Butterfly Communities Vary under Different Urbanization Types in City Parks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Categorization of Urbanization Patterns
2.3. Sampling Criteria
2.4. Butterfly Survey
2.5. Data Analysis
2.5.1. Shannon Diversity Analysis
2.5.2. β-Diversity Analysis
2.5.3. Familial Diversity and Indicator Species
3. Results
3.1. Overview
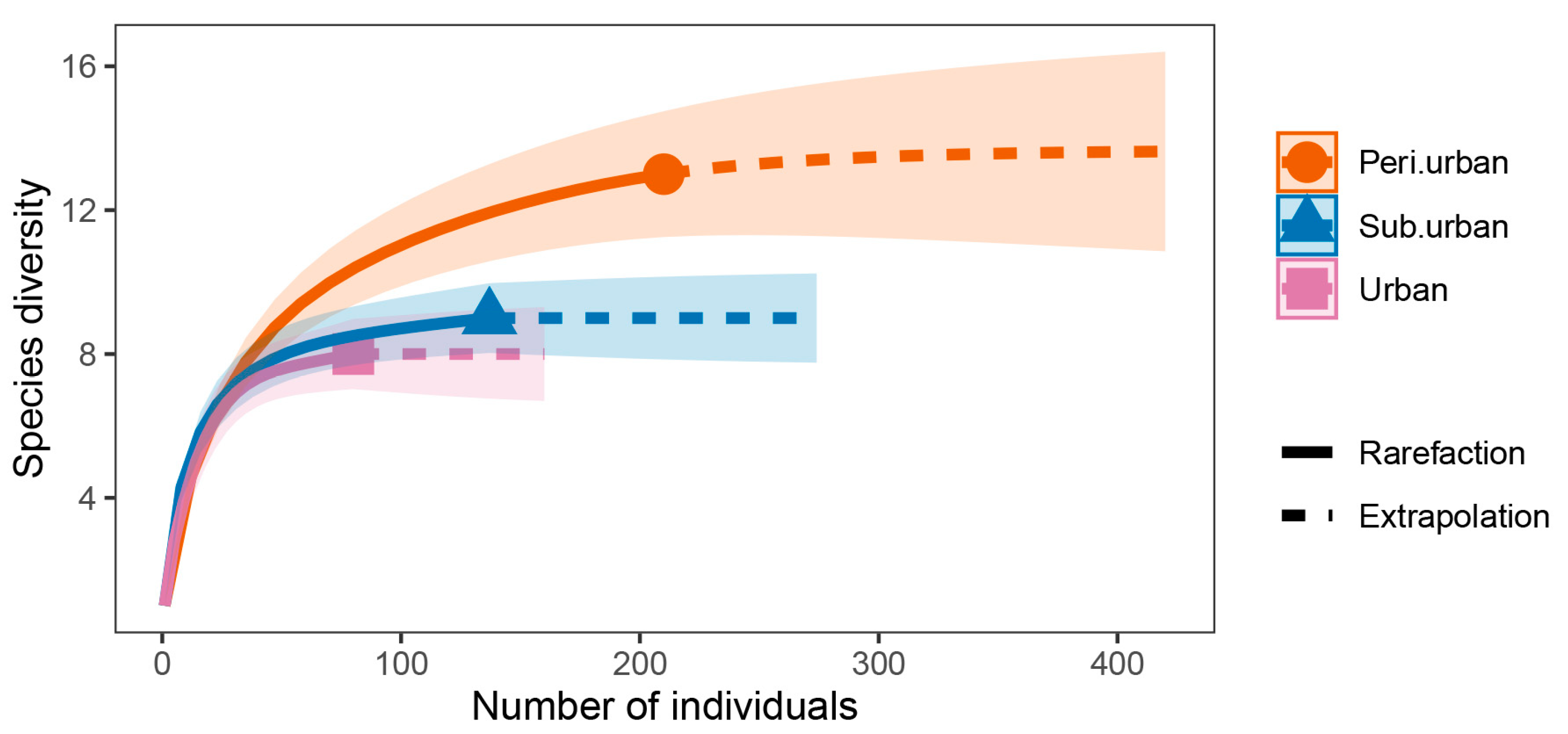
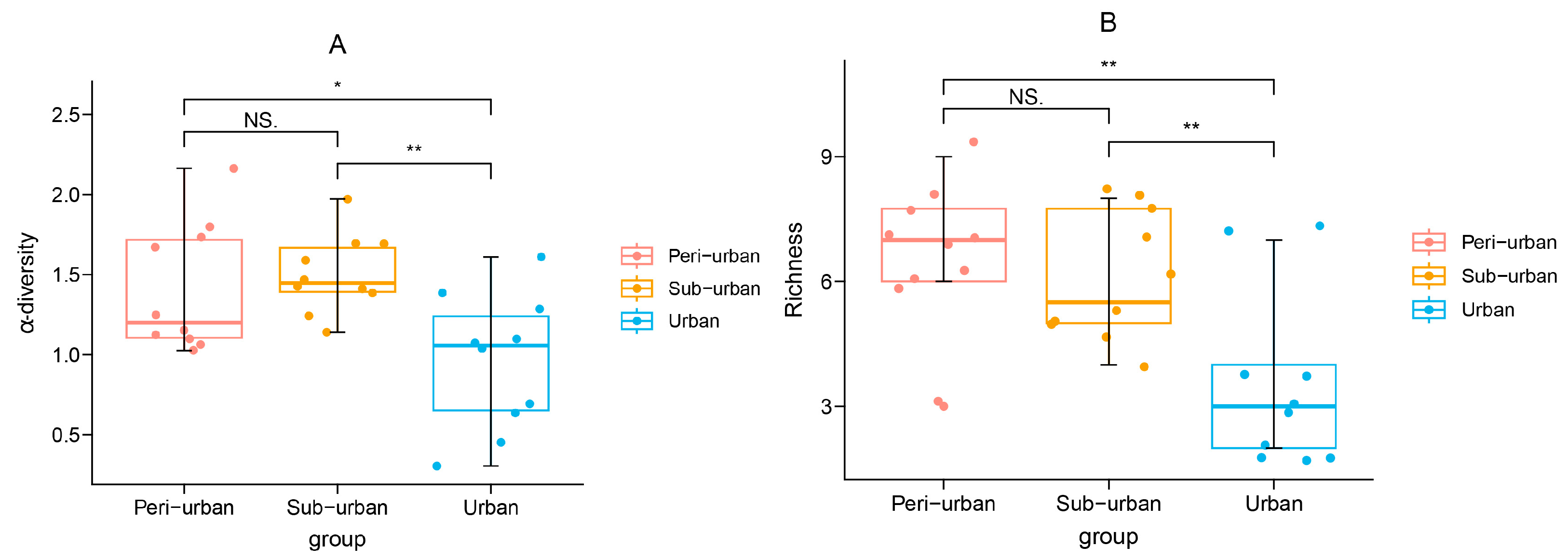
3.2. Shannon Diversity Exhibited Variation across Different Urbanization Gradients
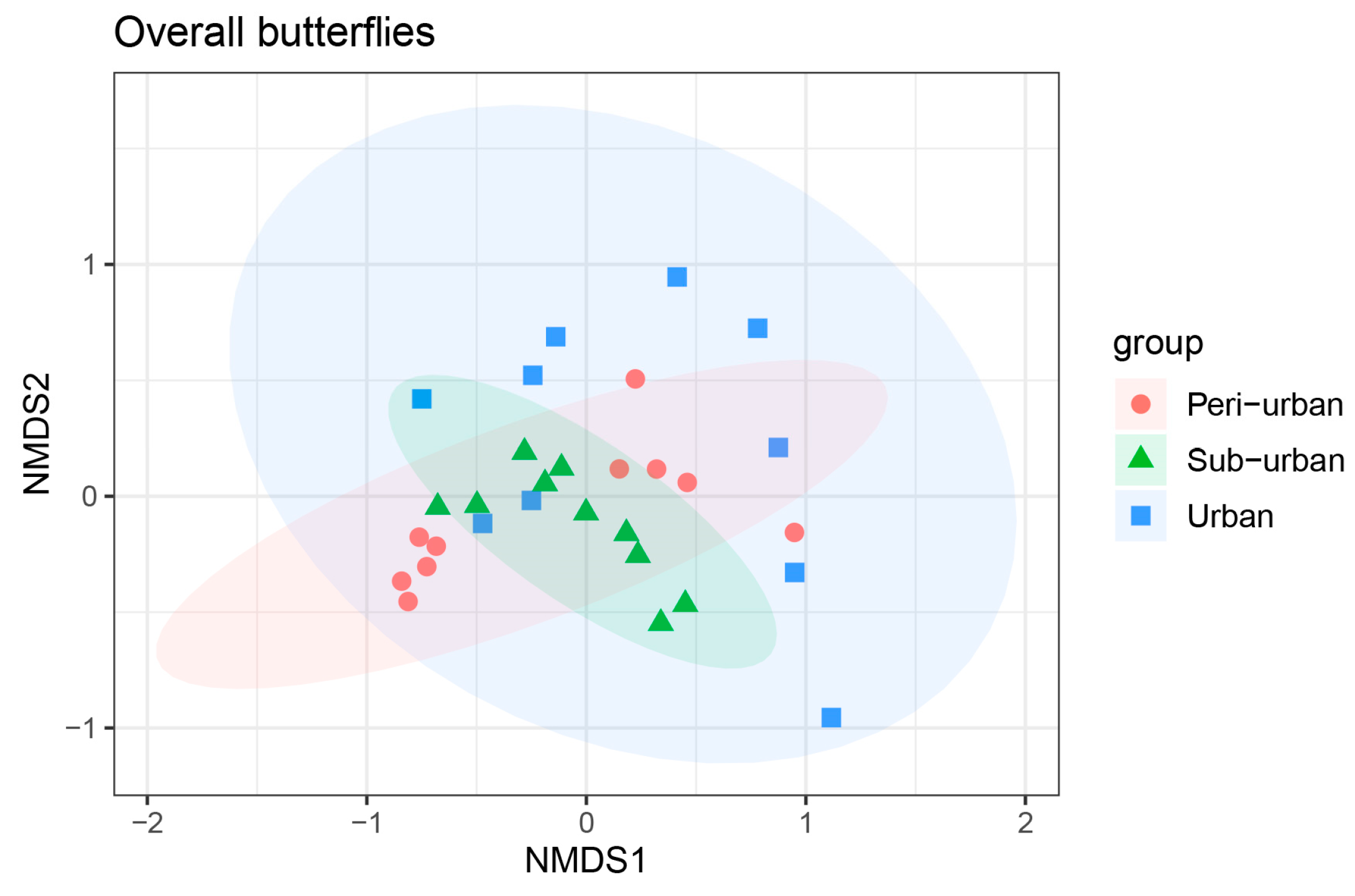
3.3. β-Diversity Exhibited Variation across Different Urbanization Gradients
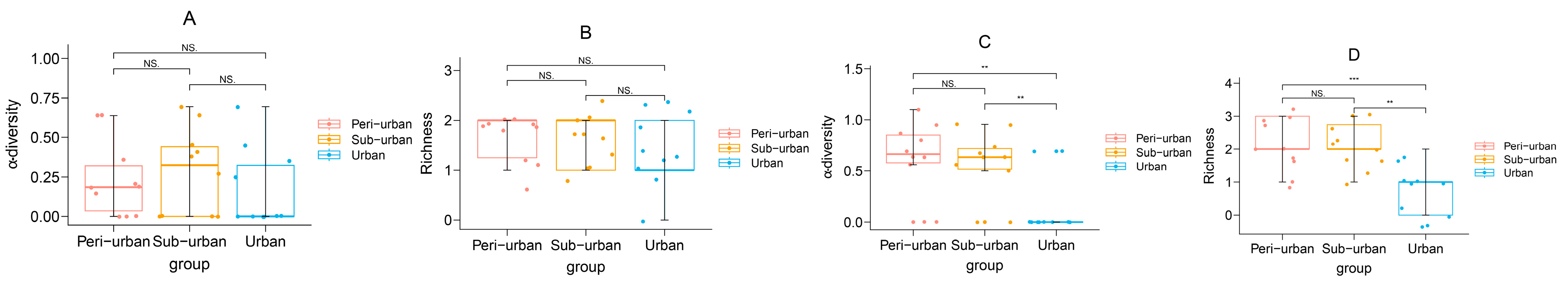
3.4. Familial Role and Indicator Species
4. Discussion
4.1. Urbanization Affects Butterfly Diversity in Urban Parks
4.2. Urbanization Affects Butterfly Families in Urban Parks
4.3. Urbanization Affects Butterfly Familial Roles and Indicator Species in Urban Parks
4.4. Insights for Enhancing Butterfly Diversity
4.5. Limitations of This Study and Possible Developments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Y. Urban Ecology; East China Normal University Press: Shanghai, China, 2000. [Google Scholar]
- Cui, S.; Li, F.; Yu, Y. Theoretical Thinking on Urbanization and Sustainable Urbanization. Urban Stud. 2010, 17, 17–21. [Google Scholar]
- Qi, R. Study on Habitat Fragmentation and Its Impacts on Biodiversity at Urban Areas: A Case of Suzhou. Ph.D. Thesis, East China Normal University, Shanghai, China, 2008. [Google Scholar]
- Liu, X. A Study on the Morphological Evolution of Fuzhou City Since 1840. Master’s Thesis, Northwest A&F University, Xianyang, China, 2003. [Google Scholar]
- Miller, J.R.; Hobbs, R.J. Conservation Where People Live and Work. Conserv. Biol. 2002, 16, 330–337. [Google Scholar] [CrossRef]
- Zhao, Y. Study on urban biodiversity conservation from the perspective of landscape ecological security pattern—A case study of Haidian District, Beijing. Master’s Thesis, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Song, Y. Biotope Evaluation and Design in High-density Urban Area—Take Huangpu District, Shanghai as an Example. In Proceedings of the 2016 Annual Conference of the Chinese Society of Landscape Architecture, Nanning, China, 23–26 September 2016; pp. 62–71. [Google Scholar]
- Liu, L.; Pan, C.; Liu, J.; He, L. Resources and fauna of butterflies in Jiulongshan Mountain National Nature Reserve. Chin. J. Ecol. 2015, 34, 3438–3442. [Google Scholar]
- Fang, L.; Guan, J. Progress in the studies of butterflies in responding to global climate change. J. Environ. Entomol. 2010, 32, 399–406. [Google Scholar]
- Kitching, R.; Orr, A.; Thalib, L.; Mitchell, H.; Hopkins, M.; Graham, A. Moth assemblages as indicators of environmental quality in remnants of upland Australian rain forest. J. Appl. Ecol. 2000, 37, 284–297. [Google Scholar] [CrossRef]
- Kremen, C. Assessing the Indicator Properties of Species Assemblages for Natural Areas Monitoring. Ecol. Appl. 1992, 2, 203–217. [Google Scholar] [CrossRef]
- Koh, L.P.; Sodhi, N.S. Importance of Reserves, Fragments, and Parks for Butterfly Conservation in A Tropical Urban Landscape. Ecol. Appl. 2004, 14, 1695–1708. [Google Scholar] [CrossRef]
- Kadlec, T.; Benes, J.; Jarosik, V.; Konvicka, M. Revisiting urban refuges: Changes of butterfly and burnet fauna in Prague reserves over three decades. Landsc. Urban Plan. 2008, 85, 1–11. [Google Scholar] [CrossRef]
- Öckinger, E.; Dannestam, Å.; Smith, H.G. The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landsc. Urban Plan. 2009, 93, 31–37. [Google Scholar] [CrossRef]
- Bergerot, B.; Fontaine, B.; Julliard, R.; Baguette, M. Landscape variables impact the structure and composition of butterfly assemblages along an urbanization gradient. Landsc. Ecol. 2010, 26, 83–94. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, L.; MacGregor-Fors, I. Butterflies in the city: A review of urban diurnal Lepidoptera. Urban Ecosyst. 2016, 20, 171–182. [Google Scholar] [CrossRef]
- Aguilera, G.; Ekroos, J.; Persson, A.S.; Pettersson, L.B.; Öckinger, E. Intensive management reduces butterfly diversity over time in urban green spaces. Urban Ecosyst. 2018, 22, 335–344. [Google Scholar] [CrossRef]
- Gu, W.; Ma, L.; Liu, Z.; Jiao, Y.; Wang, L.; Zhang, C.; Sun, H.; Sun, M. Diversity of butterflies in Liangshui nature reserves of Xiao Xing’an Mountains. Acta. Ecol. Sin. 2015, 35, 7387–7396. [Google Scholar]
- Yang, D. Studies on the structure of the butterfly community and diversity in the fragmentary tropical rainforest of Xinshuangbanan, China. Acta Entomol. Sin. 1998, 41, 49–52. [Google Scholar] [CrossRef]
- Li, C.; Liu, S. Study on the fauna of butterfly in Changbai Mountain Reserve. J. Jilin Forestry Sci. Technol. 2007, 32, 41–45. [Google Scholar]
- Zhou, G.; Gu, M.; Gong, Y.; Wang, S.; Wu, Z.; Xie, G. Diversity and fauna of butterflies in Nanling National Nature Reserve. J. Environ. Entomol. 2016, 38, 971–978. [Google Scholar]
- Ferenc, M.; Sedláček, O.; Fuchs, R.; Dinetti, M.; Fraissinet, M.; Storch, D. Are cities different? Patterns of species richness and beta diversity of urban bird communities and regional species assemblages in Europe. Glob. Ecol. Biogeogr. 2013, 23, 479–489. [Google Scholar] [CrossRef]
- Huang, Q. The Study on Greenbelt Plant Diversities in Tri-Circle Area of Fuzhou City. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2014. [Google Scholar]
- Ouyang, J. Fuzhou has a resident population of 8.42 million. Fuzhou Daily News, 16 February 2022. [Google Scholar]
- Li, X. Study on Butterfly Diversity and Endangered Mechanisms and Conservation Methods of Rare Species in Baishuijiang Natural Reserve. Ph.D. Thesis, Southwest Jiaotong University, Chengdu, China, 2006. [Google Scholar]
- Chen, Z. Research on the spatial distribution Characters of Residential Price and Its Influencing Factors in Fuzhou City. Master’s Thesis, Fuzhou University, Fuzhou, China, 2015. [Google Scholar]
- Cai, Y.; Zhang, H.; Pan, W.; Chen, Y.; Wang, X. Urban expansion and its influencing factors in Natural Wetland Distribution Area in Fuzhou City, China. Chin. Geogr. Sci. 2012, 22, 568–577. [Google Scholar] [CrossRef]
- Pollard, E.; Yates, T.J. Monitoring butterflies for ecology and conservation: The British butterfly monitoring scheme. In Conservation Biology Series; Springer Science & Business Media: Berlin, Germany, 1993. [Google Scholar]
- Chen, Y. Temperature Effect of Urban Green Space Spatiotemporal Evolution in the City of Fuzhou. Ph.D. Thesis, Fujian Normal University, Fuzhou, China, 2020. [Google Scholar]
- Pollard, E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977, 12, 115–134. [Google Scholar] [CrossRef]
- Pollard, E. Monitoring butterfly abundance in relation to the management of a nature reserve. Biol. Conserv. 1982, 24, 317–328. [Google Scholar] [CrossRef]
- Han, D.; Wang, C.; Yin, L. Characteristics of butterfly-nectar plant network in Beijing’s urban parks and identifying important nectariferous plant species. Acta Ecol. Sin. 2021, 41, 8892–8905. [Google Scholar]
- Xu, Y.; Wu, C. Butterflies of China; Straits Books Publishing House: Fuzhou, China, 2017. [Google Scholar]
- Zhou, Y. Classification and Identification of Chinese Butterflies; Henan Scientific and Technological Publishing House: Zhengzhou, China, 1998. [Google Scholar]
- Zhou, Y. Chinese Butterfly Primary Colors Illustrated; Henan Scientific and Technological Publishing House: Zhengzhou, China, 1999. [Google Scholar]
- Zhou, Y. Monographia Rhopalocerorum Sinensium; Henan Scientific and Technological Publishing House: Zhengzhou, China, 2000. [Google Scholar]
- Shou, J. On Brand-New Butterfly Classification: The Latest trends of China’s butterfly Research. J. Xi’an Univ. (Nat. Sci. Ed.) 2012, 15, 26–32. [Google Scholar]
- Yin, L. Research on the Correlation between Butterfly Diversity and Plant Landscape in Hangzhou Jiangyangfan Ecological Park. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Zhao, H. The variegated and colorful phoenix butterflies may stem from rapid genetic evolution. Science and Technology Daily, 18 April 2022. [Google Scholar]
- Lin, F. Butterfly Diversity in Different Types of Habitat under Rapid Urbanization—A Case in Liangjiang New District, Chongqing. Master’s Thesis, Chongqing University, Chongqing, China, 2012. [Google Scholar]
- Harsh, S.; Jena, J.; Sharma, T.; Sarkar, P.K. Diversity of butterflies and their habitat association in four different habitat types in Kanha-Pench corridor, Madhya Pradesh, India. Int. J. Adv. Res. 2006, 3, 779–785. [Google Scholar]
- Sarkar, V.K.; Das, D.S.; Balakrishnan, V.C.; Kunte, K. Validation of the reported occurrence of Tajuria maculata, the Spotted Royal butterfly (Lepidoptera: Lycaenidae), in the Western Ghats, southwestern India, on the basis of two new records. J. Threat. Taxa 2011, 3, 1629–1632. [Google Scholar] [CrossRef]
- Widhiono, I. Diversity of butterflies in four different forest types in Mount Slamet, Central Java, Indonesia. Biodivers. J. Biol. Divers. 2015, 16, 196–204. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-Project.Org/ (accessed on 21 February 2023).
- Shaphiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Alva, J.A.V.; Estrada, E.G. A Generalization of Shapiro-Wilk’s Test for Multivariate Normality. Commun. Stat. Theory Methods 2009, 38, 1870–1883. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package vegan: Community Ecology Package. In Community Ecology R Package Version 2.3-1; 2013; Volume 2, pp. 1–295. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Cameron, A.; Trivedi, P.K. Regression-based tests for overdispersion in the Poisson model. J. Econ. 1990, 46, 347–364. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Beauchamp, G. Time for some a priori thinking about post hoc testing. Behav. Ecol. 2008, 19, 690–693. [Google Scholar] [CrossRef]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Ricotta, C.; Pavoine, S. A new parametric measure of functional dissimilarity: Bridging the gap between the Bray-Curtis dissimilarity and the Euclidean distance. Ecol. Model. 2022, 466, 109880. [Google Scholar] [CrossRef]
- Holland, S.M. Non-Metric Multidimensional Scaling (MDS); Department of Geology, University of Georgia, Athens, Tech. Rep. Ga 30602-2501. 2008. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwic2vHzuo__AhX3h1YBHQeRChUQFnoECAkQAQ&url=https%3A%2F%2Fstrata.uga.edu%2Fsoftware%2Fpdf%2FmdsTutorial.pdf&usg=AOvVaw1GtNLMEkfApxIOknziPXwJ (accessed on 21 February 2023).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Altig, R.; Mcdiarmid, R.W. Diversity: Familial and generic characterizations. In Tadpoles the Biology of Anuran Larvae; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Jansen, F. Package ‘indicspecies’ (Version 1.7.6). Indicators 2016, 8. [Google Scholar]
- Blair, R.B. Birds and Butterflies along an Urban Gradient: Surrogate Taxa for Assessing Biodiversity? Ecol. Appl. 1999, 9, 164–170. [Google Scholar] [CrossRef]
- Blair, R.B.; Launer, A.E. Butterfly diversity and human land use: Species assemblages along an urban grandient. Biol. Conserv. 1997, 80, 113–125. [Google Scholar] [CrossRef]
- Lin, F.; Yuan, X.; Wu, Y.; Liu, H. Diversity of butterfly in different habitat types in rapid urbanization area. Chin. J. Ecol. 2012, 31, 2579–2584. [Google Scholar] [CrossRef]
- Tzortzakaki, O.; Kati, V.; Panitsa, M.; Tzanatos, E.; Giokas, S. Butterfly diversity along the urbanization gradient in a densely-built Mediterranean city: Land cover is more decisive than resources in structuring communities. Landsc. Urban Plan. 2018, 183, 79–87. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Li, Z.; Qiu, Z.; Zhao, H.; Wang, S.; Gu, M. Spatial distribution pattern and diversity analysis of skipper in middle area of Nanling Mountains, China. Ecol. Environ. Sci. 2021, 30, 117–124. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.; Wang, C.; She, J.; Sun, Z.; Zhao, D.; Bian, Q.; Han, W.; Yin, L.; Sun, R.; et al. Differences in Response of Butterfly Diversity and Species Composition in Urban Parks to Land Cover and Local Habitat Variables. Forests 2021, 12, 140. [Google Scholar] [CrossRef]
- Wu, C. Zoology of China. Insecta. Lepidoptera. Pieridae; Science Press: Beijing, China, 2010; Volume 52. [Google Scholar]
- New, T.R. Conservation Biology of Lycaenidae (Butterflies); Iucn Species Survival Commission: Gland, Switzerland, 1993; Volume 8. [Google Scholar]
- Wu, C. Zoology of China: Cnidaria, Cnidaria subfamily, Cnidaria subfamily. Insecta. Lepidoptera; Science Press: Beijing, China, 2001; Volume 25. [Google Scholar]
- Eswaran, R.; Pramod, P. Structure of butterfly community of Anaikatty hills, Western Ghats. Zoos’ Print J. 2005, 20, 1939–1942. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Kumaraguru, A.; Thiyagesan, K.; Asokan, S. Diversity of papilonid butterflies in the Indira Gandhi wildlife sanctuary, Western Ghats, Southern India. Tigerpaper 2008, 35, 1–8. [Google Scholar]
- Raut, B.N.; Pendharkar, A. Butterfly (Rhopalocera) fauna of Maharashtra Nature Park, Mumbai, Maharashtra, India. Check List 2010, 6, 22–25. [Google Scholar] [CrossRef]
- Sing, K.-W.; Luo, J.; Wang, W.; Jaturas, N.; Soga, M.; Yang, X.; Dong, H.; Wilson, J.-J. Ring roads and urban biodiversity: Distribution of butterflies in urban parks in Beijing city and correlations with other indicator species. Sci. Rep. 2019, 9, 4653. [Google Scholar] [CrossRef]
- She, J.; Han, D.; Wang, C.; Yin, Q.; Sun, Z.; Han, C. Butterfly Diversity of Pocket Parks in Central Urban Area of Beijing. J Chin. Urban For. 2022, 20, 1–6. [Google Scholar]
- Zhang, Q. How butterflies respond to climate change. Discov. Nat. 2020, 12, 42–45. [Google Scholar]
- Thomas, J.A.; Bourn, N.; Clarke, R.T.; Stewart, K.E.; Simcox, D.J.; Pearman, G.S.; Curtis, R.; Goodger, B. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc. R. Soc. B Boil. Sci. 2001, 268, 1791–1796. [Google Scholar] [CrossRef]
- Byron, C.W.; Andrew, S.P. Persistence of species in a fragmented urban landscape: The importance of dispersal ability and habitat availability for grassland butterflies. In Biodiversity and Conservation; Springer Science & Business Media: Berlin, Germany, 2002; Volume 11. [Google Scholar]
- Snep, R.; Opdam, P.; Baveco, J.; WallisDeVries, M.; Timmermans, W.; Kwak, R.; Kuypers, V. How peri-urban areas can strengthen animal populations within cities: A modeling approach. Biol. Conserv. 2006, 127, 345–355. [Google Scholar] [CrossRef]
- Matteson, K.C.; Langellotto, G.A. Determinates of inner city butterfly and bee species richness. Urban Ecosyst. 2010, 13, 333–347. [Google Scholar] [CrossRef]
- Konvicka, M.; Kadlec, T. How to increase the value of urban areas for butterfly conservation? A lesson from Prague nature reserves and parks. Eur. J. Entomol. 2011, 108, 219–229. [Google Scholar] [CrossRef]
- Ekroos, J.; Heliölä, J.; Kuussaari, M. Homogenization of lepidopteran communities in intensively cultivated agricultural landscapes. J. Appl. Ecol. 2010, 47, 459–467. [Google Scholar] [CrossRef]
- Öckinger, E.; Eriksson, A.K.; Smith, H.G. Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biol. Conserv. 2006, 133, 291–300. [Google Scholar] [CrossRef]
- Dover, J.W.; Rescia, A.; Fungariño, S.; Fairburn, J.; Carey, P.; Lunt, P.; Dennis, R.L.H.; Dover, C.J. Can hay harvesting detrimentally affect adult butterfly abundance? J. Insect Conserv. 2010, 14, 413–418. [Google Scholar] [CrossRef]
- Vergnes, A.; Le Viol, I.; Clergeau, P. Green corridors in urban landscapes affect the arthropod communities of domestic gardens. Biol. Conserv. 2012, 145, 171–178. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Manel, S.; Mauffrey, J.-F.; Tatoni, T.; Deschamps-Cottin, M. Matrix configuration and patch isolation influences override the species–area relationship for urban butterfly communities. Landsc. Ecol. 2011, 27, 159–169. [Google Scholar] [CrossRef]
- Kurylo, J.S.; Threlfall, C.G.; Parris, K.M.; Ossola, A.; Williams, N.S.G.; Evans, K.L. Butterfly richness and abundance along a gradient of imperviousness and the importance of matrix quality. Ecol. Appl. 2020, 30, e02144. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.Y.; Teo, S.; Kurukulasuriya, B.; Chung, Y.F.; Rajathurai, S.; Tan, H.T.W. Not all green is as good: Different effects of the natural and cultivated components of urban vegetation on bird and butterfly diversity. Biol. Conserv. 2014, 171, 299–309. [Google Scholar] [CrossRef]
- Clark, P.J.; Reed, J.M.; Chew, F.S. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst. 2007, 10, 321–337. [Google Scholar] [CrossRef]



| Type of Urbanization | Butterfly Family | Scientific Name | The Number of Individuals per Species | Frequency |
|---|---|---|---|---|
| Urban | Lycaenidae | Lampides boeticus (Linnaeus, 1767) | 48 | 9 |
| Lycaenidae | Taraka hamada (Druce, 1875) | 4 | 4 | |
| Pieridae | Pieris rapae (Linnaeus, 1758) | 6 | 4 | |
| Pieridae | Catopsilia pomona (Fabricius, 1775) | 6 | 4 | |
| Papilionidae | Papilio polytes (Linnaeus, 1758) | 5 | 4 | |
| Papilionidae | Graphium sarpedon (Linnaeus, 1758) | 5 | 5 | |
| Papilionidae | Papilio protenor (Cramer, 1775) | 1 | 1 | |
| Nymphalidae | Argyreus hyperbius (Linnaeus, 1763) | 5 | 5 | |
| Peri-urban | Lycaenidae | Lampides boeticus (Linnaeus, 1767) | 124 | 10 |
| Lycaenidae | Taraka hamada (Druce, 1875) | 9 | 7 | |
| Pieridae | Pieris rapae (Linnaeus, 1758) | 28 | 10 | |
| Pieridae | Catopsilia pomona (Fabricius, 1775) | 10 | 8 | |
| Pieridae | Eurema hecabe (Linnaeus, 1758) | 4 | 4 | |
| Papilionidae | Papilio polytes (Linnaeus, 1758) | 13 | 7 | |
| Papilionidae | Graphium sarpedon (Linnaeus, 1758) | 8 | 7 | |
| Papilionidae | Papilio protenor (Cramer, 1775) | 1 | 1 | |
| Papilionidae | Papilio memnon (Linnaeus, 1758) | 2 | 2 | |
| Papilionidae | Papilio helenus (Linnaeus, 1758) | 2 | 1 | |
| Nymphalidae | Argyreus hyperbius (Linnaeus, 1763) | 6 | 6 | |
| Nymphalidae | Vanessa indica (Herbst, 1794) | 1 | 1 | |
| Nymphalidae | Neptis sappho (Pallas, 1771) | 2 | 2 | |
| Suburban | Lycaenidae | Lampides boeticus (Linnaeus, 1767) | 55 | 10 |
| Lycaenidae | Taraka hamada (Druce, 1875) | 6 | 6 | |
| Pieridae | Pieris rapae (Linnaeus, 1758) | 25 | 10 | |
| Pieridae | Catopsilia pomona (Fabricius, 1775) | 22 | 8 | |
| Pieridae | Eurema hecabe (Linnaeus, 1758) | 3 | 3 | |
| Papilionidae | Papilio polytes (Linnaeus, 1758) | 10 | 10 | |
| Papilionidae | Graphium sarpedon (Linnaeus, 1758) | 6 | 6 | |
| Papilionidae | Papilio protenor (Cramer, 1775) | 1 | 1 | |
| Nymphalidae | Argyreus hyperbius (Linnaeus, 1763) | 9 | 7 |
| Type of Urbanization | Grouping Variable | Parameter | |||
|---|---|---|---|---|---|
| Shannon Diversity | Richness | Abundance | Chao1 | ||
| Urban | Butterflies overall Lycaenidae Pieridae Papilionidae Nymphalidae | 0.958 0.174 0.139 0.133 0 | 8 2 2 3 1 | 80 52 12 11 5 | 5.23 1.40 0.80 1.10 0.50 |
| Peri-urban | Butterflies overall Lycaenidae Pieridae Papilionidae Nymphalidae | 1.408 0.235 0.625 0.53 0.069 | 13 2 3 5 3 | 210 133 42 26 9 | 13.18 1.70 2.80 2.80 1.00 |
| Suburban | Butterflies overall Lycaenidae Pieridae Papilionidae Nymphalidae | 1.502 0.284 0.565 0.456 0 | 9 2 3 3 1 | 137 61 50 17 9 | 10.95 1.70 2.30 2.50 0.70 |
| Overall | Butterflies overall Lycaenidae Pieridae Papilionidae Nymphalidae | 1.298 0.231 0.443 0.373 0.023 | 13 2 3 5 3 | 427 246 104 54 23 | 9.78 1.60 1.97 2.13 0.73 |
| Variable | Parameter | Estimate | Std. Error | p | df |
|---|---|---|---|---|---|
| All butterflies | Shannon diversity Abundance Richness | 1.033 0.017 0.187 | 0.312 0.014 0.064 | 0.003 * 0.241 0.007 * | 28 |
| Lycaenidae | Shannon diversity Abundance Richness | 0.609 0.004 0.317 | 0.618 0.019 0.268 | 0.333 0.815 0.247 | 28 |
| Pieridae | Shannon diversity Abundance Richness | 0.974 0.183 0.459 | 0.360 0.047 0.133 | 0.011 * 0.001 * 0.002 * | 28 |
| Papilionidae | Shannon diversity Abundance Richness | 0.673 0.128 0.359 | 0.364 0.121 0.179 | 0.075 0.299 0.055 | 28 |
| Nymphalidae | Shannon diversity Abundance Richness | −0.004 0.352 0.241 | 1.240 0.242 0.290 | 0.997 0.157 0.413 | 28 |
| Node | Butterfly Families | Type of Urbanization | Model | F | R2 | Sig. |
|---|---|---|---|---|---|---|
| 1 | Lycaenidae | Urban Peri-urban Sub-urban | LY = −0.212 + 0.297 SR LY = −0.336 + 0.336 SR LY = −0.473 + 0.473 SR | 14.260 6.737 33.140 | 0.641 0.457 0.806 | 0.005 0.032 0.001 |
| 2 | ||||||
| 3 | ||||||
| 4 5 6 | Pieridae | Urban Peri-urban Sub-urban | PI = −0.099 + 0.297 SR PI = −0.345 + 0.441 SR PI = −0.345 + 0.297 SR | 14.400 71.490 61.380 | 0.643 0.899 0.885 | 0.005 0.000 0.000 |
| 7 8 9 | Papilionidae | Urban Peri-urban Sub-urban | PA = −0.199 + 0.332 SR PA = −0.245 + 0.430 SR PA = −0.567 + 0.602 SR | 13.250 41.370 294.400 | 0.624 0.838 0.974 | 0.007 0.000 0.000 |
| Indicator Species | Type of Urbanization | IndVal | Frequency | p-Value |
|---|---|---|---|---|
| Lampides boeticus | Peri-urban | 0.546 | 29 | 0.036 * |
| Pieris rapae | Sub-urban | 0.475 | 24 | 0.049 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Huang, S.; Fang, W.; Zhao, Y.; Huang, Z.; Zheng, R.; Huang, J.; Dong, J.; Fu, W. Butterfly Communities Vary under Different Urbanization Types in City Parks. Animals 2023, 13, 1775. https://doi.org/10.3390/ani13111775
Lin Y, Huang S, Fang W, Zhao Y, Huang Z, Zheng R, Huang J, Dong J, Fu W. Butterfly Communities Vary under Different Urbanization Types in City Parks. Animals. 2023; 13(11):1775. https://doi.org/10.3390/ani13111775
Chicago/Turabian StyleLin, Ying, Shanjun Huang, Wenqiang Fang, Yujie Zhao, Ziluo Huang, Ruoxian Zheng, Jingkai Huang, Jiaying Dong, and Weicong Fu. 2023. "Butterfly Communities Vary under Different Urbanization Types in City Parks" Animals 13, no. 11: 1775. https://doi.org/10.3390/ani13111775
APA StyleLin, Y., Huang, S., Fang, W., Zhao, Y., Huang, Z., Zheng, R., Huang, J., Dong, J., & Fu, W. (2023). Butterfly Communities Vary under Different Urbanization Types in City Parks. Animals, 13(11), 1775. https://doi.org/10.3390/ani13111775







