Simple Summary
The addition of various bioactive compounds to chicken feed has a positive effect on the health, production traits and general welfare of the animals. To use these compounds more efficiently, it is necessary to better understand the molecular relationship between the nutrients in the feed and the genes that trigger the change to a preferred phenotype. This can be achieved with the help of nutrigenomics, a science that studies the interaction between nutrients and the genome and their influence on metabolic and physiological processes in the body. By implementing the knowledge gained from nutrigenomics, it is possible to more efficiently produce high-quality chicken products that have a positive impact on human health.
Abstract
Consumer demand for high quality and safe foods that will have a positive impact on their health has increased in recent years. Today, it is possible to meet those demands by combining the genetic potential of domestic animals and applying different feeding strategies. Nutrigenomics is one of the “omics” sciences that studies the interaction between nutrients and the genome together with their influence on metabolic and physiological processes in the body. While nutrition of domestic animals is solely based on studying the influence of nutrients on animal health and production traits, nutrigenomics integrates the fields of nutrition, genomics, molecular genetics and bioinformatics. By understanding the molecular relationships between different forms and/or concentrations of nutrients in feed and genes, it is possible to answer the question of how small changes in the diet of farm animals can produce a quality product with positive effects on human health. The aim of this article is to describe how the manipulation of adding different nutrients in the feed affects the expression of different genes in chicken and consequently alters their phenotype.
1. Introduction
The importance of consuming healthy food of good quality has been known for almost two thousand years. Terms, such as “food” and “nutrition”, have been known and used since the earliest history, although the concept of nutritional science as we know it today emerged in the early 19th century [1]. The concept reached its peak after the isolation of vitamin C in 1936, when it was officially documented for the first time that this vitamin protects against scurvy [2]. Thereafter, nutrition science has continued to evolve, from the discovery of the role of fat and sugar in the body in the 1950s, through dietary supplements and nutrition adjusted to chronic diseases in the 1970s, to a science-based personalized approach to individual nutrition in the 21st century [2]. However, none of this would have been possible without the development of technology and different sciences, and in particular “omics” sciences. In the context of nutrition, this refers predominantly to nutrigenomics, which is defined as a scientific discipline that studies the relationships between nutrients, diet and gene expression [3]. The aim of nutrigenomics is to find out how food components (bioactive components) can influence gene expression in terms of enhancing or suppressing their potential [4]. With its tools, it is possible to select very precisely the nutrients that can fine-tune the expression of genes in humans or animals and improve their health or production traits [5]. Before the advent of nutrigenomics in livestock production, nutrition and genetics were studied as separate disciplines, without taking into consideration the influence of genome–nutrition interaction effect on metabolic and physiological processes in domestic animals [6]. However, with the development of epigenetics, genomics and other “omics” sciences, it became clear that the expression of certain traits in domestic animals is influenced by the synergy of genetic background and environmental factors [7]. An illustration of how “omics” science is involved in the expression of a particular phenotype can be found in Figure 1.
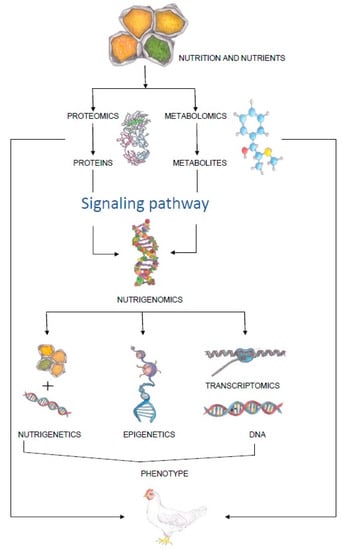
Figure 1.
Schematic representation of the influence of feed on the phenotype of animals.
Food components interact with the body at system, organ, cellular and molecular levels. They are present in complex mixtures, in which the presence and concentrations of a single compound and also the interactions of several compounds, determine their bioavailability and biological efficiency.
The interaction between nutrients and cellular/genetic processes is referred to as nutrigenomics. It is a broad term that encompasses nutrigenetics, which aims to understand how genetic background affects the body’s response to bioactive food ingredients; epigenetics, which studies stable, environmentally induced changes in gene expression; and transcriptomics, which studies all forms of RNA in the body. Proteomics in nutrition can identify and quantify bioactive proteins and peptides and address questions of nutritional biological efficiency, while metabolomics aims to determine metabolites responsible for a particular phenotype. In this way, the coherent measures of nutrigenomics, proteomics and metabolomics play a crucial role in understanding how a particular diet interacts with the body and forms a particular phenotype.
A better understanding of the physiology of domestic animals and its relationship to the nutrients in diets is a prerequisite for designing meals that not only have a positive impact on production results but also enable the realization of the animal’s full genetic potential [8]. Recent research has shown that components, such as vitamin E [9], carotenoids [10], coenzyme Q [11], organic acids [12], essential oils [13], amino acids [14] and many other bioactive compounds, affect gene expression in different species of domestic animals, confirming the existence of interactions between nutrients in the diets and their genome. With the production of 100.974 million tons of meat and 86.670 million tons of eggs in 2020, poultry breeding is the leading livestock industry in the world [15].
From the consumer’s perspective, poultry meat and products are perceived as low-fat and lean foods that are healthier than other products derived from farmed animals. This is one of the reasons why consumption of poultry meat has increased enormously and poultry meat ranks first in global meat consumption. It also has the organoleptic and qualitative characteristics desired by consumers, such as a neutral taste, a uniform and good texture and a light color [15]. From a nutritional point of view, it has a high protein content, a low cholesterol and fat content and a balanced ratio of n-6 to n-3 polyunsaturated fatty acids.
Feed is the most important element in the production system, the cost of which can account for up to 70% of total production costs. The importance of feed optimization in poultry nutrition is reflected in the optimal distribution and utilization of nutrients, which leads to improved performance of the animals and furthermore to a minimization of economic losses and an increase in profitability [16,17]. Due to the increasing consumption of poultry products, feed optimization represents one of the most important tasks of the farm, which would neither be possible nor sustainable without the application of findings from nutrigenomics.
The aim of this paper is to summarize new scientific findings on the impact of feed modulation using bioactive substances on health and productive traits of chicken.
Although there are many bioactive compounds that have been shown to have a positive effect on the health and productive traits or on the expression of various genes in chicken, only scientific research that have been shown to act on both the phenome and the genome are presented in this article.
2. Phytonutrients
Phytochemicals are substances of plant origin whose addition to animal feed positively influences growth, production traits and maintenance of intestinal microflora [18,19]. The results of numerous studies have shown that essential oils and substances can play an important role in poultry health and performance by stimulating food intake, secretion of endogenous enzymes, production of antioxidants and antibacterial activity. This group includes plant extracts and their active ingredients, such as carvacrol, thymol and capsaicin, with beneficial effects related to their bioactive compounds [20]. Phytonutrients can be added to poultry feed individually or as mixtures in varying ratios. Phytonutrients, such as oregano and cinnamaldehyde, are considered natural additives that help protect broilers from infectious diseases [21] while providing an alternative to antibiotics as growth promoters [22]. Furthermore, it has been shown that the addition of oregano and cinnamaldehyde to broiler diets stimulates the expression of the insulin-like growth factor 1 (IGF1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes and positively affects production traits, such as carcass yield, gut microflora, and also blood glucose and cholesterol levels [23]. Cinnamaldehyde also has a positive effect on interleukin (IL-10) and transforming growth factor-beta (TGF-β) gene expression in intraepithelial lymphocytes [8]. Numerous studies have shown that the addition of curcumin to poultry feed positively influences the expression of genes important for lipid and glycogen metabolism. For example, Xie et al. (2019) [24] showed that the addition of curcumin to a broiler diet significantly reduced the expression of genes for acetyl-CoA carboxylase (ACC), apoptosis antigen 1 (FAS receptor) and transcription factor SREBP-1c, all of which are involved in lipogenesis and fatty acid synthesis in poultry, playing an important role in reducing abdominal fat deposition. Hafez et al. (2022) [25] investigated the effect of curcumin on the expression of genes related to poultry growth. The authors showed that the addition of curcumin had a positive effect on poultry growth by increasing the expression of insulin-like growth factor 1 (IGF-1) and growth hormone receptor (GHR) and decreasing the expression of leptin (LEP) and myostatin (MSTN). McKnight et al. (2019) [26] analyzed the effect of the addition of fatty acids, organic acids and phytochemicals on the morphology and expression of inflammatory genes in broilers. The results showed microstructural changes in the duodenum and jejunum of broilers that consumed feed with added bacitracin methylene disalicylate (BMD) and fatty acids as well as higher expression of the cytokines interferon Ɣ (IFNƔ), interleukin 6 (IL-6) and inducible nitric oxide synthase (iNOS). Previous studies confirmed that IFNƔ has an inhibitory effect on parasitic diseases and is considered a key component of the immune response to parasitic infections in broilers [27,28].
Neem leaf extract (Azadirachta indica) is another phytochemical with a proven positive effect on broiler meat quality and also on the gene expression of antioxidant enzymes in the breast muscle. It should be, however, emphasized that fresh neem leaves can negatively affect broiler growth due to their high crude fiber content [29], because of which it is necessary to add them in the form of a dry leaf extract. The results of Nakamura et al. (2022) [30] showed that the addition of 2.0% neem dry extract to broiler feed increased the expression of genes important for the activity of antioxidant enzymes in the breast muscle (Cu/Zn superoxide dismutase—SOD1, Mn superoxidase—MnSOD, glutathione peroxidase 7—GPX 7 and catalase—CAT). Furthermore, the authors showed that the addition of the neem to broiler feed reduces lipid peroxidation and water losses in m. pectoralis major muscle.
In addition to micronutrients, the addition of plant by-products to feed can also influence gene expression in poultry. One such by-product is the water produced during the extraction of olive oil. Sabino et al. (2018) [31] showed a positive effect of adding this by-product to broiler feed on their small intestinal function and health in general. The authors reported differential expression of 280 genes related to lipid metabolism and the regulation of virus replication in the epithelial cells of the jejunum of broilers fed experimental diets.
3. Vitamins and Minerals
Vitamin A (retinol) has an important function in both avian embryonic development and adult biology [32]. It is a fat-soluble vitamin that can be stored efficiently in liver and egg yolk. However, its amounts should be carefully managed, as excessive amounts of this vitamin remaining in the bird’s body can lead to vitamin D deficiency [33], reduce the deposition of α-tocopherol in the yolk [34] or even be toxic to the brain and liver [35]. Vitamin A can be easily destroyed during feed processing, so it needs to be supplemented. The study by Yuan et al. (2014) [35] showed that supplementation of vitamin A at a dose of up to 35,000 IU/kg did not affect the reproductive performance of the birds and increased the vitamin A concentration in liver and yolk, but decreased the α-, γ- and total tocopherol concentration in yolk and the α-tocopherol in liver. In contrast, at a dose of 45,000 IU/kg and above, egg weight, yolk color, eggshell thickness and firmness, and reproductive performance decreased significantly. The authors reported that the addition of vitamin A to the diet increased mRNA expression of the vitamin D receptor in the duodenal mucosa, increased aspartate aminotransferase activity and decreased serum total bilirubin concentration.
Vitamin D3 plays an important role in maintaining phosphorus homeostasis, enhancing intestinal absorption of phosphorus and stimulating osteoblast activation and proliferation. Vitamin D requirements depend on dietary Ca and P concentrations and are estimated to be higher than recommended levels in the first two weeks of a broiler’s life [36]. The study by Shao et al. (2019) [37] showed that vitamin D3 supplementation in broiler diets promoted intestinal P absorption and bone P utilization, and concluded that this effect may be related to increased PiT-1 levels in the duodenum and PiT-1 and NaP-IIb levels in the jejunum, respectively. In addition, vitamin D supplementation was shown to have an immunomodulatory effect in chickens. Supplementation of Ca- and P-deficient diet with vitamin D increased transcription of TLR2b, TLR4, CATH1 and CATHB1 and predominantly Th2 cytokines in the spleen, while supplementation of the control diet with vitamin D downregulated TLR4 transcription and increased CATH1, CATHB1, Th1 and Th2 cytokine transcription in a dose-dependent manner [36].
Apart from being the most important vitamin related to blood coagulation, vitamin K, together with vitamin D, plays a crucial role in bone turnover and strength and also has an anti-inflammatory effect in the body [38]. The addition of vitamin K3 and probiotics has been shown to promote growth performance of broiler chickens in grower phase by synergistically improving the physical and chemical properties of the tibia through modulation of calcium and phosphorus metabolism and expression of osteogenic genes (runt-related transcription factor 2 OCN, and alkaline phosphatase) [39].
Calcium is considered one of the most important minerals in animal diet due to its crucial role in bone development and growth. Although it is generally always added to feed in its inorganic form, it is necessary to maintain calcium’s balance in the body as its deficiency can lead to bone injury and poor growth [40], and its presence in excessive amount can also reduce body weight gain and feed intake of broilers [41]. The response of the animal body through growth and bone quality to dietary calcium depends on calcium absorption in the small intestine. Han et al. (2022) [42] found that low dietary Ca stimulates transcription of the VDR (nuclear vitamin D receptor (nVDR) and membrane vitamin D receptor (mVDR)) and the combination of vitamin D with VDR to regulate Ca absorption in the small intestine, while high Ca inhibits this effect and prevents excessive absorption in the small intestine of broiler chickens.
Vitamin C is a water-soluble antioxidant compound whose main role is to protect cells from oxidative damage and to strengthen the immune system [43]. It is not a part of any metabolic pathway but acts as an essential cofactor in many enzymatic reactions, such as the synthesis of collagen, carnitine and catecholamine and the metabolism of microsomes or the synthesis and catabolism of tyrosine [44]. Due to its positive effect on maintaining the integrity of the cellular defense system during stress, vitamin C administered together with vitamin E may adverse the performance deterioration of chickens that occurs during heat stress [45]. Furthermore, a study by Shakeri et al. (2020) [44] showed that in broiler chickens exposed to summer heat stress, the addition of 200 mg/kg vitamin C and 100 mg/kg vitamin E to the basal diet significantly decreased the expression of interleukin IL-1β, IL-6, interferon (IFN)-γ, toll-like receptor (TLR)-4 and HSP70 in the liver, all of which are associated with response to stress. Similarly, Abdel-Moneim et al. (2021) [46] found that the addition of 200 mg/kg vitamin C alone provided protection for broiler chickens against the risk of high density through improved total feed intake, reduced mortality and down-regulation of HSP70 expression levels in the liver.
Vitamin E and selenium are bioactive substances with a positive influence on chicken health, production traits and the quality of their products [47,48,49]. Selenium is an essential element found in the composition of glutathione peroxidase (GPx), an enzyme important in lipid peroxidation processes, while vitamin E plays an important role in protecting cells from free radicals [48]. The recommended levels of selenium and vitamin E in broiler feed are 0.15 mg/kg and 10 IU vitamin E/kg, respectively [50]. Khalifa et al. (2021) [51] investigated the synergistic effect of selenium and vitamin E addition on the growth and expression of genes related to growth in broilers. The authors showed that selenium and vitamin E influence broiler growth by regulating IGF-1 and GHR genes. According to Kirella et al. (2021) [52], the expression of genes related to poultry growth can be increased by adding by-products of agricultural production with high concentrations of vitamin E to feed. On the other hand, the addition of vitamin E isomers, α-tocopherol and γ-tocopherol, affect the expression of genes related to lipid metabolism and genes responsible for inflammatory processes and the immune response [53]. Shehata et al. (2022) [54] reported that the addition of vitamin E in feed reduced the negative effects of poor housing conditions (e.g., high stocking density) on growth traits and stress in broiler chickens. The results of their study showed increased expression of the genes GHR and IGF-1 and a significant decrease in triiodothyronine (T3) and thyroxine (T4), circulating thyroid hormones known to be susceptible to stressful conditions [55]. According to Khalifa et al. (2021) [51], the antioxidant effects of vitamin E and selenium were also reflected in the good health of broiler chickens. The findings of Khalifa et al. (2021) [51] were confirmed by Elgendey et al. (2022) [56], who also reported an increase in CAT and SOD expression. CAT and SOD encode the levels of the antioxidant enzyme GPx. In addition, Amevor et al. (2021) [57] reported a synergistic effect of vitamin E and quercetin, due to which higher expressions of interferon γ (INF-γ) and interleukin 2 (IL-2), which are important for the immune system, were found in the liver of broilers who were fed these compounds.
The addition of selenium and vitamin E to chicken feed affects also the expression of genes involved in the transport of nutrients in the gut [58], genes expressed in the fallopian tube tissue [59], genes related to the occurrence of oxidative stress, and those related to inflammation-related disorders [56]. The combination of n-3 PUFA fatty acids and vitamin E increases the expression of genes related to peptide transport (SLC15A1, and GALNT2), oxidative stress and intestinal hypoxia, as well as some genes related to the occurrence of stress (mucin 2-MUC2, interferon gamma-IFNG, member 7 of the heat stress protein group-HSPB7 [58]. Furthermore, adding selenium and vitamin E to chicken feed affects the expression of genes involved in the transport of nutrients in the intestine [58], genes expressed in oviductal tissue [59], genes related to the occurrence of oxidative stress and genes related to inflammation-related disorders [56]. Because of their rapid growth and ability to store a large amount of abdominal fat, broiler chickens are a good model for studying carcass lipid and abdominal obesity [60]. In this context, research by Zhang et al. (2021) [9] has shown that vitamin E supplementation in broiler feed reduces the expression of genes leading to de novo synthesis of fatty acids (FASN, ACACA), thereby reducing the accumulation of abdominal adipose tissue.
4. Flavonoids and Carotenoids
Poultry, especially those used for the production of meat (broilers) and eggs (hens), due to extremely strong selection pressure on high productivity, are very sensitive to oxidative stress—a condition in which more reactive oxygen compounds (ROS) and reactive nitrogen compounds (RNS) are present than can be broken down by the animal’s organism. Furthermore, commercial production of broilers and laying hens is associated with numerous environmental stressors (vaccination, density in the facility, long photoperiods, dust, and ammonia) that predispose them to oxidative stress [61]. Previous research has shown that oxidative stress has a tremendous impact on poultry health and performance [62]. For this reason, flavonoids, in the form of leaves or fruits of various plants, are being increasingly added to animal feed in modern poultry production. Flavonoids are polyphenolic secondary metabolites of plants found in a variety of foods, including berries, grapes, onions and legumes [63,64]. They are synthesized from the amino acids phenylalanine and malonate. To date, more than 6000 different flavonoid compounds with influence on animal growth, reproduction and the immune system are recognized [65].
Ouyang et al. (2016) [66] investigated the effect of flavonoids from alfalfa (Medicago sativa) on the expression of genes related to lipid metabolism in the liver and adipose tissue of broiler chickens. The addition of alfalfa flavonoids to diets at 5, 10 and 15 mg kg−1 lowered the expression of the fatty acid synthase gene (FAS), which catalyzed the final step of the fatty acid synthesis, and increased the expression of lipoprotein lipase (LPL), peroxisome proliferator-activated receptor γ (PPARγ) and the adipose triglyceride lipase (ATGL). This suggests that supplementation of flavonoids from alfalfa have better antioxidant activity by regulating the activity of LPL, PPARy and ATGL genes compared to broiler chicken fed with basal diet.
The reproductive capacity of a laying hen decreases with age, and this is related to the weakening of the liver–blood–ovary system function together with reduced estrogen production. Research by Dai et al. (2021) [67] has shown that the addition of flavonoids from hawthorn to the diet of laying hens increases estrogen levels and slows ovarian apoptosis, by increasing the expression of the proliferating cell nuclear antigen (PCNA) gene. Studies also found increased expression of the Nrf2 gene in the ovaries, as well as the apolipoprotein genes ApoB and ApoV1. The Nrf2 gene is responsible for antioxidant activity and protection against oxidative stress in the ovaries of laying hens, while ApoB and ApoV1 are important protein components of low-density lipoproteins involved in the maintenance of lipid homeostasis in the liver. Similarly, Shi et al. (2022) [68] reported that flavonoids from wormwood increased the expression of the Nrf2 gene, which then translocated to the nucleus and bound to antioxidant elements. The authors concluded that flavonoids from wormwood could stimulate the expression of antioxidant enzymes and increase the activity of the antioxidant enzymes CAT, SOD and GPx, thus protecting the organism from oxidative stress. In addition to protecting the body from oxidative stress, flavonoids have also been shown to have an effect against osteoporosis. For example, Huang et al. (2020) [69] reported that the addition of total flavonoids from Drynariae rhizomes to the feed of laying hens influenced bone health by regulating osteoclast activity. The authors showed that the addition of 0.5 or 2.0 g/kg total flavonoids to the diets significantly increased the expression of the RUN transcription factor 2 (RUNX2) and osteoprotegerin (OPG) genes (responsible for osteoblast differentiation and bone mineral density), and decreased the expression of the receptor activator of nuclear factor kappa-Β ligand (RANKL) gene, associated with osteoclast activity.
Carotenoids are among the most widely distributed fat-soluble pigments found in various plants, microalgae, bacteria and fungi. Depending on their function, they are divided into two groups: carotenes (including lycopene, α-carotene and β-carotene) and xanthophylls (such as lutein and zeaxanthin) [70]. The animal and bird body cannot synthesize them by themselves, so they must be ingested. The role of carotenoids in improving the quality of meat and eggs in poultry production has been known for a long time [71,72], however, these pigments have recently received a lot of attention due to their bioactive properties and beneficial effects on animal health [73,74]. It has been found that carotenoids reduce oxidative stress in the host body through multiple cellular mechanisms (such as scavenging free radicals and upregulating the production of antioxidant enzymes) and thus have anti-cancer, immunomodulatory, anti-inflammatory, antibacterial, neuroprotective and anti-diabetic functions [75]. Csernus et al. (2020) [10] and Gao et al. (2012) [76] investigated the effect of carotenoid supplementation on the expression of the anti-inflammatory genes interleukin 1β (IL-1β), interleukin 6 (IL-6), interferon-α (IFN-α) and interferon-γ (IFN-γ) in poultry. Their results showed that carotenoids reduced the gene expression of inflammatory factors in stressed animals. Increased expression of anti-inflammatory cytokine genes leads to pathological states of the body’s immune system, and therefore it is important to maintain a balance between inflammatory and anti-inflammatory factors. The results of Gao et al. (2012) [76] showed that the addition of xanthophyll can reduce the expression of anti-inflammatory cytokines in the liver, duodenum and jejunum of chicken, thus helping to maintain the balance between inflammatory and anti-inflammatory factors.
5. Amino Acids
The addition of amino acids to feed has several positive effects on chicken production traits and overall health: amino acids improve feed conversion, influence growth, have a positive effect on the immune system, reduce the effects of thermal stress to which chicken are exposed and have a positive effect on the development of muscle tissue [77]. At the cellular level, their function is extremely important as they are substrates for protein synthesis and are involved in the control of gene expression through their ability to modulate the initiation phase of mRNA translation in poultry [78]. Amino acid signaling occurs through two pathways: the mechanistic target of rapamycin complex 1 (mTORC1) and the amino acid response pathway (AAR) [79]. Oral intake of nutrients, such as amino acids, induces transcription, RNA stability and processing, protein synthesis and modification. These processes influence DNA replication and the regulation of gene expression in mammalian and avian cells. Therefore, the addition or absence of exogenous amino acids can effectively regulate gene expression in mammals and birds [79].
Among all amino acids, lysine is considered to be essential and for limiting the growth of the animal. This amino acid is important for the synthesis of proteins, cytokines, gene expression and the response of the immune system to infections [79]. Analysis of mRNA expression from broiler chickens fed with low and high levels of lysine identified increased expression of 67 genes and reduced expression of 143 genes related to cell growth, respectively. Methionine is another amino acid whose deficiency affects mRNA expression and thus growth and immune function in poultry [80,81]. For example, it has been observed that broilers that grow more slowly consume larger amounts of feed containing non-essential amino acids (alanine, asparagine, and aspartic acid), while broilers that grow quickly consume more feed with the addition of essential amino acids (methionine, lysine, and threonine) [82].
In vertebrates, histidine-bound dipeptides are found in relevant concentrations in skeletal muscle, cardiac muscle and some parts of the brain. These dipeptides can act as intracellular buffers, metal ion chelators or antioxidants. In chickens, the dominant dipeptide related to histidine is called carnosine and it consists of histidine, the non-essential amino acid β-alanine and the methylated form of anserine [83]. The secondary precursor of carnosine, β-alanine, is formed as an intermediate in the metabolic pathways of aspartate and uracil [83]. Histidine deficiency leads to a decrease in body weight and an imbalance of body nitrogen [84], while reduced carnosine concentration has been found in birds suffering from pectoral myopathy [85]. Therefore, it is hypothesized that an increased intake of histidine may help to increase carnosine concentrations and thus prevent the development of pectoral myopathy [83]. However, it is important to note that the highest carnosine levels are synthesized by the addition of β-alanine and L-histidine combination, and that increased carnosine content does not negatively affect the quality of the meat, but rather improves the texture of the meat and alters the secondary protein structures [86]. In addition, increasing carnosine content in meat improves the oxidative stability of meat [87], reduces drip loss, cooking loss and tenderness of the meat [88], and also increases the initial and final pH values of M. pectoralis in chicken [89]. On the cellular level, supplementation with β-alanine resulted in multiple increases in carnosine synthase (CARNS1) and taurine transporter (SLC6A6; [89]). The same group of authors found that individual addition of β-alanine and L-histidine to broiler meal increased the expression of the histidine carboxylase, carnosine synthase, peptide transporter 1 (PEPT1) and inorganic phosphate 1 (PHT1) transporter genes, while the combination of L-histidine and β-alanine only increased the expression of the PHT1 transporter gene. The PEPT1 gene is a member of the POT family of membrane transporters that uses the proton electrochemical gradient to drive the uptake of di- and tripeptides across cell membranes [90], and is therefore capable of transporting both L-histidine and carnosine in skeletal muscle [14].
Kubota et al. (2021) [91] analyzed the RNA profile of Korat chicken supplemented with β-alanine and L-histidine, which resulted in a very tender breast muscle. The authors noted increased expression of genes involved in the regulation of myosin, intramuscular fat and calpain (LOC107051274, ACSBG1, and CAPNS2) and decreased expression of myosin VIIB (MYO7B), myosin binding protein H (MYBPH), inhibitor of serpin peptidase H (SERPINH1) and phosphoglycerate mutase 1 (PGAM1) genes. Interestingly, no carnosine synthase was detected. Functional enrichment analysis identified signaling pathways affected by dietary supplements, including the insulin signaling pathway (β-alanine supplementation) and insulin resistance, and adipocytokine signaling pathways (L-histidine supplementation).
L-carnitine is an amino acid with an irreplaceable role in intermediate metabolism. Its role in poultry production is multifunctional: it influences growth, strengthens the immune system, improves semen quality and has an antioxidant effect. Its concentration varies considerably depending on the species, tissue type and nutritional status of the animal [92]. The addition of L-carnitine to broiler water at a dose of 50 mg/kg/day during 35 days showed significant upregulation of amino acid cation transporter (CAT2), myoblast determination protein 1 (MYOD) and myogenic factor 5 (MYF5), the genes that play an important role in muscle growth and proliferation in animals and birds [93]. The results showed a significant increase in live weight and a decrease in feed intake in broilers that were supplemented with L-carnitine compared to the control group. Similarly, the introduction of a combination of L-carnitine and a methionine derivate, methyl methioninenine sulfonium chloride (MMSK), into broiler feed showed that the addition of MMSK alone or in combination with L-carnitine reduced the expression level of the MSTN gene and increased the expression of the IGF-1 gene [94]. Higher growth rates and live weight were observed in broilers with increased expression of the IGF-1 gene and reduced expression of the MSTN gene. The obtained results were expected, considering that the IGF-1 gene promotes growth and development of the organism, while the MSTN gene is associated with muscle growth, i.e., inhibition of its expression leads to an increase in muscle mass.
6. Conclusions
The addition of various bioactive compounds to the feed improves the production traits, health and metabolism of chickens by altering the expression profile of genes involved in various metabolic pathways. The addition of phytonutrients and flavonoids positively influences the expression of genes important for lipid and glycogen metabolism, together with genes related to poultry growth and oxidative mechanisms, while modification of chicken feed with amino acids, vitamins or minerals influences signaling pathways responsible for the balance of inflammatory responses, improves production traits and early embryonic development.
Future research in the field of nutrigenomics will lead to the combined use of different technologies, the results of which will contribute to a better understanding of the interaction between nutrition and biological processes in the body and improve the possibility of combating disease through the effect of nutrients on the genome of farm animals. In addition, it will be possible to better control the intake of vitamins and minerals, preventing their excessive absorption and enabling the production of functional poultry products.
Author Contributions
Conceptualization, I.D.K.; methodology, Z.K.; writing—original draft preparation, K.G. and I.D.K.; writing—review and editing, I.D.K. and M.K., visualization, I.D.K.; supervision, G.K.; project administration, Ž.R. and M.K.; funding acquisition, G.K. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Structural and Investment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care (grant #KK.01.1.1.01.0010) and by the Ministry of Science and Education of the Republic of Croatia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Mija Škrobot, student of the Faculty of Agrobiotechnical Sciences Osijek, for illustrating the figure “Schematic representation of the influence of feed on the phenotype of animals”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roberfroid, M.B. Defining functional foods. JFF 2000, 9, 9–27. [Google Scholar]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science—Implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R. Nutrigenomics, individualism and public health. Proc. Nutr. Soc. 2004, 63, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sales, N.M.R.; Pelegrini, P.B.; Goersch, M. Nutrigenomics: Definitions and advances of this new science. J. Nutr. Metab. 2014, 2014, 202759. [Google Scholar] [CrossRef] [PubMed]
- ul Haq, Z.; Saleem, A.; Khan, A.A.; Dar, M.A.; Ganaie, A.M.; Beigh, Y.A.; Ahmad, S.M. Nutrigenomics in livestock sector and its human-animal interface-a review. Vet. Anim. Sci. 2022, 17, 100262. [Google Scholar] [CrossRef]
- Benítez, R.; Núñez, Y.; Óvilo, C.; Ovilo, C. Nutrigenomics in farm animals. Int. J. Genom. 2017, 4, 1. [Google Scholar]
- Gayon, J. From Mendel to epigenetics: History of genetics. Comptes Rendus Biol. 2016, 339, 225–230. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019, 250, 41–50. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhu, Y.; Wu, Q.; Li, Y.; Huang, D.; Sun, Y. Effect of vitamin E supplementation on deposition and gene expression profiling of abdominal fat in broiler chickens. Poult. Sci. J. 2021, 58, 40–50. [Google Scholar] [CrossRef]
- Csernus, B.; Biró, S.; Babinszky, L.; Komlósi, I.; Jávor, A.; Stündl, L.; Czeglédi, L. Effect of carotenoids, oligosaccharides and anthocyanins on growth performance, immunological parameters and intestinal morphology in broiler chickens challenged with Escherichia coli lipopolysaccharide. Animals 2020, 10, 347. [Google Scholar] [CrossRef]
- Sharideh, H.; Zaghari, M. Effect of dietary L-tryptophan supplementation and light-emitting diodes on growth and immune response of broilers. Vet. Res. Forum 2021, 12, 63. [Google Scholar] [PubMed]
- Krysiak, K.; Konkol, D.; Korczyński, M. Overview of the use of probiotics in poultry production. Animals 2021, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Laptev, G.Y.; Yildirim, E.A.; Ilina, L.A.; Filippova, V.A.; Kochish, I.I.; Gorfunkel, E.P.; Romanov, M.N. Effects of essential oils-based supplement and salmonella infection on gene expression, blood parameters, cecal microbiome, and egg production in laying hens. Animals 2021, 11, 360. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J.; Hu, M.; Ma, Y.; Wu, S.; Qi, G.; Zhang, H. Influences of beta-alanine and l-histidine supplementation on growth performance, meat quality, carnosine content, and mRNA expression of carnosine-related enzymes in broilers. Animals 2021, 11, 2265. [Google Scholar] [CrossRef]
- STATISTA Chicken Meat Production Worldwide from 2012 to 2022 (in 1000 Metric Tons). Available online: https://www.statista.com/statistics/237637/production-of-poultry-meat-worldwide-since-1990/ (accessed on 1 February 2023).
- Makkar, H.P. Animal nutrition in a 360-degree view and a framework for future R&D work: Towards sustainable livestock production. Anim. Prod. Sci. 2016, 56, 1561–1568. [Google Scholar]
- Adedokun, S.A.; Olojede, O.C. Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar]
- Nowacka-Woszuk, J. Nutrigenomics in livestock—Recent advances. J. Appl. Genet. 2020, 61, 93–103. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Bravo, D. High-throughput gene expression analysis of intestinal intraepithelial lymphocytes after oral feeding of carvacrol, cinnamaldehyde, or Capsicum oleoresin. Poult. Sci. 2010, 89, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Ipcak, H.H.; Alcicek, A. Addition of Capsicum Oleoresin, Carvacrol, Cinnamaldehyde and Their Mixtures to the Broiler Mixed Feed I. Effects On Growth Performance, Carcass Characteristics, Intestinal Microflora, Some Blood Parameters and IGF-1 Gene Expression Levels. Poult. Sci. 2019, 89, 68–81. [Google Scholar]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019, 98, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; El-Sayed, Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef]
- McKnight, L.L.; Peppler, W.; Wright, D.C.; Page, G.; Han, Y. A blend of fatty acids, organic acids, and phytochemicals induced changes in intestinal morphology and inflammatory gene expression in coccidiosis-vaccinated broiler chickens. Poult. Sci. 2019, 98, 4901–4908. [Google Scholar] [CrossRef]
- Ciraci, C. Molecular Genetic Assessment of Chicken Macrophage Innate Immunity: Toll-like Receptors, Mechanisms of Action, and Kinetic Transcriptome Profile; Iowa State University: Ames, IA, USA, 2010. [Google Scholar]
- Calenge, F.; Coville, J.L.J.L.; Gourichon, D.; Trapp, S.; Bed’Hom, B.B.; Quéré, P.P. Quantification of early innate immune responses in chicken blood using microfluidic expression arrays. In IAD 2016-10. Journées du Réseau Français" Immunologie des Animaux Domestiques"; IRD Editions: Ploufragan, France, 2016; p. 51. [Google Scholar]
- Shaahu, D.T.; Uza, O.; Gege, B.M.; Awua, L.L. Comparative efficiency of antibiotics and aqueous fresh neem leaf extract on growth performance, apparent nutrient digestibility, carcass characteristics and economics of production of broiler chickens. Niger. J. Anim. Sci. Technol. 2020, 3, 1–9. [Google Scholar]
- Nakamura, K.; Shishido, M.; Shimamoto, S.; Ogawa, G.; Khandelwal, N.; Tatsugawa, K.; Ijiri, D. Effects of Supplementation with Dried Neem Leaf Extract on Lipid Peroxidation and Antioxidant Enzyme mRNA Expression in the Pectoralis Major Muscle of Broiler Chickens. Poult. Sci. J. 2022, 59, 75–80. [Google Scholar] [CrossRef]
- Sabino, M.; Cappelli, K.; Capomaccio, S.; Pascucci, L.; Biasato, I.; Verini-Supplizi, A.; Trabalza-Marinucci, M. Dietary supplementation with olive mill wastewaters induces modifications on chicken jejunum epithelial cell transcriptome and modulates jejunum morphology. BMC Genom. 2018, 19, 576. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef]
- Caire-Juvera, G.; Ritenbaugh, C.; Wactawski-Wende, J.; Snetselaar, L.G.; Chen, Z. Vitamin A and retinol intakes and the risk of fractures among participants of the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2009, 89, 323–330. [Google Scholar] [CrossRef]
- Jiang, Y.H.; McGeachin, R.B.; Bailey, C.A. α-Tocopherol, β-carotene, and retinol enrichment of chicken eggs. Poult. Sci. 1994, 73, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Roshdy, A.R.; Guo, Y.; Wang, Y.; Guo, S. Effect of Dietary Vitamin A on Reproductive Performance and Immune Response of Broiler Breeders. PLoS ONE 2014, 9, e105677. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lecompte, J.C.; Yitbarek, A.; Cuperus, T.; Echeverry, H.; Van Dijk, A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult. Sci. 2016, 95, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wen, Q.; Zhang, S.; Lu, L.; Zhang, L.; Liao, X.; Luo, X. Dietary supplemental vitamin D3 enhances phosphorus absorption and utilisation by regulating gene expression of related phosphate transporters in the small intestine of broilers. BJN 2019, 121, 9–21. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.M.; Ball, M.E.; McDonald, E.; Hull, G.L.; Danaher, M.; Cashman, K.D. Biofortification of Chicken Eggs with Vitamin K—Nutritional and Quality Improvements. Foods 2020, 9, 1619. [Google Scholar] [CrossRef]
- Guo, S.; Xv, J.; Li, Y.; Bi, Y.; Hou, Y.; Ding, B. Interactive effects of dietary vitamin K3 and Bacillus subtilis PB6 on the growth performance and tibia quality of broiler chickens with sex separate rearing. Animal 2020, 14, 1610–1618. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.; Chen, G.; Qu, H.; Zhang, J.; Shi, C.; Cheng, Y. Effects of calcium to non-phytate phosphorus ratio and different sources of vitamin D on growth performance and bone mineralization in broiler chickens. Rev. Bras. Zootec. 2016, 45, 1–7. [Google Scholar] [CrossRef]
- Tancharoenrat, P.; Ravindran, V. Influence of tallow and calcium concentrations on the performance and energy and nutrient utilization in broiler starters. Poul. Sci. 2014, 93, 1453–1462. [Google Scholar] [CrossRef]
- Han, J.C.; Wang, X.N.; Wu, L.H.; Lv, X.L.; He, L.; Qu, H.X.; Wang, Z.X. Dietary calcium levels regulate calcium transporter gene expression levels in the small intestine of broiler chickens. Br. Poult. Sci. 2022, 63, 202–210. [Google Scholar] [CrossRef]
- Abidin, Z.; Khatoon, A. Heat stress in poultry and the beneficial effects of ascorbic acid (vitamin C) supplementation during periods of heat stress. World’s Poult. Sci. J. 2013, 69, 135–152. [Google Scholar] [CrossRef]
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.K.; Ramarao, S.V.; Raju, M.V.; Chatterjee, R.N. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions. Br. Poult. Sci. 2008, 49, 592–599. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Ebeid, T.A. Nutritional manipulation to combat heat stress in poultry–A comprehensive review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, J.; Li, C. Effect of different selenium sources on production performance and biochemical parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2014, 98, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.L.; Sobolewska, S.; Rose, S.P.; Whiting, I.M.; Blanchard, A.; Ionescu, C.; Pirgozliev, V. Effect of feeding different sources of selenium on growth performance and antioxidant status of broilers. Br. Poult. Sci. 2020, 61, 274–280. [Google Scholar] [CrossRef]
- Gul, F.; Ahmad, B.; Afzal, S.; Ullah, A.; Khan, S.; Aman, K.; Ahmad, L. Comparative analysis of various sources of selenium on the growth performance and antioxidant status in broilers under heat stress. Braz. J. Biol. 2021, 83, e251004. [Google Scholar] [CrossRef]
- National Research Council, National Academies Press. NRC (National Research Council) Non-Native Oystersin the Chesapeake Bay; National Research Council, National Academies Press: Washington, DC, USA, 2004.
- Khalifa, O.A.; Al Wakeel, R.A.; Hemeda, S.A.; Abdel-Daim, M.M.; Albadrani, G.M.; El Askary, A.; Elgendey, F. The impact of vitamin E and/or selenium dietary supplementation on growth parameters and expression levels of the growth-related genes in broilers. BMC Vet. Res. 2021, 17, 251. [Google Scholar] [CrossRef]
- Kirrella, A.A.; Abdo, S.E.; El-Naggar, K.; Soliman, M.M.; Aboelenin, S.M.; Dawood, M.A.; Saleh, A.A. Use of corn silk meal in broiler diet: Effect on growth performance, blood biochemistry, immunological responses, and growth-related gene expression. Animals 2021, 11, 1170. [Google Scholar] [CrossRef]
- Korošec, T.; Tomažin, U.; Horvat, S.; Salobir, J. The diverse effects of α-and γ-tocopherol on chicken liver transcriptome. Poult. Sci. 2017, 96, 667–680. [Google Scholar] [CrossRef]
- Shehata, S.F.; Baloza, S.H.; Elsokary, M.M.; Hashem, N.M.; Khawanda, M.M. Effect of stocking density and vitamin E or zinc supplementation on growth, physiology, gene expression, and economic efficiency of growing broiler chicks. Trop. Anim. Health Prod. 2022, 54, 403. [Google Scholar] [CrossRef]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef]
- Elgendey, F.; Al Wakeel, R.A.; Hemeda, S.A.; Elshwash, A.M.; Fadl, S.E.; Abdelazim, A.M.; Khalifa, O.A. Selenium and/or vitamin E upregulate the antioxidant gene expression and parameters in broilers. BMC Vet. Res. 2022, 18, 310. [Google Scholar] [CrossRef]
- Amevor, F.K.; Cui, Z.; Ning, Z.; Du, X.; Jin, N.; Shu, G.; Zhao, X. Synergistic effects of quercetin and vitamin E on egg production, egg quality, and immunity in aging breeder hens. Poult. Sci. 2021, 100, 101481. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Clark, D.L.; Jacobi, S.K.; Velleman, S.G. Supplementation of vitamin E and omega-3 fatty acids during the early posthatch period on intestinal morphology and gene expression differentiation in broilers. Poult. Sci. 2021, 100, 100954. [Google Scholar] [CrossRef] [PubMed]
- Naji, T.A.; Amadou, I.; Zhao, R.Y.; Tang, X.; Shi, Y.H.; Le, G.W. Effects of phytosterol in feed on growth and related gene expression in muscles of broiler chickens. Trop. J. Pharm. Res. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends Food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Rafiei, F.; Khajali, F. Flavonoid antioxidants in chicken meat production: Potential application and future trends. Worlds Poult. Sci. J. 2021, 77, 347–361. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef]
- López JG, E. Flavonoids in health and disease. Curr. Med. Chem. 2019, 26, 6972–6975. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, I.H.; Bhat, S.A.; Ahmad, S. Flavonoids: Health benefits and their potential use in food systems. J. Funct. Foods. 2020, 26, 235–256. [Google Scholar]
- Ouyang, K.; Xu, M.; Jiang, Y.; Wang, W. Effects of alfalfa flavonoids on broiler performance, meat quality, and gene expression. Can. J. Anim. Sci. 2016, 96, 332–341. [Google Scholar] [CrossRef]
- Dai, H.; Lv, Z.; Huang, Z.; Ye, N.; Li, S.; Jiang, J.; Shi, F. Dietary hawthorn-leaves flavonoids improves ovarian function and liver lipid metabolism in aged breeder hens. Poult. Sci. 2021, 100, 101499. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jin, X.; Xu, Y.; Xing, Y.; Yan, S.; Guo, Y.; Shi, B. Effects of Total Flavonoids of Artemisia ordosica on Growth Performance, Oxidative Stress, and Antioxidant Status of Lipopolysaccharide-Challenged Broilers. Antioxidants 2022, 11, 1985. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, X.F.; Yu, Z.W.; Hu, Y.P.; Zhang, L.; Liu, Y.; Zhou, Z.X. Dietary supplementation of total flavonoids from Rhizoma Drynariae improves bone health in older caged laying hens. Poult. Sci. 2020, 99, 5047–5054. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. Poult. Sci. J. 2001, 38, 1–27. [Google Scholar] [CrossRef]
- Rajput, N.; Ali, S.; Naeem, M.; Khan, M.A.; Wang, T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on pigmentation, oxidative stability and quality of meat from broiler chickens affected by a coccidiosis challenge. Br. Poult. Sci. 2014, 55, 501–509. [Google Scholar] [CrossRef]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. Worlds Poult. Sci. J. 2018, 74, 89–100. [Google Scholar] [CrossRef]
- Changxing, L.; Chenling, M.; Alagawany, M.; Jianhua, L.; Dongfang, D.; Gaichao, W.; Chao, S. Health benefits and potential applications of anthocyanins in poultry feed industry. Worlds Poult. Sci. J. 2018, 74, 251–264. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Liu, J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Xie, Q.M.; Jin, L.; Sun, B.L.; Ji, J.; Chen, F.; Bi, Y.Z. Supplementation of xanthophylls decreased proinflammatory and increased anti-inflammatory cytokines in hens and chicks. Br. J. Nutr. 2012, 108, 1746–1755. [Google Scholar] [CrossRef]
- Baxter, M.F.; Greene, E.S.; Kidd, M.T.; Tellez-Isaias, G.; Orlowski, S.; Dridi, S. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. J. Anim. Sci. 2020, 98, skaa049. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Amino acids as regulators of gene expression. Nutr. Metab. 2004, 1, 3. [Google Scholar] [CrossRef]
- Khwatenge, C.N.; Kimathi, B.M.; Nahashon, S.N. Transcriptome Analysis and Expression of Selected Cationic Amino Acid Transporters in the Liver of Broiler Chicken Fed Diets with Varying Concentrations of Lysine. Int. J. Mol. Sci. 2020, 21, 5594. [Google Scholar] [CrossRef]
- Lai, A.; Dong, G.; Song, D.; Yang, T.; Zhang, X. Responses to dietary levels of methionine in broilers medicated or vaccinated against coccidia under Eimeria tenella-challenged condition. BMC Vet. Res. 2018, 14, 140. [Google Scholar] [CrossRef]
- Fagundes, N.S.; Milfort, M.C.; Williams, S.M.; Da Costa, M.J.; Fuller, A.L.; Menten, J.F.; Aggrey, S.E. Dietary methionine level alters growth, digestibility, and gene expression of amino acid transporters in meat-type chickens. Poult. Sci. 2020, 99, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Niknafs, S.; Fortes, M.R.; Cho, S.; Black, J.L.; Roura, E. Alanine-specific appetite in slow growing chickens is associated with impaired glucose transport and TCA cycle. BMC Genom. 2022, 23, 393. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.; Hess, V.; Marx, A.; Hosseini-Ghaffari, M.; Sauerwein, K. Effects of dietary supplementation with histidine and β-alanine on blood plasma metabolome of broiler chickens at different ages. PLoS ONE 2022, 17, e0277476. [Google Scholar] [CrossRef] [PubMed]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Soglia, F.; Silva, A.K.; Lião, L.M.; Laghi, L.; Petracci, M. Effect of broiler breast abnormality and freezing on meat quality and metabolites assessed by 1 H-NMR spectroscopy. Poult. Sci. 2019, 98, 7139–7150. [Google Scholar] [CrossRef] [PubMed]
- Suwanvichanee, C.; Sinpru, P.; Promkhun, K.; Kubota, S.; Riou, C.; Molee, W.; Molee, A. Effects of β-alanine and L-histidine supplementation on carnosine contents in and quality and secondary structure of proteins in slow-growing Korat chicken meat. Poult. Sci. 2022, 101, 101776. [Google Scholar] [CrossRef]
- Kralik, G.; Sak-Bosnar, M.; Grčević, M.; Kralik, Z. Effect of amino acids on growth performance, carcass characteristics, meat quality, and carnosine concentration in broiler chickens. Poult. Sci. J. 2018, 55, 239–248. [Google Scholar] [CrossRef]
- Cong, J.; Zhang, L.; Li, J.; Wang, S.; Gao, F.; Zhou, G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017, 97, 3733–3741. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J.; Ma, Y.B.; Wu, S.G.; Qi, G.H.; Zhang, H.J. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Spanier, B.; Kottra, G.; Weitz, D. From bacteria to man: Archaic proton-dependent peptide transporters at work. Physiology 2006, 21, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Promkhun, K.; Sinpru, P.; Suwanvichanee, C.; Molee, W.; Molee, A. RNA profiles of the korat chicken breast muscle with increased carnosine content produced through dietary supplementation with β-alanine or L-histidine. Animals 2021, 11, 2596. [Google Scholar] [CrossRef]
- Adabi, S.G.; Cooper, R.G.; Ceylan, N.; Corduk, M. L-carnitine and its functional effects in poultry nutrition. Worlds Poult. Sci. J. 2011, 67, 277–296. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Dorghamm, D.A.; Kahilo, K.A.; Elkattawy, A.M.; Nassef, E.; El-sawy, H.B. Impact of L-carnitine supplementation on growth of broiler chicken through determination of changes in the expression of CAT2, MYOD and MYF5 genes. Slov. Vet. Zb. 2019, 56, 665–672. [Google Scholar] [CrossRef]
- El-Saway, H.B.; Soliman, M.M.; Sadek, K.M.; Nassef, E.; Abouzed, T.K. Beneficial impact of dietary methyl methionine sulfonium chloride and/or L-carnitine supplementation on growth performance, feed efficiency, and serum biochemical parameters in broiler chicken: Role of IGF-1 and MSTN genes. Trop. Anim. Health Prod. 2022, 54, 98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).