Cell 1995, 83, 195–206. [Google Scholar] [CrossRef]
- Wysocki, C.J.; Lepri, J.J. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991, 39, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Booth, K.; Katz, L.S. Role of the vomeronasal Organ in neonatal offspring recognition in sheep. Biol. Reprod. 2000, 63, 953–958. [Google Scholar] [CrossRef]
- Booth, K.K.; Webb, E.C. Effect of blockage of the ducts of the vomeronasal Organ on LH plasma levels during the whitten effect in does. Vet. Med. Int. 2011, 2011, 22–24. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Removal of the vomeronasal organ blocks the stress-induced hyperthermia response to alarm pheromone in male rats. Chem. Senses 2007, 32, 57–64. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Cherry, J.A.; Baum, M.J. Effect of Vomeronasal Organ Removal From Male Mice on Their Preference for and Neural Fos Responses to Female Urinary Odors. Behav. Neurosci. 2006, 120, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and behaviour in feline medicine: Investigating the link between vomeronasalitis and aggression. J. Feline Med. Surg. 2016, 18, 997–1002. [Google Scholar] [CrossRef]
- Asproni, P.; Mainau, E.; Cozzi, A.; Carreras, R.; Bienboire-Frosini, C.; Teruel, E.; Pageat, P. Is There a Link between Vomeronasalitis and Aggression in Stable Social Groups of Female Pigs? Animals 2022, 12, 303. [Google Scholar] [CrossRef]
- Curtis, S.E.; Anderson, C.R.; Simon, J.; Jensen, A.H.; Day, D.L.; Kelley, K.W. Effects of aerial ammonia, hydrogen sulfide and swine-house dust on rate of gain and respiratory-tract structure in swine. J. Anim. Sci. 1975, 41, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.A.; Jiang, X.Z.; James, R.A.; Morgan, K.T.; Barrow, C.S. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol. Appl. Pharmacol. 1984, 74, 417–429. [Google Scholar] [CrossRef]
- Gaafar, H.; Tantawy, A.; Hamza, M.; Shaaban, M. The effect of ammonia on olfactory epithelium and vomeronasal organ neuroepithelium of rabbits. A histological and histochemical study. ORL 1998, 60, 88–91. [Google Scholar] [CrossRef]
- Martin, S.W.; Willoughby, R.A. Organic dusts, sulfur dioxide, and the respiratory tract of swine. Arch. Environ. Health 1972, 25, 158–165. [Google Scholar] [CrossRef]
- Riskowski, G.L.; Harrison, P.C.; Memarzadeh, F. Mass generation rates of ammonia, moisture, and heat production in mouse cages with two bedding types, two mouse strains, and two room relative humidities. ASHRAE Trans. 2006, 112, 134–144. [Google Scholar]
- Ferrecchia, C.E.; Jensen, K.; Van Andel, R. Intracage ammonia levels in static and individually ventilated cages housing C57BL/6 mice on 4 bedding substrates. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 146–151. [Google Scholar] [PubMed]
- Shi, Z.; Sun, X.; Lu, Y.; Xi, L.; Zhao, X. Emissions of ammonia and hydrogen sulfide from typical dairy barns in central China and major factors influencing the emissions. Sci. Rep. 2019, 9, 13821. [Google Scholar] [CrossRef]
- Mexas, A.M.; Brice, A.K.; Caro, A.C.; Hillanbrand, T.S.; Gaertner, D.J. Nasal histopathology and intracage ammonia levels in female groups and breeding mice housed in static isolation cages. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 478–486. [Google Scholar]
- Vogelweid, C.M.; Zapien, K.A.; Honigford, M.J.; Li, L.; Li, H.; Marshall, H. Effects of a 28-day cage-change interval on intracage ammonia levels, nasal histology, and perceived welfare of CD1 mice. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 868–878. [Google Scholar]
- Mechin, V.; Pageat, P.; Teruel, E.; Asproni, P. Histological and Immunohistochemical Characterization of Vomeronasal Organ Aging in Mice. Animals 2021, 11, 1211. [Google Scholar] [CrossRef]
- Mechin, V.; Asproni, P.; Bienboire-Frosini, C.; Cozzi, A.; Chabaud, C.; Arroub, S.; Mainau, E.; Nagnan-Le Meillour, P.; Pageat, P. Inflammation interferes with chemoreception in pigs by altering the neuronal layout of the vomeronasal sensory epithelium. Front. Vet. Sci. 2022, 9, 936838. [Google Scholar] [CrossRef]
- Zancanaro, C. Vomeronasal Organ: A Short History of Discovery and an Account of Development and Morphology in the Mouse. In Neurobiology of Chemical Communication; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Degré, A.; Verhève, D.; Debouche, C. Émissions gazeuses en élevage porcin et modes de réduction: Revue bibliographique. Biotechnol. Agron. Soc. Environ. 2001, 5, 135–143. [Google Scholar]
- Schulze, H.; Sandhoff, K. Lysosomal lipid storage diseases. Cold Spring Harb. Perspect. Biol. 2011, 3, a004804. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.D.; Shin, T.H.; Bae, C.S.; Ahn, T.; Shin, S.S.; Kim, H.J.; Lee, C.M.; Suh, G.H. Respiratory and systemic toxicity of inhaled artificial asian sand dust in pigs. Life 2021, 11, 25. [Google Scholar] [CrossRef]
- Brown, A.M.; Sickmann, H.M.; Fosgerau, K.; Lund, T.M.; Schousboe, A.; Waagepetersen, H.S.; Ransom, B.R. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 2005, 79, 74–80. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Ruth, H.; Magistretti, P.J.; Alberini, C.M. Memory Formation. Encycl. Exerc. Med. Health Dis. 2012, 144, 557. [Google Scholar]
- Duran, J.; Guinovart, J.J. Brain glycogen in health and disease. Mol. Asp. Med. 2015, 46, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Augé, E.; Cabezón, I.; Pelegrí, C.; Vilaplana, J. New perspectives on corpora amylacea in the human brain. Sci. Rep. 2017, 7, srep41807. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mu, M.; Zou, Y.; Deng, S.; Lu, Y.; Li, Q.; Li, Z.; Tao, H.; Wang, Y.; Tao, X. Glycogen metabolism reprogramming promotes inflammation in coal dust-exposed lung. Ecotoxicol. Environ. Saf. 2022, 242, 113913. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Park, B.; Palanivel, R.; Vinayachandran, V.; Deiuliis, J.A.; Gangwar, R.S.; Das, L.; Yin, J.; Choi, Y.; Al-Kindi, S.; et al. Metabolic effects of air pollution exposure and reversibility. J. Clin. Investig. 2020, 130, 6034–6040. [Google Scholar] [CrossRef]
- Dibattista, M.; Reisert, J. The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J. Neurosci. 2016, 36, 2995–3006. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Münzel, T. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Trouillet, A.C.; Keller, M.; Weiss, J.; Leinders-Zufall, T.; Birnbaumer, L.; Zufall, F.; Chamero, P. Central role of G protein Gαi2 and Gαi2 + vomeronasal neurons in balancing territorial and infant-directed aggression of male mice. Proc. Natl. Acad. Sci. USA 2019, 116, 5135–5143. [Google Scholar] [CrossRef]
- Chamero, P.; Katsoulidou, V.; Hendrix, P.; Bufe, B.; Roberts, R.; Matsunami, H.; Abramowitz, J.; Birnbaumer, L.; Zufall, F.; Leinders-Zufall, T. G protein Gαo is essential for vomeronasal function and aggressive behavior in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 12898–12903. [Google Scholar] [CrossRef]
- Loconto, J.; Papes, F.; Chang, E.; Stowers, L.; Jones, E.P.; Takada, T.; Kumánovics, A.; Lindahl, K.F.; Dulac, C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell 2003, 112, 607–618. [Google Scholar] [CrossRef]
- Mangold, C.A.; Masser, D.R.; Stanford, D.R.; Bixler, G.V.; Pisupati, A.; Giles, C.B.; Wren, J.D.; Ford, M.M.; Sonntag, W.E.; Freeman, W.M. CNS-wide sexually dimorphic induction of the major histocompatibility complex 1 pathway with aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Loike, J.D.; Sulzer, D. Neuronal mhc-i expression and its implications in synaptic function, Axonal regeneration and parkinson’s and other brain diseases. Front. Neuroanat. 2014, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.M. Ventilation and temperature criteria for pigs. In Environmental Aspects of Housing for Animal Production; Clarck, J.A., Ed.; Butterworths: Boston, MA, USA, 1981; pp. 197–216. [Google Scholar]
- Honda, A.; Matsuda, Y.; Murayama, R.; Tsuji, K.; Nishikawa, M.; Koike, E.; Yoshida, S.; Ichinose, T.; Takano, H. Effects of Asian sand dust particles on the respiratory and immune system. J. Appl. Toxicol. 2014, 34, 250–257. [Google Scholar] [CrossRef]
- McClendon, C.J.; Gerald, C.L.; Waterman, J.T. Farm animal models of organic dust exposure and toxicity: Insights and implications for respiratory health. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Bays, D.W.; Cooper, S.F.; Baker, S.P. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 57–62. [Google Scholar] [PubMed]
- Kern, R.C. Candidate’s Thesis: Chronic Sinusitis and Anosmia: Pathologic Changes in the Olfactory Mucosa. Laryngoscope 2000, 110, 1071–1077. [Google Scholar] [CrossRef]
- Turner, J.H.; May, L.; Reed, R.R.; Lane, A.P. Reversible Loss of Neuronal Marker Protein Expression in a Transgenic Mouse Model for Sinusitis-Associated Olfactory Dysfunction. Am. J. Rhinol. Allergy 2010, 24, 192–196. [Google Scholar] [CrossRef]
- Opinion, S.; Panel, E.; Health, A. Scientific Opinion on the use of animal-based measures to assess welfare in pigs. EFSA J. 2012, 10, 2512. [Google Scholar]
- HSUS The Humane Society of the United States. The Welfare of Animals in the Pig Industry. Washington. 2010. Available online: https://www.humanesociety.org/sites/default/files/docs/hsus-report-pig-industry-welfare.pdf (accessed on 1 March 2023).


| Qualitative Variables | ||||||
|---|---|---|---|---|---|---|
| Variable | N (%) Normal Housing (N tot = 20) | N (%) Ammonia 0 ppm (N tot = 16) | N (%) Ammonia 44 ppm (N tot = 17) | Model Used | Global Results | Multiple Comparisons |
| VNO alteration | MOLR | χ2 = 5.95, DF = 2, p = 0.0311 | Normal < w NH3 * Normal < w/o NH3 * | |||
| 0 = Normal | 10 (71.4) | 2 (14.3) | 2 (14.3) | |||
| 1 = Weak | 8 (32.0) | 8 (32.0) | 9 (36.0) | |||
| 2 = Moderate/strong | 2 (14.3) | 6 (42.9) | 6 (42.9) | |||
| NSE inflammation | MOLR | χ2 = 12.04, DF = 2, p = 0.0024 | Normal < w NH3 * Normal < w/o NH3 * | |||
| 0 = Normal | 13 (65.0) | 6 (30.0) | 1 (5.0) | |||
| 1 = Weak | 7 (36.8) | 4 (21.1) | 8 (42.1) | |||
| 2 = Moderate/strong | 0 (0.0) | 6 (42.9) | 8 (57.1) | |||
| Soft tissue alteration | MOLR | χ2 = 5.91, DF = 2, p = 0.0480 | Normal < w NH3 *** Normal < w/o NH3 *** w NH3 > w/o NH3 *** | |||
| 0 = Normal | 9 (81.8) | 2 (18.2) | 0 (0.0) | |||
| 1 = Weak | 9 (37.5) | 9 (37.5) | 6 (25.0) | |||
| 2 = Moderate/strong | 2 (11.1) | 5 (27.8) | 11 (61.1) | |||
| VNSE inflammation | MOLR | χ2 = 2.98, DF = 2, p = 0.2251 | NA | |||
| 0 = Normal | 9 (47.4) | 7 (36.8) | 3 (15.8) | |||
| 1 = Weak | 9 (45.0) | 4 (20.0) | 7 (35.0) | |||
| 2 = Moderate/strong | 2 (14.3) | 5 (35.7) | 7 (50.0) | |||
| Quantitative variables | ||||||
| Variable | Mean ± SD Normal housing | Mean ± SD Ammonia 0 ppm | Mean ± SD Ammonia 44 ppm | Model used | Global results | Multiple comparisons |
| PAS positivity (%) | 4.24 ± 1.95 | 19.05 ± 5.01 | 19.70 ± 6.45 | GLMM | χ2 = 47.92, DF = 2, p < 0.0001 | Normal < w NH3 *** Normal < w/o NH3 *** |
| OMP positivity (%) | 75.27 ± 7.07 | 28.77 ± 12.83 | 39.56 ± 4.22 | GLMM | χ2 = 138.48, DF = 2, p < 0.0001 | Normal > w NH3 *** Normal > w/o NH3 *** |
| Gαi2 positivity (%) | 39.09 ± 9.91 | 39.56 ± 24.48 | 37.47 ± 22.49 | GLMM | χ2 = 1.88, DF = 2, p = 0.3892 | NA |
| Gαo positivity (%) | 6.03 ± 1.95 | 27.22 ± 21.32 | 29.82 ± 13.49 | GLMM | χ2 = 8.95, DF = 2, p = 0.0114 | Normal < w NH3 Normal < w/o NH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechin, V.; Pageat, P.; Boutry, M.; Teruel, E.; Portalier, C.; Asproni, P. Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals 2023, 13, 1902. https://doi.org/10.3390/ani13121902
Mechin V, Pageat P, Boutry M, Teruel E, Portalier C, Asproni P. Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals. 2023; 13(12):1902. https://doi.org/10.3390/ani13121902
Chicago/Turabian StyleMechin, Violaine, Patrick Pageat, Marion Boutry, Eva Teruel, Céline Portalier, and Pietro Asproni. 2023. "Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming" Animals 13, no. 12: 1902. https://doi.org/10.3390/ani13121902
APA StyleMechin, V., Pageat, P., Boutry, M., Teruel, E., Portalier, C., & Asproni, P. (2023). Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals, 13(12), 1902. https://doi.org/10.3390/ani13121902






