Machine Learning Approach for Muscovy Duck (Cairina moschata) Semen Quality Assessment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds and Rearing
2.3. Semen Collection
2.4. Sperm Analysis
2.5. Enzyme Assay
2.6. DNA Methylation
2.7. Machine Learning Sperm Parameters Classification
2.8. Training of Machine Learning Models
2.9. Performance Assessment of the Models (Validation)
2.10. Model Predictions Utilization
2.11. Statistical Analysis
3. Results
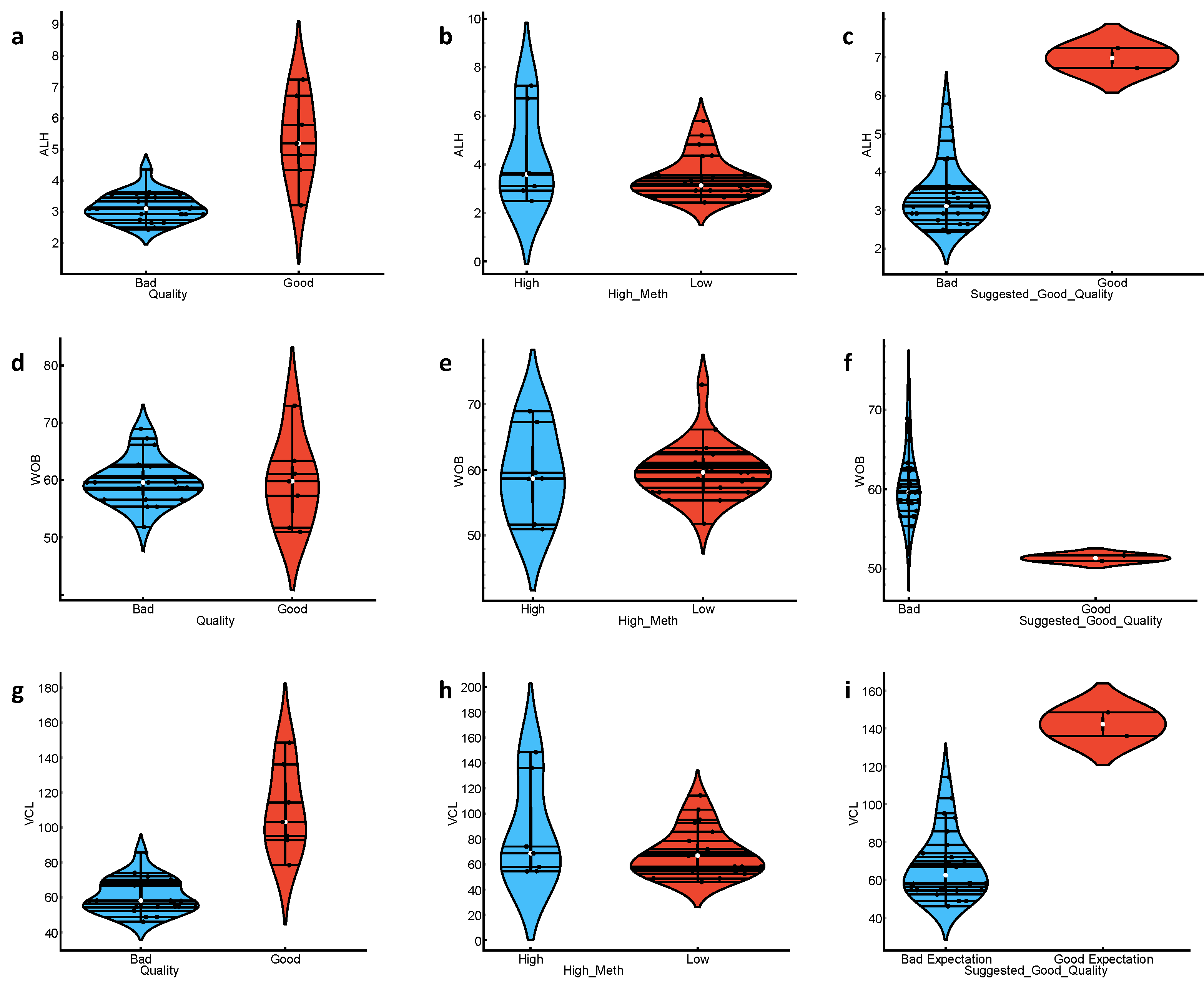
3.1. CASA Parameters Differ between Groups with High and Low Progressive Motility Labelled as “Good” vs. “Bad” Quality
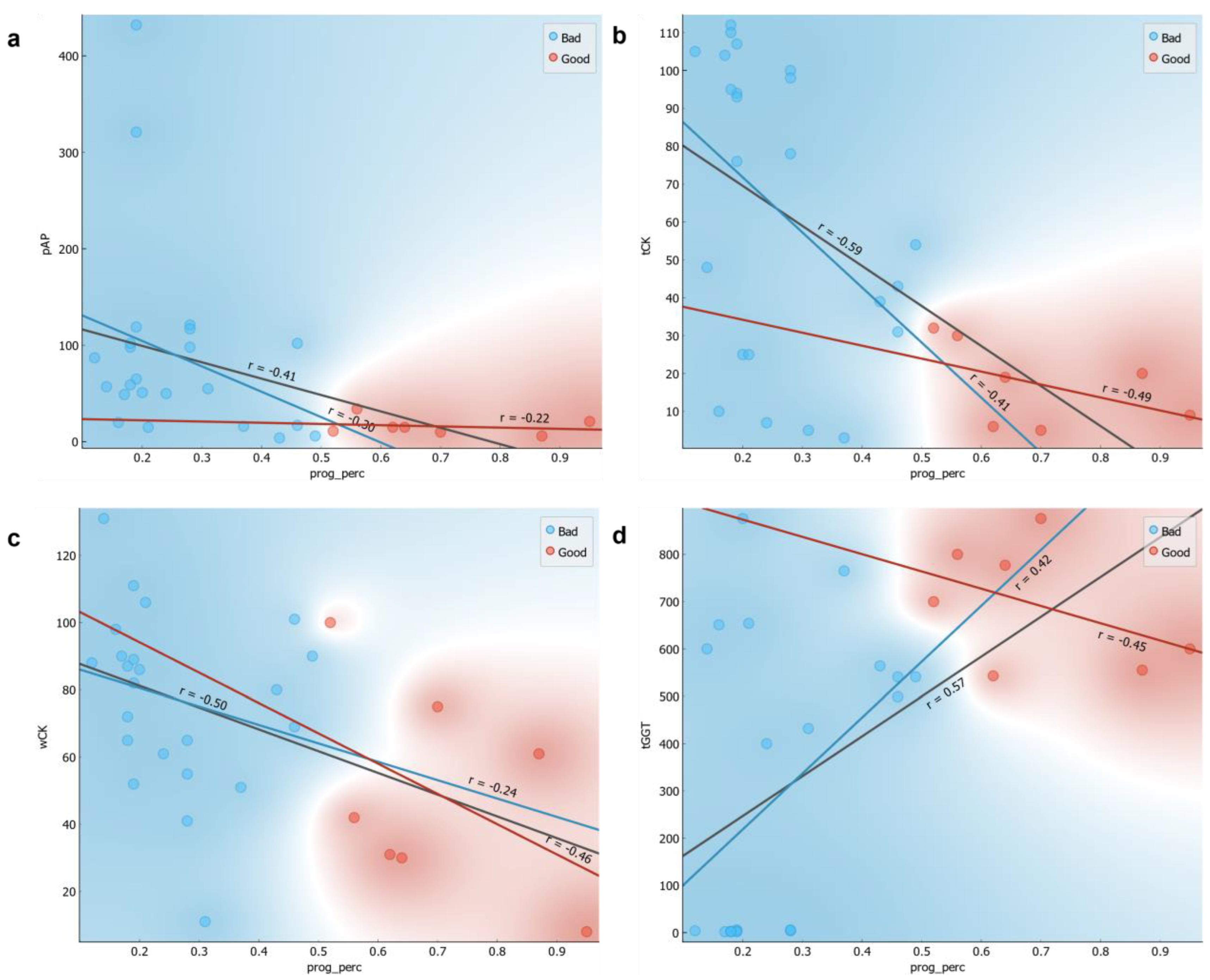
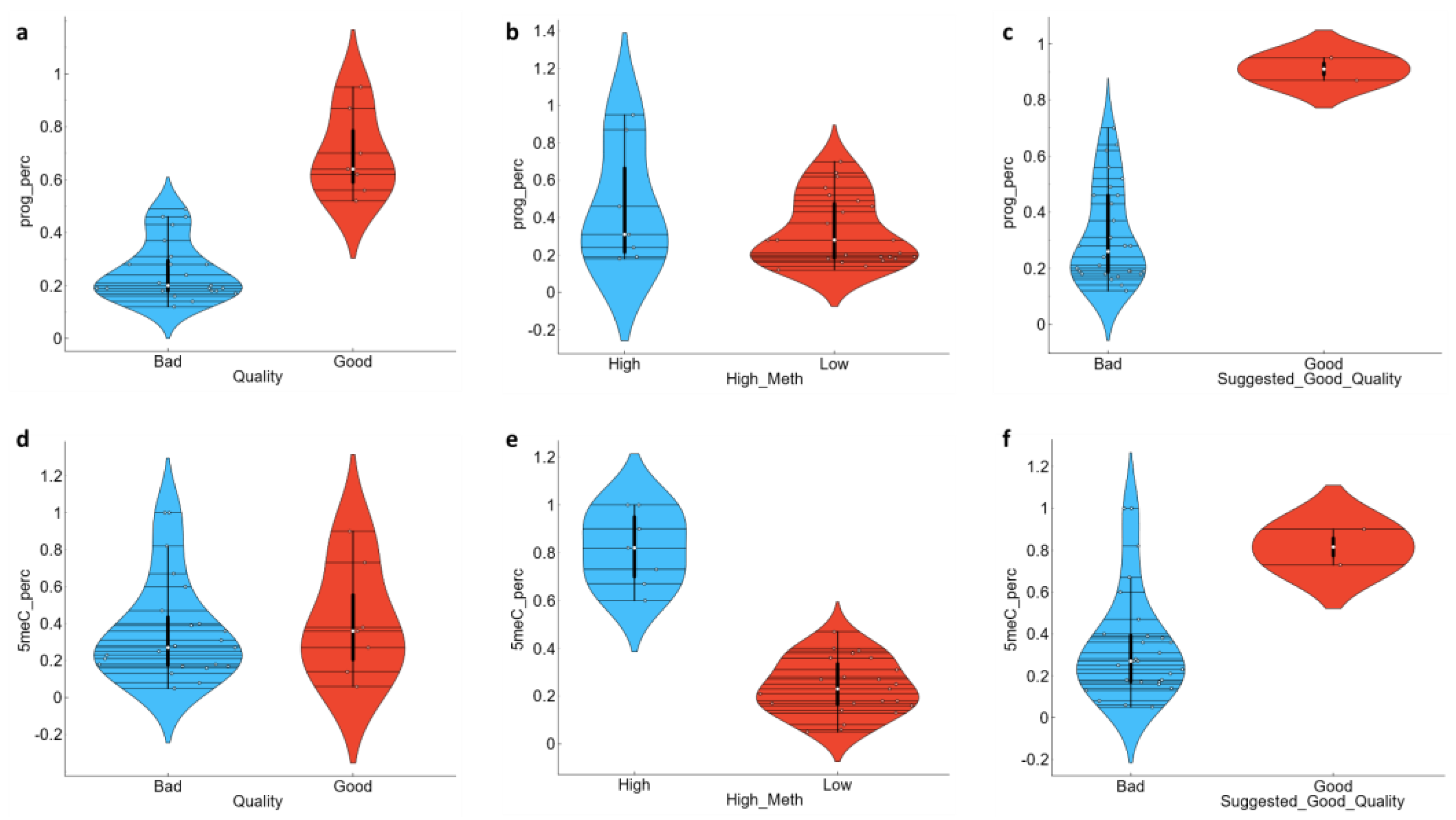
3.2. Methylation Level Could Be Used to Potentially Stratify the Sperm Characterization Parameters
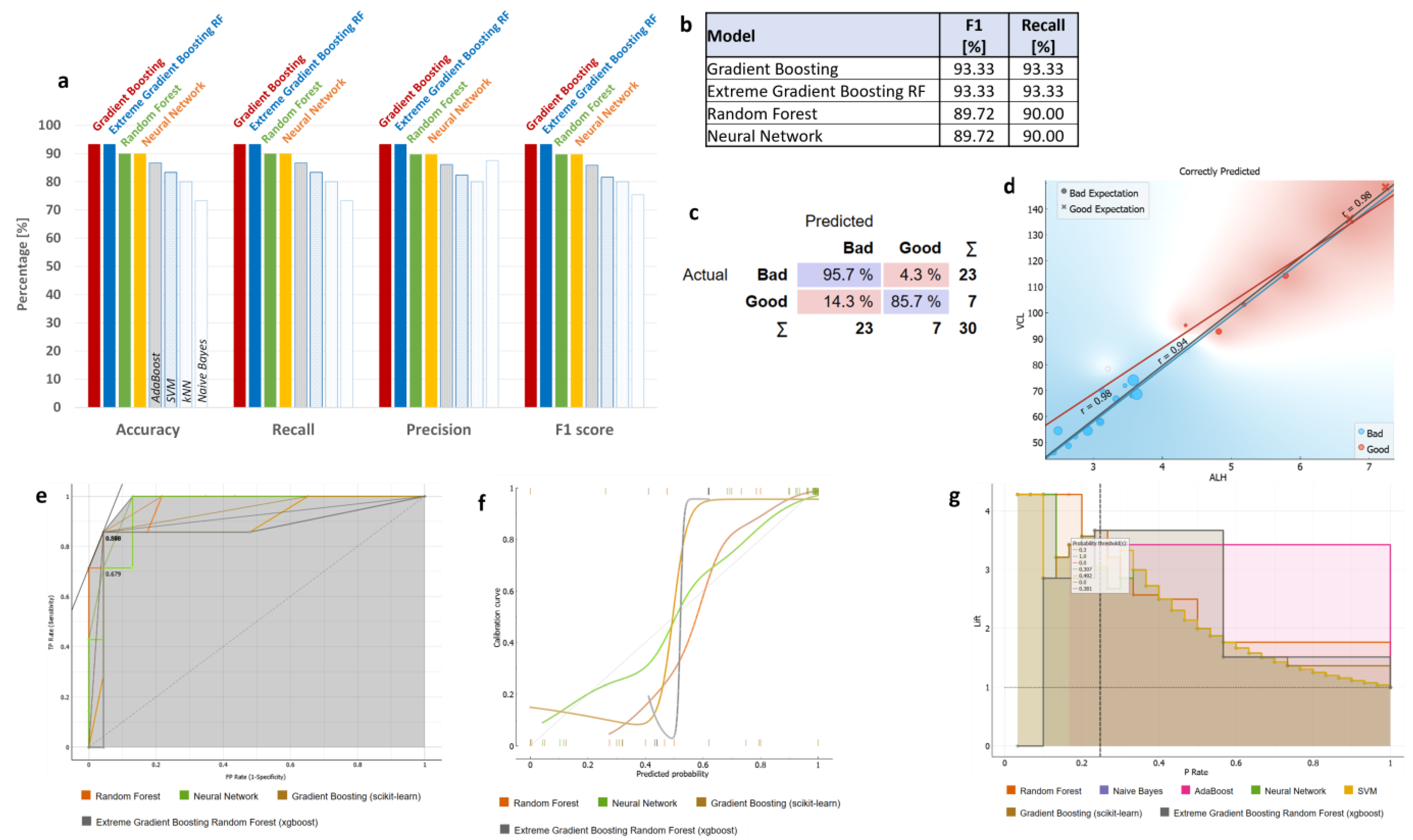
3.3. ML Classifications Suggest Which CASA and Enzyme Parameters Could Potentially Be Used Together with DNA Methylation Status for Field Preliminary Analysis of Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | adenosine diphosphate |
| ALH | amplitude of lateral head displacement |
| AMP | adenosine monophosphate |
| ANOVA | analysis of variance |
| AP | alkaline phosphatase |
| AU extender | Agraren Universitet’ extenders |
| AUC | Area Under the Curve |
| BCF | beat-cross frequency |
| CASA | computer-aided sperm analysis |
| CK | creatine kinase |
| Cr | creatine |
| DNA | deoxyribonucleic Acid |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| GGT | Gamma-Glutamyl transferase |
| kNN | k-Nearest Neighbours |
| LDH | Lactate Dehydrogenase |
| LDH-C4 | Lactate Dehydrogenase-C4 |
| LIN | linearity |
| ML | machine learning |
| MLP-NN | Multi-layered Perception Backpropagation Neural Network |
| n.s. | no significant difference |
| NADP | Nicotinamide adenine dinucleotide phosphate |
| NN | Neural Network |
| sp | seminal plasma extract |
| spAP | seminal plasma extract of alkaline phosphatase |
| spCK | seminal plasma extract of creatine kinase |
| spGGT | seminal plasma extract of gamma-glutamyl transferase |
| spLDH | seminal plasma extract of Lactate Dehydrogenase |
| RF | Random Forest |
| RNAs | Ribonucleic acids |
| ROC | receiver operating characteristic |
| SLP-NN | single layer neural network |
| sp | seminal plasma |
| spz | spermatozoids |
| STR | straightness of the curvilinear trajectory |
| SVM | Support Vector Machine map |
| t | triton sperm extract |
| tAP | triton sperm extract of alkaline phosphatase |
| tCK | triton sperm extract of creatine kinase |
| tGGT | triton sperm extract of gamma-glutamyl transferase |
| tLDH | triton sperm extract of lactate dehydrogenase |
| TP | true positive |
| VAP | velocity of the average path |
| VCL | curvilinear velocity |
| VSL | linear Velocity |
| w | water sperm extract |
| wAP | water sperm extract of alkaline phosphatase |
| wCK | water sperm extract of creatine kinase |
| wGGT | water sperm extract of gamma-glutamyl transferase |
| wLDH | water sperm extract of lactate dehydrogenase |
| WOB | wobble of the curvilinear trajectory |
| 5-meC | 5-methyl-cytosins |
References
- Waberski, D.; Suarez, S.S.; Henning, H. Assessment of Sperm Motility in Livestock: Perspectives Based on Sperm Swimming Conditions in Vivo. Anim. Reprod. Sci. 2022, 246, 106849. [Google Scholar] [CrossRef] [PubMed]
- Broekhuijse, M.L.W.J.; Šoštarić, E.; Feitsma, H.; Gadella, B.M. Application of Computer-Assisted Semen Analysis to Explain Variations in Pig Fertility1. J. Anim. Sci. 2012, 90, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, T.M.; Turri, F.; Manes, S.; Cassinelli, C.; Pizzi, F. The Combination of Kinetic and Flow Cytometric Semen Parameters as a Tool to Predict Fertility in Cryopreserved Bull Semen. Animal 2017, 11, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Liu, H.-C.; Wei, L.-Y.; Huang, J.-F.; Lin, C.-C.; Blesbois, E.; Chen, M.-C. Sperm Quality Parameters and Reproductive Efficiency in Muscovy Duck (Cairina moschata). J. Poult. Sci. 2016, 53, 223–232. [Google Scholar] [CrossRef]
- Kamphuis, C.; Duenk, P.; Veerkamp, R.F.; Visser, B.; Singh, G.; Nigsch, A.; de Mol, R.M.; Broekhuijse, M.L.W.J. Machine Learning to Further Improve the Decision Which Boar Ejaculates to Process into Artificial Insemination Doses. Theriogenology 2020, 144, 112–121. [Google Scholar] [CrossRef]
- Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R.D. Sperm Morphology: Assessment, Pathophysiology, Clinical Relevance, and State of the Art in 2017. Andrology 2017, 5, 845–862. [Google Scholar] [CrossRef]
- McSwiggin, H.M.; O’Doherty, A.M. Epigenetic Reprogramming during Spermatogenesis and Male Factor Infertility. Reproduction 2018, 156, R9–R21. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Carrell, D.T. The Paternal Epigenome and Embryogenesis: Poising Mechanisms for Development. Asian J. Androl. 2011, 13, 76–80. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell. Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Disease. Environ. Epigenet 2018, 4, dvy016. [Google Scholar] [CrossRef] [PubMed]
- Head, J.A. Patterns of DNA Methylation in Animals: An Ecotoxicological Perspective. Integr. Comp. Biol. 2014, 54, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, N.; Hu, X.; Li, J.; Du, Z.; Chen, L.; Yin, G.; Duan, J.; Zhang, H.; Zhao, Y.; et al. Genome-Wide Mapping of DNA Methylation in Chicken. PLoS ONE 2011, 6, e19428. [Google Scholar] [CrossRef] [PubMed]
- Denomme, M.M.; McCallie, B.R.; Parks, J.C.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Alterations in the Sperm Histone-Retained Epigenome Are Associated with Unexplained Male Factor Infertility and Poor Blastocyst Development in Donor Oocyte IVF Cycles. Hum. Reprod. 2017, 32, 2443–2455. [Google Scholar] [CrossRef]
- ben Maamar, M.; Sadler-Riggleman, I.; Beck, D.; McBirney, M.; Nilsson, E.; Klukovich, R.; Xie, Y.; Tang, C.; Yan, W.; Skinner, M.K. Alterations in Sperm DNA Methylation, Non-Coding RNA Expression, and Histone Retention Mediate Vinclozolin-Induced Epigenetic Transgenerational Inheritance of Disease. Environ. Epigenet 2018, 4, dvy010. [Google Scholar] [CrossRef]
- Du, Y.; Li, M.; Chen, J.; Duan, Y.; Wang, X.; Qiu, Y.; Cai, Z.; Gui, Y.; Jiang, H. Promoter Targeted Bisulfite Sequencing Reveals DNA Methylation Profiles Associated with Low Sperm Motility in Asthenozoospermia. Hum. Reprod. 2015, 31, 24–33. [Google Scholar] [CrossRef]
- Zhang, J.J.; Do, H.L.; Chandimali, N.; Lee, S.B.; Mok, Y.S.; Kim, N.; Kim, S.B.; Kwon, T.; Jeong, D.K. Non-Thermal Plasma Treatment Improves Chicken Sperm Motility via the Regulation of Demethylation Levels. Sci. Rep. 2018, 8, 7576. [Google Scholar] [CrossRef]
- Salehi, M.; Mahdavi, A.H.; Sharafi, M.; Shahverdi, A. Cryopreservation of Rooster Semen: Evidence for the Epigenetic Modifications of Thawed Sperm. Theriogenology 2020, 142, 15–25. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chandimali, N.; Kim, N.; Kang, T.Y.; Kim, S.B.; Kim, J.S.; Wang, X.Z.; Kwon, T.; Jeong, D.K. Demethylation and MicroRNA Differential Expression Regulate Plasma-Induced Improvement of Chicken Sperm Quality. Sci. Rep. 2019, 9, 8865. [Google Scholar] [CrossRef]
- Wu, H.; Hauser, R.; Krawetz, S.A.; Pilsner, J.R. Environmental Susceptibility of the Sperm Epigenome During Windows of Male Germ Cell Development. Curr. Environ. Health Rep. 2015, 2, 356–366. [Google Scholar] [CrossRef]
- Łukaszewicz, E.; Jerysz, A.; Chełmońska, B. Effect of Semen Extenders and Storage Time on Quality of Muscovy Duck (Cairina moschata) Drake Semen during the Entire Reproductive Season. Reprod. Domest. Anim. 2020, 55, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, Y.; Sun, Y.; Mehaisen, G.M.K.; Ma, T.; Chen, J. Chicken Sperm Cryopreservation: Review of Techniques, Freezing Damage, and Freezability Mechanisms. Agriculture 2023, 13, 445. [Google Scholar] [CrossRef]
- Gerzilov, V.; Andreeva, M. Effect of Three Extenders on the Motility and Morphological Characteristics of Spermatozoa in Diluted Muscovy Semen Stored at 4 °C up to 120 Hours. Bulg. J. Agric. Sci. 2021, 27, 1187–1193. [Google Scholar]
- Ribas-Maynou, J.; Barranco, I.; Salas-Huetos, A. Sperm Quality and Fertility of Livestock Animals. Animals 2023, 13, 604. [Google Scholar] [CrossRef]
- Galdiero, G.; D’Anza, E.; de Angelis, C.; Albarella, S.; Peretti, V.; Pivonello, R.; Ciotola, F. Sperm Global DNA Methylation (SGDM) in Semen of Healthy Dogs. Vet. Sci. 2021, 8, 50. [Google Scholar] [CrossRef]
- Ammar, O.; Mehdi, M.; Tekeya, O.; Neffati, F.; Haouas, Z. Novel Association between Apoptotic Sperm Biomarkers with Seminal Biochemical Parameters and Acetylcholinesterase Activity in Patients with Teratozoospermia. J. Assist. Reprod. Genet. 2019, 36, 2367–2378. [Google Scholar] [CrossRef]
- Hallak, J.; Sharma, R.K.; Pasqualotto, F.F.; Ranganathan, P.; Thomas, A.J.; Agarwal, A. Creatine Kinase as an Indicator of Sperm Quality and Maturity in Men with Oligospermia. Urology 2001, 58, 446–451. [Google Scholar] [CrossRef]
- Matsuura, K.; Huang, H.-W.; Chen, M.-C.; Chen, Y.; Cheng, C.-M. Relationship between Porcine Sperm Motility and Sperm Enzymatic Activity Using Paper-Based Devices. Sci. Rep. 2017, 7, 46213. [Google Scholar] [CrossRef]
- Mohammad Mostakhdem Hashemi, M.; Tabandeh, A.; Tajari, H.; Behnampour, N.; Joshaghani, H.R. Evaluation of LDH Activity and Its Relationship with Fructose Levels in Seminal Plasma of Normospermic and Asthenospermic Males. Med. Lab. J. 2016, 10, 18–23. [Google Scholar] [CrossRef]
- Pero, M.E.; Lombardi, P.; Longobardi, V.; Boccia, L.; Vassalotti, G.; Zicarelli, L.; Ciani, F.; Gasparrini, B. Influence of γ-Glutamyltransferase and Alkaline Phosphatase Activity on in Vitro Fertilisation of Bovine Frozen/Thawed Semen. Ital. J. Anim. Sci. 2017, 16, 390–392. [Google Scholar] [CrossRef]
- Bucci, D.; Isani, G.; Giaretta, E.; Spinaci, M.; Tamanini, C.; Ferlizza, E.; Galeati, G. Alkaline Phosphatase in Boar Sperm Function. Andrology 2013, 2, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.S. The Training of Drakes for Semen Collection. Ann. Zootech. 1980, 29, 93–102. [Google Scholar] [CrossRef]
- Gerzilov, V. A Method for Obtaining of Semen from the Species Muscovy Dick (Cairina moschata). Journal of Animal Science. 2000, 37, 56–63. [Google Scholar]
- Gerzilov, V.; Alexandrova, A.; Andreeva, M.; Tsvetanova, E.; Georgieva, A.; Petrov, P.; Stefanov, R. Effect of Prooxidants and Chelator Desferal on the Oxidative Status and Sperm Motility of Muscovy Semen. Toxicol. Rep. 2022, 9, 276–283. [Google Scholar] [CrossRef]
- Atanasov, V.; Gerzilov, V.; Dyshlianova, E. Comparison of Biochemical Parameters of Muscovy Drake Semen Diluted and Stored at 4 °C in Three Buffers. Anim. Reprod. Sci. 2007, 100, 329–337. [Google Scholar] [CrossRef]
- Nasrallah, F.; Hammami, M.; Omar, S.; Aribia, H.; Sanhaji, H.; Feki, M. Semen Creatine and Creatine Kinase Activity as an Indicator of Sperm Quality. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Bird, A. DNA Methylation Patterns and Epigenetic Memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Watson, H.; Salmón, P.; Isaksson, C. Dynamic Changes in DNA Methylation during Embryonic and Postnatal Development of an Altricial Wild Bird. Ecol. Evol. 2019, 9, 9580–9585. [Google Scholar] [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA Methylation Dynamics during Epigenetic Reprogramming in the Germline and Preimplantation Embryos. Genes. Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef]
- Demšar, J.; Erjavec, A.; Hočevar, T.; Milutinovič, M.; Možina, M.; Toplak, M.; Umek, L.; Zbontar, J.; Zupan, B. Orange: Data mining toolbox in python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Usui, F.; Nakamura, Y.; Yamamoto, Y.; Bitoh, A.; Ono, T.; Kagami, H. Analysis of Developmental Changes in Avian DNA Methylation Using a Novel Method for Quantifying Genome-Wide DNA Methylation. J. Poult. Sci. 2009, 46, 286–290. [Google Scholar] [CrossRef]
- Saeed, B.; Baban, R.; Al-Nasiri, U. Lactate Dehydrogenase C4 (LDH-C4) Is Essential for the Sperm Count and Motility: A Case-Control Study. Baghdad J. Biochem. Appl. Biol. Sci. 2021, 2, 146–159. [Google Scholar] [CrossRef]
- Odet, F.; Duan, C.; Willis, W.; Goulding, E.; Kung, A.; Eddy, M.; Goldberg, E. Lactate Dehydrogenase-C4 (LDH-C4) Is Essential for Sperm Function. Biol. Reprod. 2008, 78, 187. [Google Scholar] [CrossRef]
- Badura, A.; Marzec-Wróblewska, U.; Kamiński, P.; Łakota, P.; Ludwikowski, G.; Szymański, M.; Wasilow, K.; Lorenc, A.; Buciński, A. Prediction of Semen Quality Using Artificial Neural Network. J. Appl. Biomed. 2019, 17, 167–174. [Google Scholar] [CrossRef]
- McCallum, C.; Riordon, J.; Wang, Y.; Kong, T.; You, J.B.; Sanner, S.; Lagunov, A.; Hannam, T.G.; Jarvi, K.; Sinton, D. Deep Learning-Based Selection of Human Sperm with High DNA Integrity. Commun. Biol. 2019, 2, 250. [Google Scholar] [CrossRef]
- Althnian, A.; AlSaeed, D.; Al-Baity, H.; Samha, A.; Dris, A.B.; Alzakari, N.; Abou Elwafa, A.; Kurdi, H. Impact of Dataset Size on Classification Performance: An Empirical Evaluation in the Medical Domain. Appl. Sci. 2021, 11, 796. [Google Scholar] [CrossRef]
- Lee, B.D.; Gitter, A.; Greene, C.S.; Raschka, S.; Maguire, F.; Titus, A.J.; Kessler, M.D.; Lee, A.J.; Chevrette, M.G.; Stewart, P.A.; et al. Ten Quick Tips for Deep Learning in Biology. PLoS Comput. Biol. 2022, 18, e1009803. [Google Scholar] [CrossRef] [PubMed]
- Ratner, A.; de Sa, C.; Wu, S.; Selsam, D.; Ré, C. Data Programming: Creating Large Training Sets, Quickly. Adv. Neural Inf. Process. Syst. 2016, 29. [Google Scholar] [CrossRef]
- Kusonmano, K.; Netzer, M.; Pfeifer, B.; Baumgartner, C.; Liedl, K.R.; Graber, A. Evaluation of the Impact of Dataset Characteristics for Classification Problems in Biological Applications. Int. J. Biomed. Biol. Eng. 2009, 3, 309–313. [Google Scholar]
- Ruparel, N.H.; Shahane, N.M.; Bhamare, D.P. Learning from Small Data Set to Build Classification Model: A Survey. In IJCA Proceedings on International Conference on Recent. Trends in Engineering and Technology 2013; IJCA Journal: New York, NY, USA, 2013; pp. 23–26. [Google Scholar]
- Zhang, G.P. Neural Networks for Classification: A Survey. IEEE Trans. Syst. Man. Cybern. Part. C Appl. Rev. 2000, 30, 451–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, Y.; Li, Q.; Ma, J.; Li, S.; Lv, X.; Lv, W. Empirical Study of Seven Data Mining Algorithms on Different Characteristics of Datasets for Biomedical Classification Applications. Biomed. Eng. Online 2017, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Zupan, B. Hands-on training about overfitting. PLOS Comput. Biol. 2021, 17, e1008671. [Google Scholar] [CrossRef]
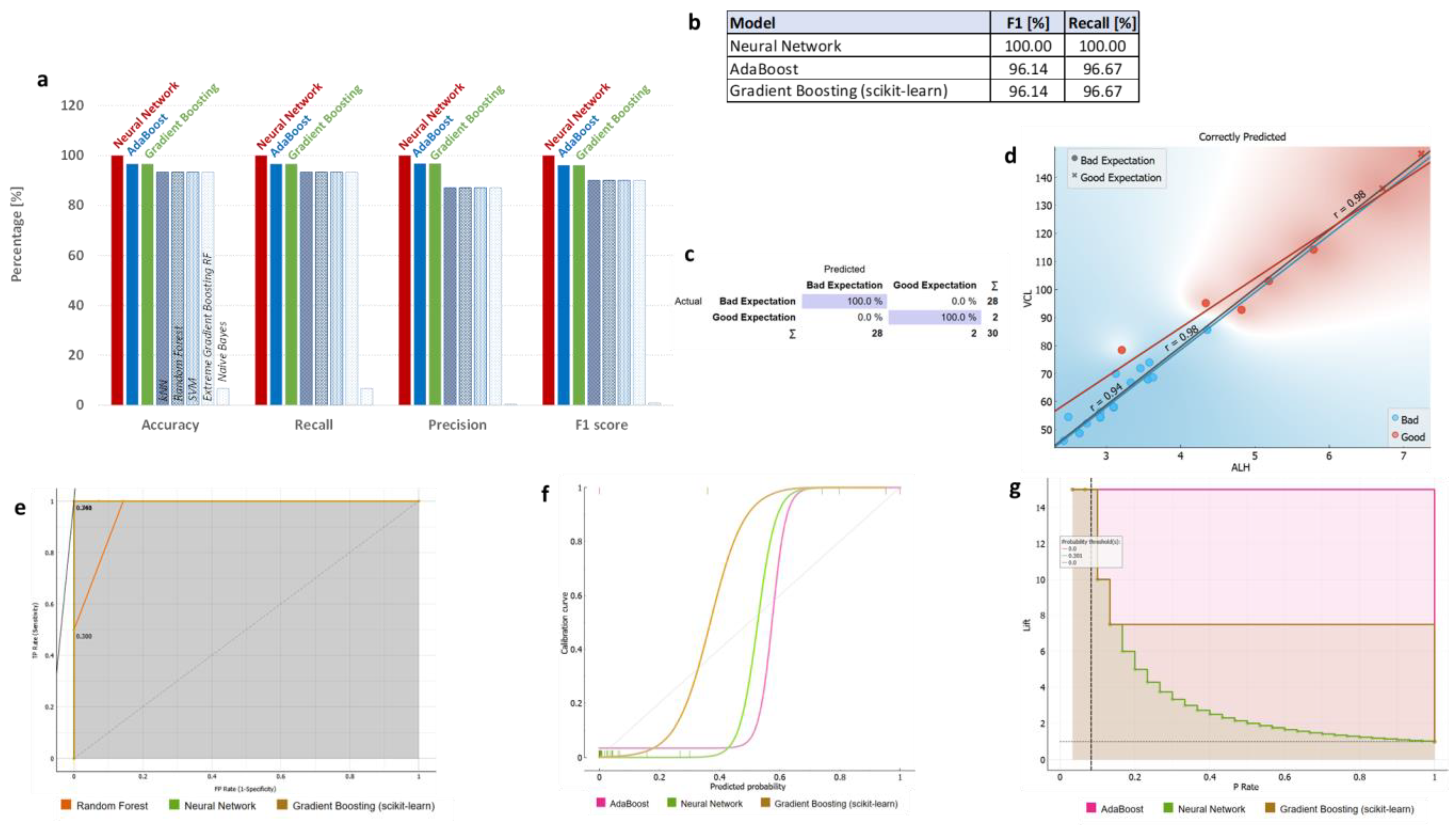
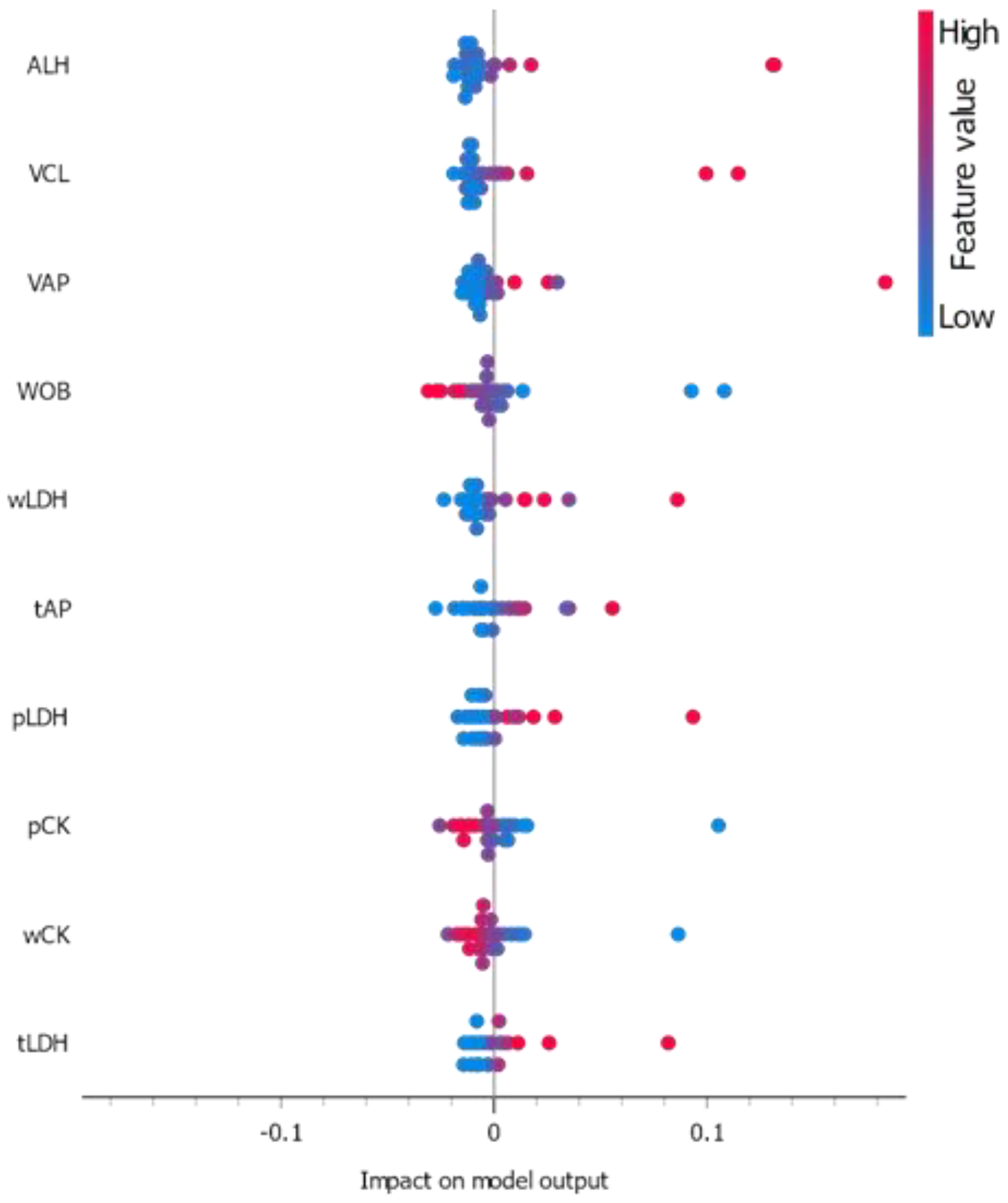
| Traits | Parameter “Quality”: “Good” | Parameter “Quality”: “Bad” | Significant Differences | p-Value |
|---|---|---|---|---|
| Motility | ||||
| Total motility, % | 99.23 ± 0.34 | 75.32 ± 2.71 | 0.000 | p < 0.001 |
| Progressive motility, % | 69.42 ± 6.05 | 25.68 ± 2.32 | 0.000 | p < 0.001 |
| Non-progressive motility, % | 29.81 ± 5.81 | 49.64 ± 1.06 | 0.000 | p < 0.001 |
| Static spz, % | 0.77 ± 0.34 | 24.68 ± 2.71 | 0.000 | p < 0.001 |
| Velocity motion parameters | ||||
| Rapid spz, | 56.57 ± 8.91 | 13.41 ± 1.44 | 0.000 | p < 0.001 |
| Medium spz, | 31.94 ± 5.38 | 26.50 ± 1.98 | 0.248 | n.s. |
| Slow spz, | 10.72 ± 3.57 | 35.32 ± 1.71 | 0.000 | p < 0.001 |
| Velocity parameters | ||||
| VCL, µm/s | 109.75 ± 9.43 | 60.78 ± 2.03 | 0.000 | p < 0.001 |
| VAP, µm/s | 46.35 ± 6.65 | 24.67 ± 1.65 | 0.000 | p < 0.001 |
| VSL, µm/s | 52.86 ± 4.85 | 34.00 ± 1.67 | 0.000 | p < 0.001 |
| STR, % | 44.18 ± 6.64 | 41.64 ± 1.84 | 0.606 | n.s. |
| LIN, % | 47.44 ± 4.00 | 55.22 ± 1.73 | 0.051 | n.s. |
| WOB, % | 59.59 ± 2.84 | 59.76 ± 0.83 | 0.936 | n.s. |
| ALH, µm | 5.33 ± 0.52 | 3.14 ± 0.09 | 0.000 | p < 0.001 |
| BCF, Hz | 5,43 ± 0.32 | 4.68 ± 0.17 | 0.041 | p < 0.05 |
| Morphological characteristics | ||||
| Live normal spz, % | 85.86 ± 2.29 | 80.14 ± 1.23 | 0.033 | p < 0.05 |
| Head defects spz, % | 4.14 ± 1.03 | 5,68 ± 0.79 | 0.333 | n.s. |
| Midpiece defects spz, % | 5.88 ± 1.58 | 9.65 ± 0.84 | 0.040 | p < 0.05 |
| Tail defects spz, % | 4.14 ± 1.32 | 4.37 ± 0.58 | 0.862 | n.s. |
| Factor | AP (Alkaline Phosphatase) | LDH (Lactate Dehydrogenase) | CK (Creatine Kinase) | GGT (Gamma-Glutamyl Transferase) | |
|---|---|---|---|---|---|
| Semen “Quality” Parameter | Semen Fractions | ||||
| Good | seminal plasma (sp) | 16.00 ± 3.49 b | 271.29 ± 81.23 | 65.29 ± 13.35 a | 385.29 ± 56.29 ab |
| water sperm extract (w) | 36.86 ± 8.84 ab | 241.70 ± 74.35 | 49.57 ± 11.79 ab | 308.00 ± 53.90 b | |
| Triton sperm extract (t) | 19.29 ± 2.64 b | 132.86 ± 32.51 | 17.29 ± 4.19 b | 693.00 ± 49.34 a | |
| Bad | seminal plasma (sp) | 89.61 ± 20.75 ab | 244.17 ± 53.39 | 77.44 ± 7.24 a | 286.16 ± 64.35 b |
| water sperm extract (w) | 59.44 ± 6.94 ab | 179.60 ± 43.99 | 77.45 ± 5.41 a | 227.61 ± 55.51 b | |
| Triton sperm extract (t) | 26.74 ± 3.25 ab | 85.99 ± 21.22 | 63.57 ± 8.19 a | 285.44 ± 65.78 b | |
| mean ± SEM | 50.53 ± 6.30 | 180.50 ± 21.21 | 66.10 ± 3.77 | 312.07 ± 30.51 | |
| Semen “Quality” (Bad × Good) | 0.020 (p < 0.05) | 0.369 (n.s.) | 0.001 (p < 0.01) | 0.06 (n.s.) | |
| Semen fractions (sp × w × t) | 0.07 (n.s.) | 0.010 (p < 0.05) | 0.039 (p < 0.05) | 0.200 (n.s.) | |
| Semen “Quality” × Semen fractions | 0.001 (p < 0.01) | 0.075 (n.s.) | 0.001 (p < 0.01) | 0.008 (p < 0.05) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abadjieva, D.; Georgiev, B.; Gerzilov, V.; Tsvetkova, I.; Taushanova, P.; Todorova, K.; Hayrabedyan, S. Machine Learning Approach for Muscovy Duck (Cairina moschata) Semen Quality Assessment. Animals 2023, 13, 1596. https://doi.org/10.3390/ani13101596
Abadjieva D, Georgiev B, Gerzilov V, Tsvetkova I, Taushanova P, Todorova K, Hayrabedyan S. Machine Learning Approach for Muscovy Duck (Cairina moschata) Semen Quality Assessment. Animals. 2023; 13(10):1596. https://doi.org/10.3390/ani13101596
Chicago/Turabian StyleAbadjieva, Desislava, Boyko Georgiev, Vasko Gerzilov, Ilka Tsvetkova, Paulina Taushanova, Krassimira Todorova, and Soren Hayrabedyan. 2023. "Machine Learning Approach for Muscovy Duck (Cairina moschata) Semen Quality Assessment" Animals 13, no. 10: 1596. https://doi.org/10.3390/ani13101596
APA StyleAbadjieva, D., Georgiev, B., Gerzilov, V., Tsvetkova, I., Taushanova, P., Todorova, K., & Hayrabedyan, S. (2023). Machine Learning Approach for Muscovy Duck (Cairina moschata) Semen Quality Assessment. Animals, 13(10), 1596. https://doi.org/10.3390/ani13101596





